
Advances in Sleep EEG Signaling in Alzheimer's Disease Prediction
Yulin Jiang
College of Chemical Engineering, Huaqiao University, Xiamen, 362021, China
Keywords: Alzheimer’s Disease, Sleep EEG, NREM Sleep.
Abstract: With the acceleration of global aging, Alzheimer's Disease (AD) has emerged as a grave public health issue.
At present, the commonly employed diagnostic methods have certain drawbacks. In contrast, sleep
electroencephalography (EEG) signals have garnered significant attention in the area of AD prediction,
mainly because of their non - invasive nature, repeatability, and low cost. In this paper, we review the research
progress of sleep EEG signals in AD prediction, elaborate the pathological mechanisms of AD, compare the
advantages and disadvantages of traditional detection methods, and analyze the current status and
development of sleep stage classification system technology is ongoing. When concentrating on the
connection between non - rapid eye movement (NREM) sleep stages and AD, it has been discovered that in
AD patients, the σactivity shows a decline and the EEG undergoes a slowdown during NREM sleep, and that
σ power during NREM sleep is positively correlated with cognitive ability, which may be used as a reference
standard for AD detection. Future research efforts should be dedicated to optimizing the algorithm in order to
enhance the precision of sleep stage classification, integrate multimodal data to explore the relationship
between sleep and AD, and carry out a large-scale longitudinal study to validate the sleep EEG indexes, so as
to promote the development of early warning and precise intervention for AD.
1 INTRODUCTION
In today's society, with the acceleration of global
aging, Alzheimer's Disease (AD), as a
neurodegenerative disease, is the most common form
of dementia, the third most expensive disease and the
sixth leading cause of death worldwide. It has become
a serious public health challenge.
Following the deposition of insoluble amyloid-β
(Aβ), tau accumulates in neocortical cells, leading to
neuronal cell death, synapse loss, brain volume
reduction, and cognitive impairment. In the absence
of cognitive symptoms, the progression of
Alzheimer's disease (AD) involves the gradual
accumulation of pathological changes, creating a
critical window for timely therapeutic intervention.
Sleep patterns are now emerging as a potential
biomarker for AD pathology and a predictor of future
cognitive decline Lucey, et al.,2019).
AD is difficult to diagnose, and symptoms can be
easily misinterpreted as a normal consequence of
aging, requiring multiple investigations and the
exclusion of other causes. Two significant
pathological changes occur: the deposition of β -
amyloid plaques and the formation of
hyperphosphorylated tau neurofibrillary tangles.
Biomarkers like cerebrospinal fluid (CSF) analysis
and positron emission tomography (PET) imaging,
when integrated with clinical evaluations, are
commonly employed to diagnose the disease, but the
former is an invasive procedure that causes
physiological discomfort to the patient such as the
risk of infection and pain at the puncture site, and the
latter is expensive to perform with expensive
equipment and a high cost of learning, which greatly
limits its popularity. The latter is expensive and costly
to learn, which greatly limits its popularity. On the
other hand, sleep electroencephalography (EEG)
signal acquisition EEG devices are affordable. In
recent years, the progress of sleep stage recognition
technology has been remarkable. Due to the
limitation of manual scoring, the development of
automatic sleep stage classification system (ASSC)
has been accelerated, using the PhysioNet Sleep EDF
database and a decision tree classifier, the model
achieved an average sensitivity of 89.06%, specificity
of 98.61%, and accuracy of 93.13%., which improved
the feasibility and speed of practical application of
ASSC (Lucey, et al.,2019). feasibility and speed of
practical application (Aboalayon, et al., 2016).
Jiang, Y.
Advances in Sleep EEG Signaling in Alzheimer’s Disease Prediction.
DOI: 10.5220/0014386400004933
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 1st International Conference on Biomedical Engineering and Food Science (BEFS 2025), pages 39-45
ISBN: 978-989-758-789-4
Proceedings Copyright © 2026 by SCITEPRESS – Science and Technology Publications, Lda.
39

During sleep, the electrical activity of the brain
exhibits a rich variety of regular changes, which
contain a vast amount of information regarding the
state of brain health. Sleep is mainly classified into
two stages: Rapid Eye Movement (REM) sleep and
Non - Rapid Eye Movement (NREM) sleep, and each
of them has unique electrical characteristics and
physiological functions, and may play different but
interrelated roles in the development of AD.
