
Research Advances in mRNA Vaccine Manufacturing Processes
Jianyao Sun
College of Agriculture, Yanbian University, Yanji City, Jilin, China
Keywords: mRNA Vaccines, Lipid Nanoparticles (LNPs), Manufacturing Process.
Abstract: The rapid development and deployment of mRNA vaccines have revolutionized the field of vaccinology,
particularly highlighted by their pivotal role in combating the COVID-19 pandemic. This review
systematically explores the technological advancements in mRNA vaccine production, emphasizing key
processes such as plasmid DNA (pDNA) preparation, in vitro transcription (IVT), mRNA purification, and
lipid nanoparticle (LNP) encapsulation. Innovations in nucleotide modification, codon optimization, and LNP
formulation have enhanced mRNA stability, translation efficiency, and delivery precision. Despite these
breakthroughs, challenges persist, including scalability limitations, batch variability in IVT and LNP
production, intellectual property disputes, and the need for harmonized regulatory frameworks. Emerging
delivery systems—such as polymer-based carriers, inorganic nanomaterials, and peptide derivatives—offer
promising alternatives to LNPs, potentially improving tissue targeting and reducing immunogenicity.
Furthermore, the application of mRNA technology extends beyond infectious diseases to cancer
immunotherapy and protein replacement therapies. The review underscores the importance of standardized
quality control protocols, sustainable supply chains, and global collaboration to address manufacturing
bottlenecks and ensure equitable access. By resolving these challenges, mRNA vaccines are poised to become
a cornerstone of 21st-century medicine, offering versatile solutions for evolving health crises.
1 INTRODUCTION
1.1 Type Area Introduction
The advent of mRNA vaccines represents a paradigm
shift in biomedical innovation, combining rapid
development cycles, precise antigen design, and
adaptable manufacturing processes to address global
health challenges. Since the discovery of mRNA’s
role as a protein synthesis template in the 1960s,
decades of research have focused on overcoming its
inherent instability and inefficient delivery. Early
breakthroughs, such as Wolff et al.’s 1990
demonstration of in vivo protein expression via
injected naked mRNA, highlighted its therapeutic
potential but underscored the need for robust delivery
systems (Wolff et al., 2020). The emergence of lipid
nanoparticles (LNPs) in the 2010s revolutionized
mRNA technology by enabling efficient cytoplasmic
delivery while enhancing stability and reducing
immunogenicity. These advancements paved the way
for mRNA vaccines to transition from experimental
platforms to clinical reality, exemplified by the rapid
deployment of COVID-19 vaccines like BNT162b2
and mRNA-1273 during the pandemic (Teo et
al.,2022.). Their success, however, hinged on
establishing robust manufacturing frameworks
capable of producing these genetic vaccines at
unprecedented scales.Central to mRNA vaccine
production is a multi-step process that begins with
plasmid DNA (pDNA) template preparation. pDNA,
amplified in bacterial hosts, undergoes linearization
and serves as the blueprint for in vitro transcription
(IVT). During IVT, RNA polymerase synthesizes
mRNA strands incorporating modified nucleotides
(e.g., pseudouridine) to minimize innate immune
activation. Subsequent purification steps, including
tangential flow filtration and chromatographic
methods, remove enzymatic residues and ensure
mRNA integrity. The final formulation relies on
LNPs, which encapsulate mRNA via microfluidic
mixing, optimizing particle size and encapsulation
efficiency. Each stage demands stringent quality
control, from verifying pDNA supercoiling ratios to
assessing 5’ capping efficiency and poly(A) tail
length, as outlined by regulatory bodies like the FDA
and NMPA (Verbeke et al., 2021). Despite having
these advances, challenges still remain. Scalability
458
Sun, J.
Research Advances in mRNA Vaccine Manufacturing Processes.
DOI: 10.5220/0014499200004933
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 1st International Conference on Biomedical Engineering and Food Science (BEFS 2025), pages 458-464
ISBN: 978-989-758-789-4
Proceedings Copyright © 2026 by SCITEPRESS – Science and Technology Publications, Lda.

