
The CNN and ViT Fusion Model Based on Hierarchical Adaptive
Token Refinement Method in Pneumonia X-ray Image Classification
Peiqi Zhang
a
Computing Science, Aberdeen Institute of Data Science and Artificial Intelligence, South China Normal University,
Guangdong Foshan, China
Keywords: Pneumonia Diagnosis, X-ray Images, CNN, ViT, Fusion Model.
Abstract: Convolutional Neural Networks (CNN) and Vision Transformers (VIT) each have their own advantages in
medical image analysis, particularly in the automatic classification of X-ray images. Many studies have
contributed to the effective combination of these two.This paper proposes a CNN and ViT merging method -
Hierarchical Adaptive Token Refinement (HATR), combining the local feature extraction capability of CNN
with the global modeling ability of ViT. The experimental results show that the accuracy rate of the fusion
model based on ResNet (HATR-ResNet) is 91.4%, which is significantly better than that of ResNet alone
(87.3%). The accuracy rate of the fusion model based on Conv2D (HATR-Conv2D) is 88.2%, which is
approximately 5% higher than that of Conv2D alone (82.7%). The superiority of HATR-ResNet stems from
the deep residual network structure of ResNet, which can better extract complex features and capture details,
while the shallower network structure of Conv2D is relatively insufficient when dealing with complex
patterns.This study proposes a new fusion method for CNN and ViT, and compares the performance
differences of the fusion models based on different CNN backbones. It contributes to the subsequent research
on new model structures and the exploration of new fusion methods.
1 INTRODUCTION
Be advised that papers in a technically unsuitable
form will be returned for retyping. After returned the
manuscript must be appropriately modified.
The field of Chest X-ray (CXR) image analysis
plays a crucial role in the early diagnosis of
pneumonia. Traditional methods for diagnosing
pneumonia rely on doctors' experience and imaging
examinations. However, in some high-load and
resource-tight environments, this may lead to
diagnostic delays or even errors (Litjens et al., 2017).
A study published in JAMA Internal Medicine in
2024 analyzed 17,290 inpatients from 48 Michigan
hospitals and found that 12.0% of the patients were
misdiagnosed as having community-acquired
pneumonia (Gupta et al., 2024). With the
advancement of artificial intelligence technology,
Computer-Aided Diagnosis (CAD) systems based on
deep learning can extract complex features from a
large number of medical images, greatly improving
the efficiency and accuracy of diagnosis.
a
https://orcid.org/0009-0003-4096-7208
Convolutional Neural Network (CNN) is the core
architecture of deep learning and has performed
exceptionally well in the field of medical image
analysis, especially in the task of pneumonia
detection (Litjens et al., 2017; Kermany et al., 2018).
ResNet solved the degradation problem in deep
network training through residual connections,
enabling the network to extract deeper image features
(He et al., 2016). Models based on Conv2D are often
used to identify detailed features in medical images,
such as abnormal textures in X-ray images of the
lungs (Ronneberger et al., 2015). However, CNN has
limitations in capturing long-term dependencies and
global features, which may restrict its performance in
complex medical image tasks(Raghu et al., 2019).
To overcome the shortcomings of CNN in global
feature modeling, the Vision Transformer (ViT) has
become a research hotspot in recent years. ViT uses
the self-attention mechanism to achieve global image
modeling, which can more effectively capture long-
term dependencies compared to traditional CNN
(Dosovitskiy et al., 2021; Vaswani et al., 2017).
504
Zhang, P.
The CNN and ViT Fusion Model Based on Hierarchical Adaptive Token Refinement Method in Pneumonia X-ray Image Classification.
DOI: 10.5220/0014361900004718
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 2nd International Conference on Engineering Management, Information Technology and Intelligence (EMITI 2025), pages 504-510
ISBN: 978-989-758-792-4
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

Studies have shown that ViT performs well on large-
scale datasets, but its performance is usually inferior
to CNN on small sample datasets, and it requires
higher annotation data and computing resources
(Touvron et al., 2021; Chen et al., 2021). This limits
the application of ViT in resource-constrained
scenarios.