Given the unique advantage of sleep EEG signal in
reflecting the functional state of the brain, as well as
its noninvasive, reproducible, and relatively low-cost
features, investigating the use of sleep EEG signals
for AD prediction not only broadens our
comprehension of AD pathogenesis but also offers
innovative approaches for its early detection. The
present study aims to review the research progress of
sleep EEG signaling in AD prediction in recent years,
focusing on the changes of EEG characteristics
during REM and NREM sleep stages and their
correlation with the pathophysiological process of
AD, evaluating the strengths and weaknesses of
current research methods and exploring future
directions, this study aims to establish a solid
theoretical foundation and practical guidance for
early AD detection and targeted intervention.
2 AD PATHOGENESIS
2.1 Mechanisms of Aβ and Tau in the
Induction of AD
Aβ is produced by cleavage of amyloid precursor
protein (APP) by β - secretase and γ - secretase.
Under normal conditions, Aβ can be cleared, but in
AD patients, there is an imbalance between the
production and clearance of Aβ, leading to the
abnormal deposition of Aβ in the brain and the
formation of senile plaques. Aβ oligomers have
neurotoxicity, which can bind with receptors on the
cell membrane of neurons, these disruptions impair
neural signaling and synaptic plasticity, while
simultaneously promoting the generation of reactive
oxygen species. This leads to oxidative stress, which
damages cell membranes, proteins, and mitochondria,
ultimately causing the death of neurons.
Normally, tau protein promotes the assembly of
microtubules and maintains their stability to ensure
intra-neuronal substance transportation. In AD, tau
protein is abnormally hyperphosphorylated and its
ability to bind to microtubules decreases, resulting in
microtubule depolymerization, which destroys the
cytoskeleton structure of neurons, affects axonal
transport, and prevents neurons from taking up
nutrients and transmitting signals normally. Over-
phosphorylated tau protein aggregates to form
neurogenic fiber tangles. These tangles accumulate in
neurons, hindering normal physiological activities of
neurons, and can spread among neurons, accelerating
neurodegeneration.
2.2 Other Relevant Pathological
Factors and Interactions
Aβ deposition, tau protein abnormalities, impaired
mitochondrial function, and cerebrovascular
pathology all play key roles in the complex
pathogenesis of AD.
Aβ is produced by cleavage of APPs by specific
enzymes and is normally cleared. In AD patients, the
balance between Aβ production and clearance is
disrupted, leading to its excessive accumulation in the
brain. tau proteins become abnormally
hyperphosphorylated, disrupting their ability to bind
to microtubules and interfering with intra-neuronal
transport of substances. the deposition of Aβ and the
abnormalities of the tau proteins activate microglial
cells and astrocytes. Activated microglia release pro -
inflammatory cytokines, such as interleukin - 1β (It
seems there might be a mistake in your original
"interleukin - 1 Aβ", perhaps you meant interleukin -
1β) and tumor necrosis factor - α, while astrocytes
expand in reaction to inflammatory signals, releasing
a variety of cytokines and chemokines, which triggers
neuroinflammation and damage to neuronal cells.
Mitochondrial function is also impaired in the brain
of AD patients. Mitochondrial dysfunction can trigger
oxidative stress, partially clarifying the intricate
mechanisms behind oxidative damage in AD. Beyond
ATP production, mitochondria play a key role in
controlling cell death by storing various apoptotic
factors, which are released during apoptosis. In AD
patients, mitochondrial impairment, elevated
oxidative stress, and neuronal apoptosis have been
observed (Moreira, et al., 2012). These findings imply
that mitochondrial malfunction could be the impetus
behind neuronal degeneration and demise in AD.
Disruptions in the mitochondrial respiratory chain
and impaired electron transport lead to a decline in
membrane potential and diminished energy
generation. At the same time, reactive oxygen species
production increases, exceeding the cellular
antioxidant capacity and oxidatively damaging lipids,
BEFS 2025 - International Conference on Biomedical Engineering and Food Science
40

proteins, and other biomolecules, making neurons
more susceptible to oxidative stress damage.