remains hindered by the lack of standardized
protocols for large-scale IVT and LNP formulation.
Patent disputes over critical components, such as
ionizable lipids, further complicate global
accessibility. Additionally, long-term stability studies
and harmonized regulatory frameworks for
intermediate products are urgently needed to ensure
batch consistency. Innovations in alternative delivery
systems—such as polymer-based carriers or
inorganic nanoparticles—and advancements in codon
optimization algorithms promise to address these
gaps. Beyond infectious diseases, mRNA platforms
are being repurposed for cancer immunotherapy and
protein replacement therapies, underscoring their
versatility. This review examines the technological
milestones in mRNA vaccine production, analyzes
current bottlenecks, and explores future directions to
enhance manufacturability, safety, and therapeutic
scope.
2 mRNA VACCINES
2.1 Introduction to the Background of
mRNA Vaccines
In 1961, research revealed that mRNA could serve as
a direct template guiding protein biosynthesis
(BRENNER et al.,1961, SMULL et al.,1962) In 1963,
Isaacs et al. (ISAACS et al., 1963.) discovered that
mRNA could induce the production of interferon.
mRNA can induce the production of interferon;
however, due to its inherent instability, it was not
until 1969 that Lockard and Lingrel successfully
synthesized proteins using isolated mRNA in vitro
(Lockard et al.,1969). In 1990, Wolff et al. (Wolff et
al., 1990) injected naked mRNA obtained through in
vitro transcription directly into the skeletal muscle of
mice, leading to the successful expression of the
corresponding protein in the mice and the generation
of an immune response. This groundbreaking
experiment laid the foundation for further research
into the therapeutic potential of mRNA.
2.1.1 Classification of mRNA Vaccines
mRNA can be classified into two categories based on
genetic characteristics: non-replicating mRNA and
self-amplifying mRNA (saRNA).
Non-replicating mRNA, in addition to containing
a 5' cap structure, a 5' untranslated region (5'-UTR), a
3' untranslated region (3'-UTR), and a Poly(A) tail,
solely encodes the target antigen.(Furuichi et
al.,1975)mRNA technology primarily encompasses
sequence design, delivery system construction, in
vitro transcription (IVT), and formulation
development. The complete molecular structure of
mRNA includes an open reading frame (ORF) that
encodes the target protein, flanked by 5'UTR and
3'UTR regions, a 5' cap structure (or its substitute),
and a 3' sequence [such as a poly(A) tail].Each
component has its own function, and the components
can work in an organic and coordinated manner to
jointly regulate the translation efficiency, stability,
and immunogenicity of mRNA within the body (Zong
et al.,2023, Sahin et al.,2014, Lu et al.,2024).
2.2 The Production Process of mRNA
Vaccines
The production of mRNA active pharmaceutical
ingredients (APIs) primarily involves two
fundamental steps: upstream enzymatic processes
and downstream chromatographic and ultrafiltration
purification. The upstream enzymatic processes
mainly include three enzymatic reactions: plasmid
DNA linearization, mRNA in vitro transcription, and
template DNA digestion. The downstream process
begins with the collection of in vitro transcription
(IVT) reaction products and primarily includes
dilution with nuclease-free water, chromatographic
purification, ultrafiltration diafiltration concentration,
filtration, and filling. The production of mRNA
formulations mainly involves the preparation and
encapsulation of lipid nanoparticles (LNPs), followed
by sterile filtration and formulation filling (Teo et al.,
2022). The specific production process for mRNA
vaccines can be divided into five steps: ①
Preparation of plasmid DNA (pDNA), which includes
the production and purification of pDNA. ② IVT and
modification. First, linearize the pDNA, then use the
linearized plasmid DNA as a template to transcribe
and synthesize mRNA in a cell-free system.