To combine the advantages of CNN and ViT,
researchers proposed a hybrid model. Swin
Transformer enhances the feature representation
ability through the hierarchical window self-attention
mechanism, and in the ImageNet classification task,
it increased the Top-1 accuracy to 83.5%, which is
approximately 3% higher than the baseline model
(Liu et al., 2021). CoAtNet integrates convolution
and self-attention mechanisms, achieving a balance
between computational efficiency and performance.
Experimental results show that it has an accuracy
improvement of approximately 2.5% compared to the
traditional Transformer model under the same
computational cost (Dai et al., 2021).
The main objective of this study is to design and
compare the performance of different CNN and ViT
fusion models in pneumonia classification tasks. By
combining the local feature extraction advantages of
CNN and the global information modeling
capabilities of ViT, this study proposes a modular
fusion framework and analyzes the performance of
different CNN backbone networks in this framework.
Through experiments, this study explored how to
optimize the structure of the fusion model to improve
the accuracy of pneumonia classification and provide
a theoretical basis and practical guidance for the
design of future automatic diagnosis systems for
pneumonia.
2 DATASET AND METHODS
2.1 Dataset Dscription
The dataset used in this study is from the Kaggle chest
X-ray image (Pneumonia) dataset, which is widely
used in pneumonia classification tasks. The dataset
contains normal lung images and pneumonia lung
images. All the images are grayscale and have typical
medical imaging features. The composition of the
dataset is as follows: The training set approximately
contains 5,232 images, among which 1,341 are
normal lung images and 3,891 are pneumonia images.
The validation set consists of 16 images, including 8
normal and 8 pneumonia samples respectively. The
test set contains 624 images, which are divided into
234 normal images and 390 pneumonia images. The
sample of normal and pneumonia images is shown in
Figure 1 and Figure 2. Although the sample size of
the training set is large, the number of pneumonia
samples is significantly higher than that of normal
samples, resulting in an imbalance in the data. This
problem is common in many medical image analysis
tasks.
Figure 1: Samples of Normal (Data from: Kaggle).
Figure 2: Samples of Pneumonia (Data from: Kaggle).
2.2 Dataset Preprocessing
Since the original image is a grayscale image, it is
necessary to convert the image into a three-channel
RGB image suitable for deep learning models
(especially pretrained models) during subsequent
processing. This conversion is usually accomplished
by replicating the channels of grayscale images or
through pseudo-colorization processing, thereby
making the images conform to the input requirements
of models such as CNN and ViT on the channels. To
meet the size requirements of the model input, all
images have been uniformly adjusted to a size of
224x224. This size setting is not only compatible with
common convolutional neural network architectures
such as ResNet and VGG, but also meets the input
size requirements of ViT models.
Another key step in image preprocessing is
normalization. First, normalize the pixel values to the
[0, 1] interval to reduce the influence of different
image brightness and contrast on model training.
Then, based on the statistics from ImageNet, the
images were standardized, using the mean ([0.485,
0.485, 0.485]) and standard deviation ([0.229, 0.229,
0.229]) of the images. This standardized approach
helps the model adapt to the input distribution of the
pretrained model, enhancing the stability and
convergence speed of the training.
To enhance the robustness and generalization
ability of the model, various data augmentation
operations were also performed on the training set
data. Specifically, the training images are enhanced
through random horizontal flipping, random rotation
(up to ± 10 degrees), and random brightness
adjustment ( ± 10%), among other methods.
The CNN and ViT Fusion Model Based on Hierarchical Adaptive Token Refinement Method in Pneumonia X-ray Image Classification
505
However, to ensure the fairness and accuracy of the
model evaluation, the validation set and test set were
not subjected to data augmentation processing to
avoid the impact of augmentation operations on the
evaluation results.