Certain gene mutations are closely related to AD,
such as mutations in APP, progerin 1 (PS1) and
progerin 2 (PS2), which can lead to familial AD; and
the ε4 allele of the apolipoprotein E (APOE) gene
significantly heightens the risk of developing
sporadic AD (Scheltens, et al., 2021).
Cerebrovascular lesions are equally important in
the development of AD. Chronic hypertension also
impairs the integrity of the blood-brain barrier (BBB),
leading to cerebral edema and the introduction of
systemic elements into the brain parenchyma, and
chronic hypertension also impairs the integrity of the
BBB, resulting in brain swelling and the infiltration
of systemic components into brain tissue (Santos, et
al., 2012). Cerebrovascular endothelial dysfunction
affects vasodilatation and vasoconstriction, resulting
in reduced cerebral blood flow, inadequate nutrient
supply to brain tissue, and accumulation of metabolic
wastes. The damage to the blood-brain barrier is even
more serious, its permeability increases, harmful
substances enter the brain tissue, triggering
inflammation and immune damage, and it also affects
the removal of Aβ, prompting the further deposition
of Aβ and accelerating the development of AD
disease. These pathologic processes interact with
each other and jointly promote the development and
deterioration of AD.
3 PROGRESS IN DETECTION
RESEARCH
3.1 Traditional Testing Methods and
Limitations
3.1.1 Mini-Mental State Examination
(MMSE)
The MMSE is a widely used clinical instrument for
AD detection. This 30-question test evaluates
cognitive abilities, including attention, orientation,
memory, calculation, language, and visuospatial
skills, such as drawing complex shapes (Arevalo, et
al., 2012). The MMSE score offers a quantitative
measure of cognitive decline in older adults, aiding
doctors in diagnosis and treatment planning.
However, the assessment dimensions of MMSE are
limited, mainly focusing on several major aspects of
cognitive function, and the assessment of some
complex cognitive functions, such as executive
function and social cognition, is not comprehensive
enough. If the elderly has a high level of education,
there may be cases where the MMSE score is still in
the normal range even though there is some cognitive
impairment, thus masking the condition. For some
specific cognitive dysfunctions, such as executive
dysfunction, the MMSE may not be able to detect
them accurately, which may lead to an incomplete
assessment of the patient's cognitive function.
Patients with AD may develop these impairments
during the course of the disease, but the difficulty of
detecting them on MMSE may affect the overall
judgment of the patient's condition.
3.1.2 CSF
Biomarkers in CSF can directly reflect the
pathophysiologic process of AD in the brain.
Pathologic changes, such as Aβ deposition, may
occur in the brain before the onset of clinical
symptoms of AD, and biomarker levels in the CSF
may change accordingly. The most intensively
investigated biomarkers of Alzheimer's disease (AD)
are the cerebrospinal fluid proteins that are
pathologically related, namely β - amyloid 42 (Aβ
1 - 42), total tau (t - tau), and tau phosphorylated at
amino acid 181 (p - tau181). Many laboratories use
enzyme-linked immunosorbent assays (ELISA) to
detect these proteins (Wang et al., 2012). By
immobilizing an antibody that specifically recognizes
Aβ42 on a solid-phase carrier and adding it to a CSF
sample, the Aβ42 in the sample will bind to the
antibody, and then an enzyme-labeled secondary
antibody will be added, which will produce a color
change through the reaction between the enzyme and
the substrate, and then the absorbance will be
measured by using an enzyme marker, and compared
with a standard curve. If the level of Aβ42 in the
CSF decreases significantly, it suggests that
Alzheimer's disease may be present. However, the
CSF test requires lumbar puncture to obtain CSF,
which is an invasive operation that may bring some
pain and risk to patients, and the CSF test involves
special testing equipment, reagents, and specialized
technicians, and the overall cost is relatively high,
which may bring some financial burden to patients
and the health insurance system, and to a certain
extent, limit its wide application.