Nucleotide modification can be applied to enhance
the functionality of the mRNA. ③ Filtration and
purification of mRNA. This primarily involves
exchanging the buffer to remove impurities such as
enzymes and nucleotides. ④ Preparation and
encapsulation of LNPs. Currently, microfluidic
mixing technology is primarily used to precisely mix
the mRNA bulk solution with LNPs in a specific
ratio, thereby forming uniformly sized lipid
nanoparticles that encapsulate the mRNA. After steps
such as ultrafiltration and buffer exchange to remove
impurities, the intermediate product of mRNA-LNPs
formulation is ultimately prepared. ⑤ Filling of
Research Advances in mRNA Vaccine Manufacturing Processes
459
mRNA vaccines. After the intermediate product of
the mRNA-LNP formulation has passed the quality
inspection, it is aseptically filled to obtain the finished
product of the mRNA vaccine (Teo et al.,2022).
2.3 Delivery Vehicles
The main carriers for mRNA drug delivery include
lipid-based materials, polymers, protein derivatives,
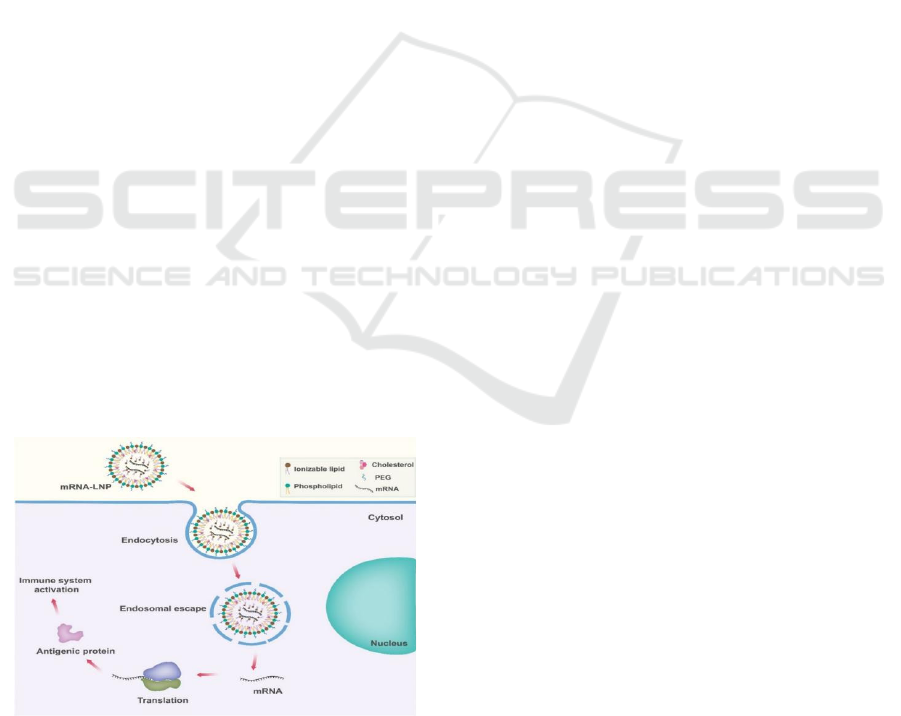
and exosomes. As shown in Figure 1, intracellular
delivery process of mRNA Vaccines (Using LNA-
mRNA Vaccine as an Example): 1. Endocytosis: the
LNA-modified mRNA vaccine, encapsulated in a
lipid nanoparticle (LNP) composed of ionizable
lipid, phospholipid, cholesterol, and PEG, is
internalized by host cells via endocytosis. The
ionizable lipid facilitates membrane interaction,
while PEG stabilizes the nanoparticle and reduces
immune recognition. 2. Endosomal Escape: within
the acidic environment of the endosome, the ionizable
lipid becomes positively charged, destabilizing the
endosomal membrane. This promotes fusion of the
LNP with the endosomal membrane, enabling the
release of the LNA-mRNA into the cytosol.3. LNA-
enhanced Stability and Translation: the incorporation
of Locked Nucleic Acid (LNA) modifications in the
mRNA backbone enhances its stability against
enzymatic degradation and improves translational
efficiency. This ensures prolonged availability of
intact mRNA in the cytosol.4. Translation of
Antigenic Protein: the released mRNA is recognized
by ribosomes, which translate it into the antigenic
protein (e.g., viral spike protein). No nuclear entry is
required, as mRNA vaccines function entirely in the
cytosol.5. Immune Activation: the antigenic protein
is processed and presented on major
histocompatibility complex (MHC) molecules,
Figure 1: This Diagram Illustrates the Mechanism of
mRNA-LNP Vaccines, Highlighting Key Steps from
Cellular Uptake to Immune Activation.