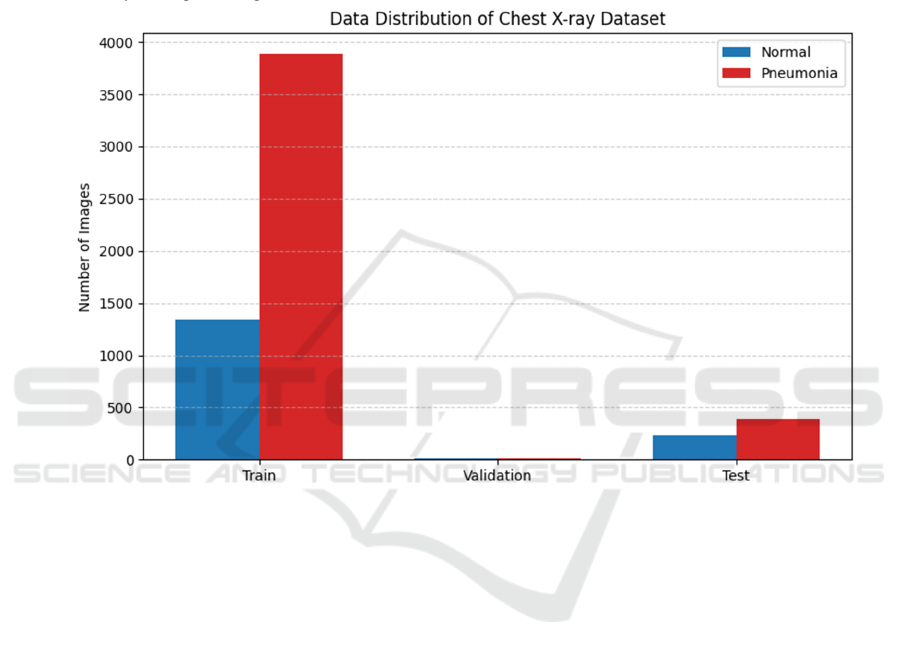
As shown in Figure 3, the imbalance problem of
the dataset is one of the key factors affecting the
performance of the model. In this study, a weighted
cross-entropy loss function was adopted to alleviate
this problem. By setting the weight ratio of the normal
category and the pneumonia category to 3:1, the
model can pay more attention to the samples of a few
categories (i.e., the normal category) during the
training process, avoiding the model being overly
biased towards the pneumonia category. In addition,
according to the experimental requirements,
oversampling of normal samples or undersampling of
pneumonia samples can also be selected to further
improve the model's adaptability to imbalanced data.
Figure 3: Distribution of the dataset (Picture credit: Original).
2.3 Model
To fully utilize the sensitivity of a convolutional
neural network (CNN) to local features and the
advantages of Vision Transformer (ViT) in global
modelling, this study designs a fusion method. It is
named HATR (Hierarchical Adaptive Token
Refinement). This method uses CNN as the feature
extractor and maps its output to sequence inputs
suitable for ViT. By introducing multi-scale feature
partitioning and adaptive weighting mechanisms, it
achieves efficient feature integration from local to
global.
This section first briefly introduces the basic
functions and output features of each sub-model, and
then focuses on elaborating the design logic and
implementation details of the fusion method.
2.3.1 Basic Model
ResNet-50 was used to extract the representation of
X-ray images of the lungs, and the final output was a
feature map of size [batch, 2048, 7, 7]. This feature
map is mapped to 512 channels through
dimensionality reduction and convolution to adapt to
subsequent module processing.
Conv2D is a lightweight convolutional network
composed of three layers of convolutional stacks,
with channels of 64, 128, and 256 in sequence. The
final output feature map is [batch, 256, 14, 14].
Compared with ResNet, it has a simpler structure and
faster training, but its semantic expression ability is
relatively weak.
ViT represents images as a series of patches and
models the global relationships among different
regions through a self-attention mechanism. It does
not have the inductive bias of convolution, and thus
is more sensitive to the organizational structure of the
EMITI 2025 - International Conference on Engineering Management, Information Technology and Intelligence
506
input features. The input of ViT is a sequence in the
form of [batch, N, d], where each element
corresponds to an embedded representation of an
image Patch, and the output is used for classification
through CLS tokens.