3.1.3 PET
PET technology allows for the evaluation of various
functional processes in the brain of AD patients
Advances in Sleep EEG Signaling in Alzheimer’s Disease Prediction
41

during their survival. This method allows for the 3D
visualization and quantification of metabolic (glucose
metabolism) and neurotransmitter activity. It also
provides insights into the pathological mechanisms of
AD. PET scans enable clinicians to visually analyze
results through color coding and, crucially, gather
quantitative data on brain regions. This data supports
objective evaluation of diagnostic precision and
treatment outcomes. PET can identify early metabolic
and pathological brain changes before noticeable
clinical symptoms appear. With specific tracers, such
as the glucose analog of brain glucose metabolism, 2-
[18F]-fluoro-2-deoxygenase, PET is able to detect
subtle metabolic and pathological changes in the
brain before they become clinically apparent.
Oxygen-d-glucose (18F-FDG) can be used to
monitor cerebral glucose metabolism (Nordberg,et
al., 2012). This tracer has been widely used in
radiopharmaceutical imaging studies and clinics of
AD, which can clearly show the metabolic or
pathological changes in different regions of the brain
and help doctors accurately determine the site and
extent of lesions. In AD diagnosis, it can clarify the
functional abnormality of brain areas closely related
to cognitive function, such as hippocampus, internal
olfactory cortex, etc., which can provide an important
basis for localized diagnosis of the disease and
evaluation of the disease, and help to differentiate it
from other diseases that may lead to cognitive
disorders. PET test not only shows the anatomical
structure of the brain, but also more importantly
reflects the functional state of the brain, such as the
metabolic activity of the neurons, neurotransmitter
changes and so on, neurotransmitter changes, etc.
However, the PET test itself is expensive, and with
the cost of the tracer, the overall cost of the test is
usually high. PET equipment is expensive, with high
maintenance costs and high requirements for
installation environment and technicians, resulting in
its limited popularity in medical institutions. At the
same time, the analysis and interpretation of PET
images require specialized nuclear medicine doctors
or specially trained personnel who are not only
familiar with the normal anatomy and physiological
functions of the brain, but also understand the
characteristics of PET performance in various disease
states.
Therefore, in Alzheimer's disease detection, EEG
has outstanding advantages over mainstream
methods. Firstly, it is non-invasive. CSF requires
lumbar puncture, which is risky, while EEG only
places electrodes on the scalp. Secondly, it has a
higher detection accuracy and can capture early
abnormalities in neuronal electrical activity.
Furthermore, in terms of economy and popularity,
CSF and PET testing equipment and process costs are
high, while EEG equipment is cheap, with low
learning costs, and can be operated by primary
healthcare professionals after short-term training,
which is more conducive to popularization, and more
patients can benefit from early diagnosis, which has a
great potential for the detection of AD.
3.2 State of the Art and Development
of Sleep Stage Classification System
Technology
Classifying sleep stages is essential for studying
sleep, diagnosing sleep disorders, and assessing
treatments. It enhances our understanding of sleep
mechanisms and offers a foundation for managing
sleep-related conditions. At present, the sleep stage
classification system technology presents diverse
characteristics in methods and applications, and also
faces many challenges, and the future development
direction is becoming clearer.
Sleep specialists usually perform manual sleep
stage scoring through the analysis of
neurophysiological signals gathered in sleep
laboratories. This process is often challenging,
monotonous, and time-intensive. Scoring is usually
based on polysomnographic (PSG) data recorded
during overnight hospital stays. In traditional
practice, overnight PSG recordings consist of EEG,
electrooculogram (EOG), electromyogram (EMG),
and electrocardiogram (ECG) data. These recordings
are manually assessed by sleep specialists based on
the 1968 guidelines established by Rechtschaffen and
Kales (R&K) (Konkoly, et al., 2012). PSG recordings
are divided into 20- or 30-second intervals and
classified into wakefulness (W), REM sleep, and
NREM sleep. Due to their multi-channel signals and
expert-based visual analysis, PSG remains the gold
standard for assessing sleep in laboratory studies.
Polysomnography offers comprehensive insights into
sleep architecture, duration, and quality. However, it
is costly, labor-intensive, and unsuitable for field
applications, as it requires a sleep technician to install
equipment and place multiple electrodes on the face
and scalp (Arevalo, et al., 2012).