activating adaptive immune responses (T cells and B
cells). This primes the immune system to recognize
and neutralize future pathogen exposure.
2.3.1 Liposomes and Lipid Complexes
Lipid nanoparticles (LNPs) are commonly used
advanced mRNA delivery carriers. Their emergence
is a key factor in advancing the approval of mRNA
vaccines and has also provided a direction for other
mRNA therapeutic fields (Kimura et al., 2020).
Although mRNA-based therapies possess the a
forementioned advantages, mRNA also faces
challenges such as poor stability and difficulty in
entering the cytoplasm to exert its function
(Kubiatowicz et al., 2022). Therefore, an appropriate
delivery carrier is crucial for mRNA therapy. Lipid
nanoparticles (LNPs) are the most widely used
carriers for nucleic acid drugs in clinical applications.
It can deliver mRNA to the cytoplasm to express
target proteins. Compared to other delivery carriers,
LNP has many advantages such as high encapsulation
efficiency, high transfection efficiency, simple
preparation, stable structure, and good safety (Cullis
et al., 2017, Eygeris et al., 2022). LNPs comprise four
lipids: ionizable, helper, cholesterol, and
PEGylated—each vital for stability, transfection, and
safety (Zong et al., 2023). Ionizable lipids (primary
component) are neutral at physiological pH but
protonate in acidic conditions, enabling electrostatic
mRNA encapsulation during self-assembly (Hald
Albertsen et al., 2022). In serum (neutral pH),
uncharged LNPs avoid protein binding, reducing
macrophage uptake and prolonging circulation. This
pH-responsive charge lowers toxicity versus
permanent cationic lipids. In endosomes, acid-
induced positivity enhances endosomal membrane
interaction, driving mRNA cytoplasmic release and
boosting transfection efficiency (Samaridou et al.,
2020). Helper lipids (primarily phospholipids)
stabilize LNPs and promote endosomal membrane
fusion for mRNA release. Common phospholipids
include DSPC (high phase transition temperature,
enhancing stability) and DOPE (cone-shaped
structure, driving hexagonal phase formation to boost
mRNA transfection efficiency) (Ahmed et al.,
2019). Cholesterol, the second most abundant LNP
component, localizes in the outer shell. It improves
stability, prolongs blood circulation, and increases
membrane fluidity/permeability to facilitate mRNA
release (Sebastiani et al., 2021).
BEFS 2025 - International Conference on Biomedical Engineering and Food Science
460

2.3.2 High Molecular Weight Polymers
Polymer carriers themselves are easy to prepare, and
fine-tuning their surface properties can achieve the
desired effects, promoting cross-presentation of
antigens. Modifying the surface of polymeric
nano/microspheres can mediate targeted delivery to
immune cells, enhancing antigen utilization and
activating the immune system with lower antigen
doses (Malla et al., 2024). Polymer nanocarrier
materials can be categorized into synthetic
macromolecular polymers and natural polymers.
Synthetic polymers are polymer materials
synthesized through chemical methods, which can be
custom-designed according to specific requirements.
Common synthetic macromolecular polymers include
polyethylene, polypropylene, and polystyrene,
among others. These materials are characterized by
their ease of synthesis, low cost, and lack of
immunogenicity (Charbe et al., 2020).