2.3.2 Fusion Method: HATR (Hierarchical
Adaptive Token Refinement)
The traditional CNN-VIT fusion methods often adopt
direct Patch segmentation and input connection,
ignoring the differences and spatial continuity of
features in each region in the CNN output, which is
prone to lead to information fragmentation or
redundant transmission. To this end, the research
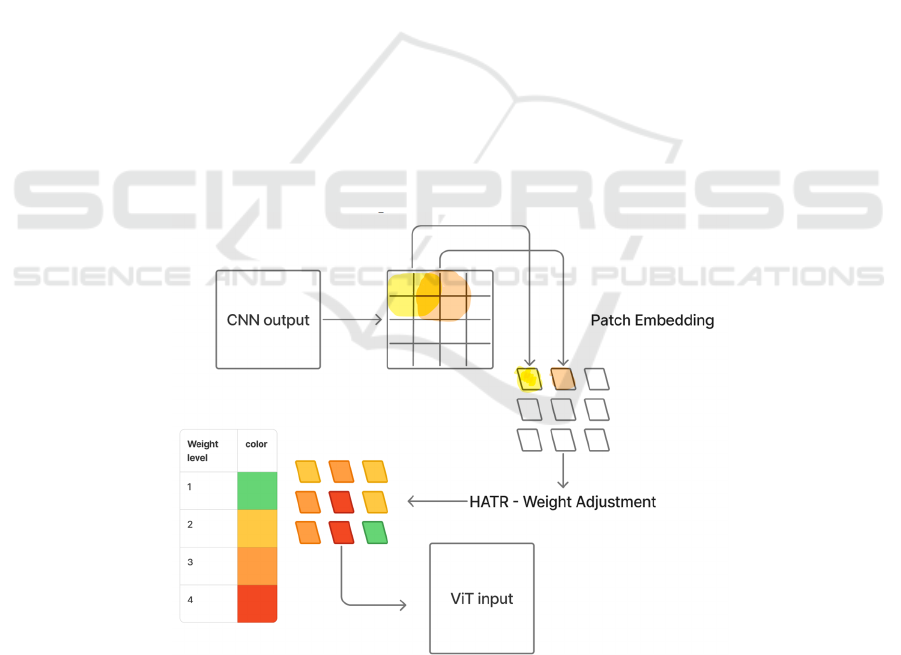
proposes a hierarchical adaptive fusion method,
HATR, which optimizes this connection process from
two aspects: multi-scale overlapping embedding and
Token adaptive weighting. The structure of this
method is shown in Figure 4.
Firstly, after the CNN output, the conventional
non-overlapping Patch segmentation method is no
longer adopted. Instead, an overlapping window
(stride < patch size) is introduced to perform
convolutional partitioning on the feature map. This
approach can retain more context information
between patches and slow down the fragmentation of
the spatial structure. For instance, for the output of
Conv2D [256, 14, 14], by dividing it with a sliding
window of kernel size=2 and stride=1, a large number
of patches with overlapping areas can be generated,
which can then be flattened to form the sequence
input [batch, N, 512] required for ViT. The output
resolution of ResNet is relatively low. The method
first performs a 1×1 convolution dimension reduction
on it and then applies the same sliding partitioning
strategy.
Secondly, the study introduces a lightweight
attention module to perform feature weighting on all
patches. This module combines channel attention and
spatial attention mechanisms to identify the
importance of different patches. Each Patch is
assigned a weight coefficient. High-response regions
(such as patches containing lesion features) will be
high-lighted, while the information of low-response
regions will be compressed. The final Patch sequence
already possesses the characteristics of spatial
continuity and significant regional enhancement
before being input into the ViT.
The sequences received by ViT already contain
hierarchical details and importance annotations. The
long-distance dependencies between various regions
are modelled through their self-attention mechanism,
and finally, the global information is aggregated by
the CLS token for pneumonia classification
Figure 4: Structure of HATR method (Picture credit: Original).
The HATR method resolves the structural
mismatch issue between convolutional features and
the Transformer input. The multi-scale overlapping
embedding method enhances the correlation between
patches and alleviates feature fragmentation. The
Token weighting mechanism enables the model to
learn to filter the most valuable regions when
information is overloaded, thereby enhancing the
model's discriminative ability.
The CNN and ViT Fusion Model Based on Hierarchical Adaptive Token Refinement Method in Pneumonia X-ray Image Classification
507
Compared with the directly connected fusion
structure, HATR can more fully leverage the
respective advantages of CNN and ViT while
maintaining the overall computational complexity
within a controllable range. For scenes like medical
images with low contrast and high redundancy, this
refined connection and weighting method has higher
practical value.