Consequently, the process of sleep stage scoring
incurs high costs, is prone to human mistakes, and is
frequently tiresome and demands a significant
amount of time. Analyzing overnight sleep recordings
usually requires 2 to 4 hours, and in some studies,
there has been a 90% expert agreement on sleep stage
BEFS 2025 - International Conference on Biomedical Engineering and Food Science
42

classification (Konkoly, et al., 2012). In addition,
sleep stage scoring using PSG usually requires a
hospital setting where subjects have to wait on a
waiting list for some time. Due to the limitations of
manual sleep stage scoring, there is an increasing
demand for the development of automated sleep stage
classification systems (ASSC).
The research divides dual-channel EEG signals
into quasi-steady-state segments, extracts features
using Short-Time Fast Fourier Transform (STFT),
reduces dimensionality with the fuzzy C-Means
algorithm, and constructs an ASSC system
employing a multi-class SVM. The system achieved
an accuracy of 70.92% (Al-Aloqaly, et al., 2012). To
improve the classification accuracy, the researchers
tried to fuse multiple physiological signals. In
addition to EEG, EOG, and EMG, signals such as
heart rate, respiratory rate, and oxygen saturation
were incorporated. Multimodal data fusion can
provide more comprehensive sleep information,
adopt more advanced algorithms, study new deep
learning architectures, such as models based on the
attention mechanism, which can better focus on key
sleep signal features; and explore the application of
generative adversarial networks (GANs) in sleep data
augmentation and model optimization, to improve
model performance. We will further improve the
multimodal data fusion method, combine artificial
intelligence and big data analysis technology, mine
the complex relationship between sleep data, and
realize the comprehensive assessment of sleep
quality, early warning of sleep disorders, and the
formulation of personalized treatment plans.
3.3 Association between NREM Sleep
Stages and AD and Research
Progress
3.3.1 The Relationship between non Rapid
Eye Movement (NREM) and AD
Starting from the prodromal phase of AD, patients
exhibit slower EEG rhythms while awake, potentially
linked to poor sleep quality. To explore the
connection between arousal and sleep, we analyzed
EEG activity during sleep, as well as before and after
sleep, in patients suffering from Alzheimer's disease
(AD), those with mild cognitive impairment (MCI),
as well as healthy individuals used as controls. It was
found that individuals with AD, as well as those
suffering from mild cognitive impairment, presented
a longer sleep latency and less slow-wave sleep. The
NREM sleep phase is typically characterized by
reduced σ activity, which reflects the absence of
the sleep spindle. For both AD and MCI patients,
EEG slowing is characteristic of REM sleep and
wakefulness, and there is a strong correlation between
these two phenomena, suggesting a common
neuropathological mechanism.
Furthermore, EEG changes from evening to early
morning during wakefulness revealed a gradual
reduction in nocturnal δ activity in both MCI and
AD patients. This suggests a progressive decline in
the restorative effects of sleep on circadian rhythms,
aligning with the impaired high-frequency sleep
activity observed in AD patients.
In this process, NREM stage sleep is crucial for
memory consolidation. It plays a facilitating role in
transforming short - term memory into long - term
memory. Moreover, it is of great significance in
maintaining learning ability and cognitive functions.
3.3.2 Power of EEG During NREM Sleep
The histograms of the spectral power at cortical sites
and bands from Figure1 show the power of the two
groups in the α and σ bands. Looking at the graph as a
whole, there is a difference in the α and σ band power
between the AD and HC groups at different cortical
sites. The α and σ bands were chosen because the σ
band is associated with the relaxation and attentional
states of the brain, and in AD patients, altered brain
function may affect their relaxation and attentional
regulation mechanisms. σ band is associated with the
sleep spindle wave, which is critical for memory
consolidation, and AD patients with impaired memory
may have characteristic changes in this band. Other
frequency bands, such as the δ band, are potentially
related to AD, but the two more distinguishable
frequency bands are discussed briefly here. α and σ
power in AD patients at T3 and T5 correspond to the
temporal lobe region of the brain, and the reduced α
and σ power at these locations may indicate abnormal
neuronal activity in the temporal lobe region. α power
is reduced, reflecting the impaired function of
relaxation and attentional regulation of the brain, and
the reduced α power suggests a weakening of the
activity of sleep spindles and impairs memory
consolidation. Reduced α power suggests that sleep
spindle wave activity is impaired, affecting memory
consolidation. This difference may reflect the
alteration of cortical function in AD patients, which to
some extent provides data support for the study of the
neurophysiological mechanism of AD, and helps to
further explore the characteristics and patterns of the
abnormalities in the brain function of AD patients.