2.3.3 Protein/Peptide Derivative-mRNA
Complex
Peptide/protein vaccines represent one of the most
common types of vaccines, offering advantages such
as well-defined antigenic sequences, low production
costs, and ease of preparation (Mai et al., 2020). Lou
et al. (Lou et al., 2019) developed a straightforward
method for peptide-functionalized mRNA polyplexes
to enhance dendritic cell presentation of mRNA
antigens, demonstrating high cellular uptake and no
cytotoxicity. Mai et al. (Mai et al., 2020) constructed
a delivery carrier based on protamine, which prevents
mRNA degradation, promotes antigen presentation
by antigen-presenting cells, and simultaneously
induces anti-tumor immune responses. Lin et al. (LIN
et al.,2022) developed a novel compound by
conjugating peptides with protoporphyrin for targeted
therapy and photodynamic therapy. Such
formulations provide a strong foundation for the
development of mRNA vaccine delivery systems.
3 mRNA VACCINE
PRODUCTION
3.1 Synthesis of mRNA Fragments
Currently, there are multiple methods for preparing
DNA transcription templates for mRNA vaccines,
such as templates produced using PCR technology or
non-linearized plasmids containing terminator
sequences. These drug templates can be amplified
and expanded in host cells, such as E. coli. However,
in all cases, each batch of DNA plasmids used for the
production of mRNA vaccines must undergo release
testing to confirm their sequence, purity, and quality
(Naveed et al.,2023). For the quality control of
transcription template plasmid DNA, specific
requirements have been outlined in the latest
guideline documents issued by China's NMPA, the
WHO, and the U.S. FDA. The pre-release testing of
plasmid DNA primarily includes five quality control
aspects: identification, concentration, purity, safety,
and others. Each aspect encompasses corresponding
testing items. Regarding the detection of the
percentage of supercoiled content for plasmid DNA
purity, the second edition of the draft guidance issued
by the USP in 2023 has included this item in the pre-
release testing of plasmid DNA. However, the NMPA
and WHO have not yet proposed requirements for this
specific test. The guideline documents from various
countries also recommend corresponding testing
methods, summarized as follows: for the
identification of plasmid DNA, methods include
direct sequencing, next-generation sequencing
(NGS), and restriction enzyme agarose gel
electrophoresis analysis; The detection methods for
the concentration and purity of plasmid DNA include
ultraviolet spectroscopy (UV), among others.The
detection methods for the superhelical percentage
content include capillary electrophoresis (CE) and
high-performance liquid chromatography (HPLC),
among others. The detection methods for host RNA
residual content include agarose gel electrophoresis
and HPLC, among others. The detection methods for
host DNA template residual content include
quantitative real-time PCR (qPCR) Baden et al.,2021,
Liu et al., 2022, Verbeke et al., 2021, Cortese et al.,
2024).
3.2 mRNA Production and
Optimization
The plasmid DNA template is enzymatically
linearized and purified, then used to synthesize
mRNA through in vitro transcription in a cell-free
system. After further purification, the mRNA stock
solution is obtained. Due to the degeneracy of the
genetic code, mRNA can be optimized in terms of
codon usage to achieve more efficient translation and
enhanced stability. Furthermore, modified
nucleotides can be utilized to reduce the
immunogenicity of mRNA, suppress innate immune
activation, and mitigate adverse effects such as
Research Advances in mRNA Vaccine Manufacturing Processes
461

inflammation (Maruggi et al., 2019). The 5' cap can
be introduced co-transcriptionally by adding a
capping reagent to the IVT (in vitro transcription)
mixture. The 3' poly(A) tail can be added
enzymatically or encoded directly within the DNA
template. Therefore, the release testing of mRNA
stock solutions should include specific assessments
such as the 5' capping efficiency and the length of the
3' poly(A) tail (US Pharmacopeia et al., 2023).
3.3 The Intermediate Product of the
Formulation
The NMPA (National Medical Products
Administration) has introduced the concept of
intermediate products in formulations and
emphasized that the definition of these intermediates,
as well as the establishment of quality standards for
them, should be based on the actual conditions of the
mRNA delivery system preparation process. These
intermediates may include products resulting from
the complexation of mRNA with positively charged
polymer materials, nanoparticle intermediates, and
others. The NMPA also pointed out that the testing of
intermediate products is part of process control.