3 EXPERIMENTS
3.1 Experimental Configuration
Table 1: Experimental Configuration.
Ite
m
Details
Hardware NVIDIA RTX 5060 GPU
Software
Framewor
k
PyTorch 1.10Python 3.8CUDA
11.2
O
p
timize
r
Adam
Learnin
g
Rate 1e-4
Batch Size 32
Loss Function Weighted cross-entropy loss (for
class imbalance
)
Trainin
g
E
p
ochs 20
The experimental environment of this study is
shown in Table 1. This study was conducted in a GPU
environment with strong image processing
capabilities (RTX 5060), using the PyTorch
framework to implement all model training and
inference processes. During the training process, the
Adam optimizer was used, with an initial learning rate
of 1e-4 and a batch size of 32. The total number of
training rounds was 20. To address the sample
imbalance problem where the proportion of the
pneumonia category in the dataset is relatively high,
a weighted cross-entropy loss function was employed
to adjust the category weights.
3.2 Experimental Result
The study evaluated the performance of different
models in classification tasks on the Kaggle chest X-
ray pneumonia classification dataset, including
individual convolutional neural networks (ResNet-
50, Conv2D), Vision Transformer (ViT), and the
HATR model based on the fusion of these two CNN
trunks and ViT. The comparison results are in Table
2.
Table 2: Performance of each model.
Model Accurac
y
Precision Recall F1 Score AUC-ROC
ResNet-50 87.3% 86.5% 88.1% 87.3% 0.945
Conv2D 82.7% 81.2% 84.5% 82.8% 0.920
ViT 85.1% 84.3% 86.7% 85.4% 0.930
HATR (ResNet) 91.4% 90.8% 92.1% 91.4% 0.960
HATR (Conv2D) 88.2% 87.5% 89.0% 88.2% 0.950
It can be seen from the results that the overall
performance of the fusion model is superior to that of
its respective individual backbone models. Among
them, HATR (ResNet) achieved the best performance
in terms of accuracy, recall rate, and AUC indicators,
indicating that the fused structure can more
effectively extract and integrate multi-level
information in lung images.
ResNet and ViT respectively have advantages in
local feature extraction and global structure
modelling. The fused HATR model connects the two
through overlapping Patch embedding and adaptive
Token weighting mechanism, enabling the model to
retain the sensitivity of convolution to details while
introducing ViT's ability to understand the overall
image structure.
Although HATR (Conv2D) is based on a
relatively lightweight convolutional network, its
performance is still significantly improved after
fusion, indicating that even if the basic network
capability is weak, a reasonable fusion design can still
bring significant gains.
This research focused on comparing the models
constructed by fusing two different CNN trunks
(ResNet-50 and Conv2D) with ViT, namely HATR-
ResNet and HATR-Conv2D. Under the same training
configuration and fusion structure, both perform
better than their respective single backbone networks
in the task of classifying pneumonia X-ray images,
but there are still significant differences in specific
performance.
From the perspective of overall performance
indicators, the accuracy rate of HATR-ResNet is
91.4%, while that of HATR-Conv2D is 88.2%. Not
only that, in terms of precision, recall rate, F1 score
and AUC and other indicators, HATR-ResNet has
always outperformed HATR-Conv2D, demonstrating
its stronger classification ability. Especially in terms
of AUC (0.960 vs 0.950) and recall rate, the former is
EMITI 2025 - International Conference on Engineering Management, Information Technology and Intelligence
508
more suitable for use in medical scenarios with high
sensitivity requirements.