Advances in Sleep EEG Signaling in Alzheimer’s Disease Prediction
43
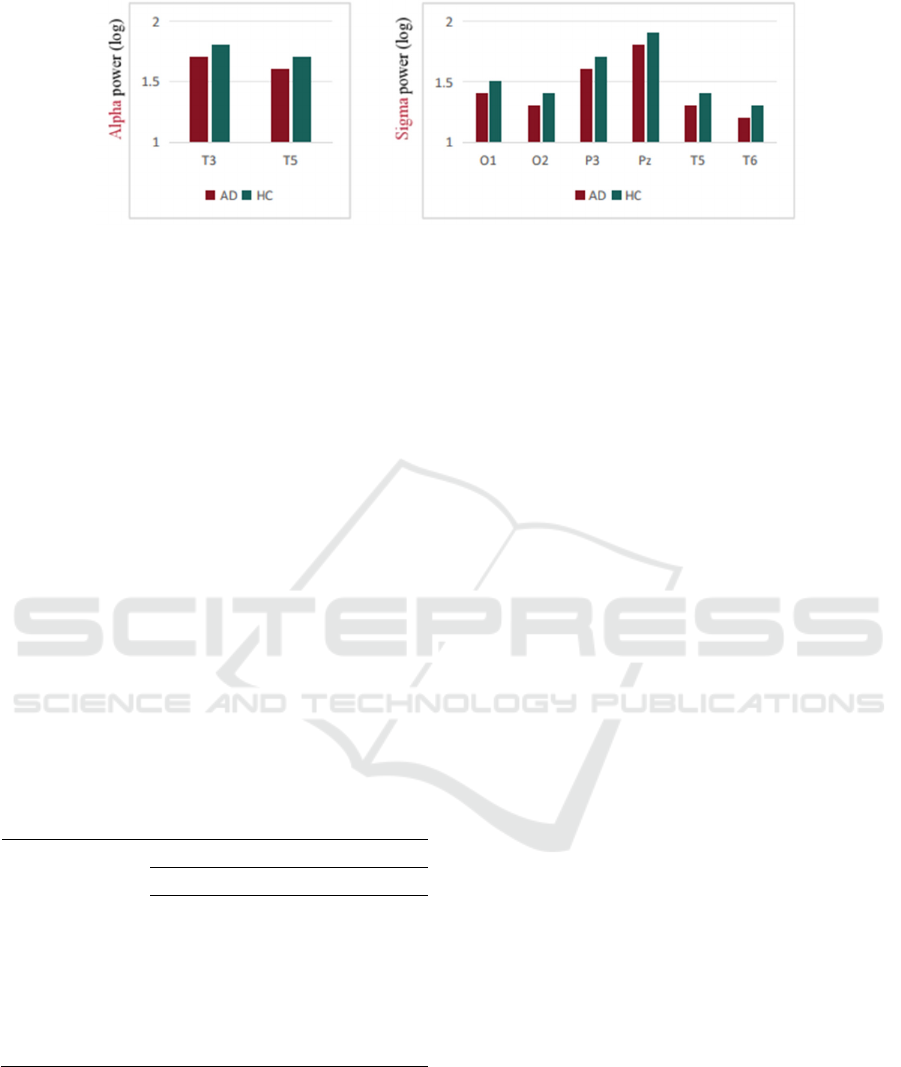
Figure 1: Spectral Power Histograms of Cortical Sites and Frequency Bands in AD (Alzheimer's Disease) and HC (Healthy
Control) Groups.
3.3.3 Correlation between σ Power and
Cognitive Ability
From Table 1, it is possible to evaluate the association
between the level of cognitive ability and the σ power
(sigma power) during NREM sleep. The graph clearly
shows that there is a significant correlation (p ≤
0.0054) between MMSE scores and σ power during
NREM sleep at different EEG loci (O1, O2, P3, Pz,
T5, T6) (D’Atri, et al., 2012). The correlation
coefficients r values ranged from 0.28 - 0.32 and the
p values were extremely small, indicating that this
correlation was highly statistically significant. Based
on this, we can basically conclude that there is a
positive correlation between σ power and cognitive
ability, i.e., the higher the σ power during NREM
sleep, the higher the corresponding MMSE score and
the stronger the cognitive ability.