Whether to define a product as an intermediate and
the corresponding testing requirements should
consider the following factors. ①Whether this stage
is the most sensitive phase for the corresponding
testing items. ②Whether subsequent production
processes and formulation compositions have an
impact on the active components, such as whether
lyophilization is performed.③Whether subsequent
process steps require testing at this stage, such as
using the content of active ingredients to guide
formulation preparation (Baden et al., 2021).
3.4 mRNA Vaccine Final Product
After precise mixing of mRNA original solution and
lipid nanoparticles (LNPs) and other delivery systems
in a certain proportion through jet impact mixing
method, microfluidic mixing method and other
technologies, nanoliposomes encapsulating mRNA
with uniform particle size are formed. After that,
through steps such as ultrafiltration and buffer
exchange to remove impurities, and then adding
buffer, sugars, and dispersants, followed by quality
inspection, aseptic filling is carried out to obtain the
final product of mRNA vaccine (Naveed et al.,2023).
4 APPLICATIONS OF mRNA
VACCINES
The COVID-19 pandemic, caused by the SARS-
CoV-2 virus, continues to persist, exerting a
significant impact on human health and the global
economy. Vaccines represent the primary approach to
combating the COVID-19 pandemic. According to
data released by the World Health Organization on
January 24, 2023, there are over 300 COVID-19
vaccine candidates globally, with 176 of them having
entered the clinical trial phase. Leveraging the
advantages of mRNA vaccines in preventing
infectious diseases, COVID-19 mRNA vaccines were
rapidly developed and iterated during the pandemic,
making significant contributions to the fight against
COVID-19. The Biologics License Application
(BLA) was also approved in the US in August 2021,
making it the first mRNA vaccine to be launched
globally. Moderna’s mRNA-1273 (Spikevax) was
granted an Emergency Use Authorization (EUA) in
the US in December 2020, with its Biologics License
Application (BLA) being approved in the US on
February 1, 2022, becoming the second mRNA
vaccine to be launched globally. AWcorna, co-
developed by the Academy of Military Medical
Sciences, Suzhou Abio Biology, and Yunnan Walvax
Biology, was granted an EUA in Indonesia in
September 2022, marking the first EUA obtained by
China overseas. SYS6006 from China
Pharmaceutical Group was granted an EUA in China
in March 2023, becoming the first of its kind in the
country (Cheng et al., 2020).
5 CHALLENGES AND
PROSPECTS IN mRNA
VACCINE PRODUCTION
With the advancement of technology, researchers
have developed some new quality control methods
and strategies. For instance, Packer et al., created a
kinetic model for predicting the shelf life of mRNA
vaccines and utilized this model to estimate the
expiration period of mRNA vaccines (Packer et al.,
2021). BioNTech has developed a ribozyme-based
method combined with denaturing polyacrylamide
gel electrophoresis or liquid chromatography and
mass spectrometry to quantitatively analyze and
detect the capping efficiency of in vitro transcribed
mRNA (Vlatkovic et al., 2022). The production
BEFS 2025 - International Conference on Biomedical Engineering and Food Science
462

of mRNA vaccines, from plasmid templates to final
filling, lacks standardized scale-up and quality
control, posing challenges in manufacturing. In
China, mRNA vaccine development focuses on
COVID-19, with expansion into vaccines for other
infectious diseases (e.g., RSV, influenza) and cancer.
Patent limitations in sequence design and delivery
systems, along with the need for localized raw
materials and equipment, hinder progress. Despite
these challenges, mRNA vaccines show promise in
infectious disease prevention, cancer vaccines, and
protein replacement therapies. In summary, mRNA
vaccines have significant potential in
immunotherapy, relying on stable synthesis,
immunogenicity regulation, and effective delivery
systems. They enhance our ability to combat
infectious diseases and offer new approaches in
cancer treatment by optimizing existing therapies
(Packer et al., 2021). However, addressing ethical,
regulatory, and societal acceptance issues is crucial to
ensure their safe and responsible contribution to
human health and scientific progress.