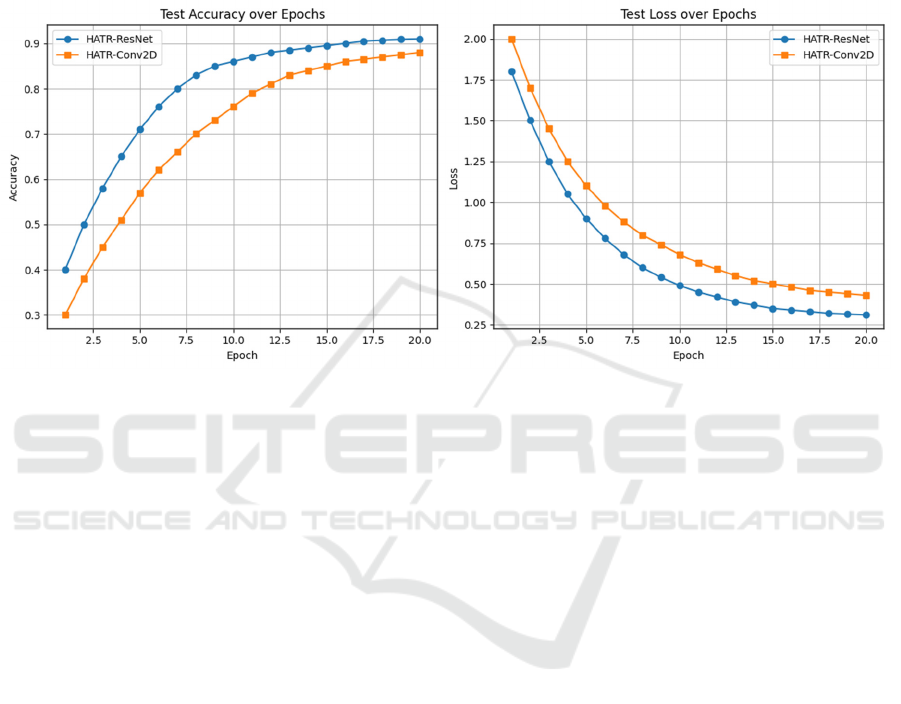
Further analysis of the training process reveals
that HATR-ResNet also has more advantages in
convergence speed and stability of the validation set.
In the first 10 epochs, the training losses of both
decreased rapidly, but the validation loss of HATR-
ResNet decreased more steadily and the overfitting
phenomenon was milder. Although the training loss
of HATR-Conv2D decreases rapidly, the
performance fluctuation on the validation set is
greater, indicating that its generalization ability is
slightly inferior. Figure 5 shows the accuracy and loss
of HATR models.
Figure 5: Accuracy and Loss of HATR Models (Picture credit: Original).
The essential reason for this difference lies in the
varying capabilities of the backbone CNN structure
itself. ResNet features a deeper network structure and
residual connections, enabling it to effectively
capture complex edges, textures and spatial layouts in
images. It is particularly suitable for areas with
blurred details and low contrast in pneumonia images.
Although Conv2D has a lightweight structure and is
suitable for deployment in resource-constrained
environments, its shallow network lacks the ability to
extract high-level semantic features, which makes it
difficult for the fused ViT to receive rich enough
information and affects the quality of subsequent
modelling.
In addition, in terms of training duration, the
training time per round of HATR-ResNet is slightly
higher (about 1.2 times), but overall, the performance
improvement is significant, making it particularly
suitable for use in medical image scenarios where
performance takes precedence over computing
resources. HATR-Conv2D is more suitable for
deployment in edge devices or systems with strict
latency requirements, serving as a lightweight
alternative.
4 CONCLUSIONS
This study focuses on the automatic recognition task
of pneumonia X-ray images and proposes a feature
modelling method based on the fusion of CNN and
ViT. By constructing the modular structure HATR
(Hierarchical Adaptive Token Refinement), the study
has achieved the efficient integration of local
convolutional features and the global attention
mechanism. The experimental results show that,
regardless of whether ResNet or Conv2D is used as
the backbone, the fusion model outperforms the
single structure in key indicators such as accuracy, F1
score and AUC, verifying the feasibility and
effectiveness of this design in medical image
classification tasks.
The key to the fusion strategy lies in the handling
of two aspects: One is to adopt the overlapping Patch
embedding method, which alleviates the feature
fragmentation problem caused by the traditional
segmentation method; Second, an adaptive Token
weighting mechanism is introduced, enabling the
model to complete the initial screening of features
before inputting them into the ViT. This structural
design enables the model to retain the convolutional
network's ability to pay attention to detailed regions
The CNN and ViT Fusion Model Based on Hierarchical Adaptive Token Refinement Method in Pneumonia X-ray Image Classification
509

while also leveraging the global modelling
advantages of the Transformer to enhance the
accuracy of overall judgment.