Table 1: Correlation (Pearson's R) between MMSE Score
and Sigma Power During NREM Sleep, and EEG Slowing
Index During REM Sleep (P ≤ 0.0054) (D’Atri, et al.,
2012).
EEG site σ power in NREM sleep
r p
O1 0.32 0.000077
O2 0.32 0.000055
P3 0.28 0.0006
Pz 0.31 0.000093
T5 0.31 0.00014
T6 0.29 0.00033
Given this association, σ power during NREM
sleep has the potential to be used as a reference
standard for EEG to detect AD levels. In clinical
practice and research, detecting the power in this
frequency band of the EEG may be able to assist in
determining the cognitive state of an individual and
provide valuable information for early screening and
assessment of AD. However, more studies are needed
to further validate its accuracy and reliability.
4 CONCLUSION
With the limitations of traditional AD detection
methods, sleep EEG signaling has become a hot
research topic due to its unique advantages. This
paper comprehensively analyzed the pathological
mechanisms of AD, the advantages and
disadvantages of traditional detection methods, and
the current state of the art of sleep stage classification
system, and focused on the association between
NREM sleep stages and AD, and found that the
cortical functional activities of AD patients differed
from those of healthy controls in terms of α and σ
band power, and clarified the positive correlation
between the σ power and the cognitive ability.
However, the accuracy and reliability of sleep
EEG signal for AD prediction still need to be
improved. In the future, we can focus on conducting
large-scale and multi-center clinical trials to further
validate the relevant indexes, exploring more
frequency bands and brain regions, optimizing the
detection techniques and analysis algorithms to
enhance the precision and steadiness of the
prediction, and promoting the development of AD
early diagnosis and intervention techniques.
REFERENCES
Aboalayon, K. A. I., Faezipour, M., Almuhammadi, W. S.,
& Moslehpour, S. 2016. Sleep stage classification using
EEG signal analysis: A comprehensive survey and new
investigation. Entropy, 18(8), 272.
BEFS 2025 - International Conference on Biomedical Engineering and Food Science
44

D'Atri, A., et al. 2021. EEG alterations during wake and
sleep in mild cognitive impairment and Alzheimer's
disease. iScience, 24(4), 102330.
Lucey, B. P., McCullough, A., Landsness, E. C.,
Toedebusch, C. D., McLeland, J. S., Zaza, A. M.,
Fagan, AA. M., McCue, L., Xiong, C., Morris, J. C.,
Benzinger, T. L. S., & Holtzman, D. M. 2019. Reduced
nonrapid eye movement sleep is associated with tau
pathology in early Alzheimer's disease. Science
Translational Medicine, 11(474), eaau6550.
Moreira, P. I., Cardoso, S. M., Santos, M. S., & Oliveira, C.
R. 2005. The key role of mitochondria in Alzheimer's
disease. Journal of Alzheimer's Disease, 8(2), 101–110.
Nordberg, A., et al. 2010. The use of PET in Alzheimer
disease. Nature Reviews Neurology, 6(2), 82–94.
Santos, C. Y., et al. 2017. Pathophysiologic relationship
between Alzheimer's disease, cerebrovascular disease,
and cardiovascular risk: A review and synthesis.
Alzheimer's & Dementia: Diagnosis, Assessment &
Disease Monitoring, 7, 69–87.
Scheltens, P., De Strooper, B., Kivipelto, M., Holstege, H.,
Chételat, G., Teunissen, C. E., ... & van der Flier, W.
M. 2021. Alzheimer's disease. The Lancet, 397(10284),
1577–1590.
Wang, L.-S., et al. 2012. Comparison of xMAP and ELISA
assays for detecting CSF biomarkers of Alzheimer's
disease. Journal of Alzheimer's Disease, 31(2), 439–
445.
Advances in Sleep EEG Signaling in Alzheimer’s Disease Prediction
45