6 CONCLUSION
The evolution of mRNA vaccine manufacturing has
been marked by transformative innovations, from
nucleotide modifications to LNPs, enabling
unprecedented speed and precision in vaccine
development. The COVID-19 pandemic catalyzed
the industrialization of these technologies, proving
their viability in global health crises. However,
scalability and standardization challenges—such as
inconsistent IVT yields and LNP batch variability—
highlight the need for automated, closed-loop
production systems and universal quality
benchmarks. Regulatory agencies must prioritize
harmonizing guidelines for intermediate products,
particularly mRNA-LNP complexes, to streamline
commercialization. Future progress hinges on
diversifying delivery platforms, such as peptide-
based carriers or stimuli-responsive nanomaterials, to
improve tissue targeting and reduce off-target effects.
Equally critical is addressing intellectual property
barriers and fostering open-access collaborations to
democratize mRNA technology. Investments in
sustainable raw material supply chains and localized
manufacturing infrastructure will enhance global
equity, particularly for low-resource regions. As
mRNA platforms expand into oncology and genetic
disorders, rigorous long-term safety assessments and
public education initiatives are essential to build trust.
In summary, mRNA vaccines epitomize the
convergence of molecular biology and
bioengineering, offering a dynamic toolset against
evolving pathogens and complex diseases. By
resolving existing technical and logistical hurdles,
this technology can fulfill its promise as a cornerstone
of 21st-century medicine.
REFERENCES
Ahmed, K. S. 2019. Liposome: composition,
characterisation, preparation, and recent innovation in
clinical applications. Journal of drug targeting, 27(7),
742–761.
Baden, L. R. 2021. Efficacy and Safety of the mRNA-1273
SARS-CoV-2 Vaccine. The New England journal of
medicine, 384(5), 403–
416. https://doi.org/10.1056/NEJMoa2035389
Brenner, S. 1961. An unstable intermediate carrying
information from genes to ribosomes for protein
synthesis. Nature, 190, 576–581.
Charbe, N. B. 2020. Small interfering RNA for cancer
treatment: overcoming hurdles in delivery. Acta
pharmaceutica Sinica. B, 10(11), 2075–2109.
Cheng, Q. 2020. Selective organ targeting (SORT)
nanoparticles for tissue-specific mRNA delivery and
CRISPR-Cas gene editing. Nature nanotechnology,
15(4), 313–320.
Cortese, S. 2024. Psychopharmacology in children and
adolescents: unmet needs and opportunities. The lancet.
Psychiatry, 11(2), 143–
154. https://doi.org/10.1016/S2215-0366(23)00345-0
Cullis, P. R. 2017. Lipid Nanoparticle Systems for Enabling
Gene Therapies. Molecular therapy, 25(7), 1467–1475.
Eygeris, Y. 2022. Chemistry of Lipid Nanoparticles for
RNA Delivery. Accounts of chemical research, 55(1),
2–12.
Furuichi, Y. 1975. A blocked structure at the 5' terminus of
mRNA from cytoplasmic polyhedrosis virus. Nature,
253(5490), 374–375.
Hald Albertsen, C. 2022. The role of lipid components in
lipid nanoparticles for vaccines and gene therapy.
Advanced drug delivery reviews, 188, 114416.
Isaacs, A., & Cox, R. A. 1963. Foreign nucleic acids as the
stimulus to make interferon. Lancet, 2(7299), 113–116.
Kimura, N. 2020. Development of a Microfluidic-Based
Post-Treatment Process for Size-Controlled Lipid
Nanoparticles and Application to siRNA Delivery.
ACS applied materials & interfaces, 12(30), 34011–
34020.
Kubiatowicz, L. J. 2022. mRNA nanomedicine: Design and
recent applications. Exploration, 2(6), 20210217.