Although this method performs well on medium
and small-scale datasets, there are still some
limitations and directions worthy of further
exploration:
Firstly, the selection of the CNN backbone is still
relatively fixed and lacks structural adaptability.
Under different tasks or data distributions, the
currently used ResNet or Conv2D may not always
maintain stable performance. In the future, more
flexible and adjustable backbone structures, such as
automatic neural architecture search (NAS), can be
explored to enhance generalization capabilities.
Secondly, due to ViT's strong reliance on large-
scale data, the fusion model is still prone to
overfitting or performance fluctuations when the data
volume is insufficient. In addition, to maintain a
lightweight configuration, this study has adopted a
shallow ViT structure. Although this reduces
computing costs, it may still limit the expressive
power in more complex data environments.
Finally, the robustness of the current model still
needs to be enhanced when dealing with large-scale,
multi-category or cross-device collected data. In the
future, the stability and practical application value of
the model can be further enhanced by integrating
transfer learning, domain adaptation or multimodal
information (such as clinical text data).
REFERENCES
Chen, X., Xie, S., & He, K. (2021). An empirical study of
training self-supervised vision transformers.
Proceedings of the IEEE/CVF International Conference
on Computer Vision (ICCV), 9620–9629.
Dai, Z., Liu, H., Le, Q. V., & Tan, M. (2021). CoAtNet:
Marrying convolution and attention for all data sizes.
Advances in Neural Information Processing Systems
(NeurIPS), 3965–3977.
Dosovitskiy, A., Beyer, L., Kolesnikov, A., Weissenborn,
D., Zhai, X., Unterthiner, T., ... & Houlsby, N. (2021).
An image is worth 16×16 words: Transformers for
image recognition at scale. Proceedings of the
International Conference on Learning Representations
(ICLR), 1–22.
Gupta, A. B., Wang, Y., Smith, J., Lee, D., Chen, M., &
Johnson, T. (2024). Inappropriate diagnosis of
community-acquired pneumonia among hospitalized
adults. JAMA Internal Medicine, 184(5), 548–556.
He, K., Zhang, X., Ren, S., & Sun, J. (2016). Deep residual
learning for image recognition. Proceedings of the
IEEE Conference on Computer Vision and Pattern
Recognition (CVPR), 770–778.
Kermany, D. S., Zhang, K., & Goldbaum, M. (2018).
Identifying medical diagnoses and treatable diseases by
image-based deep learning. Cell, 172(5), 1122–1131.
Litjens, G., Kooi, T., Bejnordi, B. E., Setio, A. A. A.,
Ciompi, F., Ghafoorian, M., ... & van Ginneken, B.
(2017). A survey on deep learning in medical image
analysis. Medical Image Analysis, 42, 60–88.
Liu, Z., Lin, Y., Cao, Y., Hu, H., Wei, Y., Zhang, Z., Lin,
S., & Guo, B. (2021). Swin Transformer: Hierarchical
vision transformer using shifted windows. Proceedings
of the IEEE/CVF International Conference on
Computer Vision (ICCV), 10012–10022.
Raghu, M., Zhang, C., Kleinberg, J., & Bengio, S. (2019).
Transfusion: Understanding transfer learning for
medical imaging. Advances in Neural Information
Processing Systems (NeurIPS), 3347–3357.
Ronneberger, O., Fischer, P., & Brox, T. (2015). U-Net:
Convolutional networks for biomedical image
segmentation. Proceedings of the International
Conference on Medical Image Computing and
Computer-Assisted Intervention (MICCAI), 234–241.
Touvron, H., Cord, M., Douze, M., Massa, F., Sablayrolles,
A., & Jégou, H. (2021). Training data-efficient image
transformers & distillation through attention.
Proceedings of the International Conference on
Machine Learning (ICML), 10347–10357.
Vaswani, A., Shazeer, N., Parmar, N., Uszkoreit, J., Jones,
L., Gomez, A. N., Kaiser, Ł., & Polosukhin, I. (2017).
Attention is all you need. Advances in Neural
Information Processing Systems (NeurIPS), 5998–
6008.
EMITI 2025 - International Conference on Engineering Management, Information Technology and Intelligence
510