Lin, H. X. 2022. An acid-targeting peptide can be used as a
carrier for photodynamic therapy (PDT). Mater Today
Commun, 31, 103659.
Liu, M. A. 2022. WHO informal consultation on regulatory
considerations for evaluation of the quality, safety and
Research Advances in mRNA Vaccine Manufacturing Processes
463

efficacy of RNA-based prophylactic vaccines for
infectious diseases, 20-22 April 2021. Emerging
microbes & infections, 11(1), 384–391.
Lockard, R. E. 1969. The synthesis of mouse hemoglobin
beta-chains in a rabbit reticulocyte cell-free system
programmed with mouse reticulocyte 9S RNA.
Biochemical and biophysical research communications,
37(2), 204–212.
Lou, B. 2019. mRNA Polyplexes with Post-Conjugated
GALA Peptides Efficiently Target, Transfect, and
Activate Antigen Presenting Cells. Bioconjugate
chemistry, 30(2), 461–475.
Lu, C. 2024. Safety, Immunogenicity, and Mechanism of a
Rotavirus mRNA-LNP Vaccine in Mice. Viruses,
16(2), 211.
Mai, Y. 2020. Intranasal delivery of cationic liposome-
protamine complex mRNA vaccine elicits effective
anti-tumor immunity. Cellular immunology, 354,
104143.
Malla, R. 2024. mRNA vaccines and their delivery
strategies: A journey from infectious diseases to cancer.
Molecular therapy, 32(1), 13–31.
Maruggi, G. 2019. mRNA as a Transformative Technology
for Vaccine Development to Control Infectious
Diseases. Molecular therapy, 27(4), 757–772.
Naveed, M. 2023. A one-health approach to design an
mRNA-based vaccine candidate against the lumpy skin
disease virus as an alternative to live-attenuated
vaccines. European review for medical and
pharmacological sciences, 27(13), 6401–6413.
Packer, M. 2021. A novel mechanism for the loss of mRNA
activity in lipid nanoparticle delivery systems. Nature
communications, 12(1), 6777.
Sahin, U. 2014. mRNA-based therapeutics--developing a
new class of drugs. Nature reviews. Drug discovery,
13(10), 759–780.
Samaridou, E. 2020. Lipid nanoparticles for nucleic acid
delivery: Current perspectives. Advanced drug delivery
reviews, 154-155, 37–63.
Sebastiani, F. 2021. Apolipoprotein E Binding Drives
Structural and Compositional Rearrangement of
mRNA-Containing Lipid Nanoparticles. ACS nano,
15(4), 6709–6722.
Shi, S. 2015. VEGFR targeting leads to significantly
enhanced tumor uptake of nanographene oxide in vivo.
Biomaterials, 39, 39–46.
Smull, C. E. 1962. Enhancement of the plaque-forming
capacity of poliovirus ribonucleic acid with basic
proteins. Journal of bacteriology, 84(5), 1035–1040.
Teo, S. P. 2022. Review of COVID-19 mRNA Vaccines:
BNT162b2 and mRNA-1273. Journal of pharmacy
practice, 35(6), 947–951.
Verbeke, R. 2021. The dawn of mRNA vaccines: The
COVID-19 case. Journal of controlled release, 333,
511–520.
Vlatkovic, I. 2022. Ribozyme Assays to Quantify the
Capping Efficiency of In Vitro-Transcribed mRNA.
Pharmaceutics, 14(2), 328.
Wolff, J. A. 1990. Direct gene transfer into mouse muscle
in vivo. Science, 247(4949 Pt 1), 1465–1468.
Yang, A. 2021. Hydrogel/nanoadjuvant-mediated
combined cell vaccines for cancer immunotherapy.
Acta biomaterialia, 133, 257–267.
Zong, Y. 2023. Lipid Nanoparticle (LNP) Enables mRNA
Delivery for Cancer Therapy. Advanced materials,
35(51), e2303261.
BEFS 2025 - International Conference on Biomedical Engineering and Food Science
464
