
Cancer Care Nexus
S. Radha Rani, T. Praveen, G. Srihitha, K. Teja Surya Narayana and N. Harika
Vignan’s Foundation for Science, Technology and Research, Guntur -Tenali Rd, Vadlamudi, Andhra Pradesh 522213,
India
Keywords: Machine Learning, Deep Learning, Convolutional Neural Networks (CNN), Random Forest, Medical
Diagnostics.
Abstract: The Cancer Care Nexus is an AI diagnostics plat- form for early cancer detection of breast, lung, skin, and
blood cancer through machine learning and deep learning algorithms. The conventional diagnosis of cancer
is invasive, time-consuming, and costly, making it inaccessible. The project fills these gaps with the
application of Random Forest algorithms for text diagnosis and Convolutional Neural Networks (CNNs) for
image diagnosis, which are efficient and accurate. Cancer Care Nexus offers a unified and easy-to-use
interface, where health workers can input text or image and obtain reliable predictions, enabling multi- cancer
diagnosis in a single platform. The system is scalable, adaptive, and privacy-friendly, providing secure
processing of medical information. Future enhancement includes the expansion of cancer detection, model
performance enhancement, and real- time predictive analysis integration. The project is a key milestone in
AI-based healthcare making cancer detection faster, more accessible, and more accurate, which translates to
improved patient outcomes in the world.
1 INTRODUCTION
Cancer is still one of the leading reasons for deaths
across the world with millions of fresh cases
diagnosed each year. According to the World Health
Organization (WHO), cancer accounted for nearly 10
million deaths in the year 2020 alone and thus the
urge for early and accurate detection methods is
paramount. Early cancer detection significantly
boosts the chances of survival by providing for timely
and efficacious treatment. However, standard cancer
detection methods, such as biopsies, imaging tests,
and histopathological evaluation, are often invasive,
tedious, costly, and call for specialized infrastructure
available at all times in low-resource settings.
Besides, types of cancers such as breast, lung,
skin, and blood cancer require individual-specific
diagnostic methods due to variance in symptoms, data
structures, and clinical manifestations and thus
rendering an integrated approach for multi-cancer
detection unfruitful.
Cancer Care Nexus solves these problems by
creating a single, AI-based diagnostic platform that
employs machine learning and deep learning
algorithms. This project com- bines several models
to diagnose cancers of different types efficiently and
accurately, providing a scalable and flexible solution
for medical professionals. The system applies
Random Forest models to text-based classification
(breast and lung cancer) and Convolutional Neural
Networks (CNNs) for image-based diagnosis (skin
and blood cancer). By offering a single platform
that supports both text and image inputs, Cancer
Care Nexus makes cancer screening easier and more
accessible to healthcare providers.
Conventional cancer diagnostic approaches are
very much dependent on laboratory tests, imaging,
and biopsies, with some drawbacks. Cancer diagnosis
through biopsies can be several days to weeks long,
hindering treatment decisions, while sophisticated
imaging methods such as MRI and CT scans are
expensive and require high-end equipment and
skilled personnel, thus being out of reach for many in
resource- poor areas. Moreover, tests such as biopsies
and tissue extrac-tions are invasive, are
uncomfortable, and also increase the chances of
complications, with some tests requiring repeated
analyses, further weighing down patients. Cancer
detection is also type-dependent, with breast and lung
cancer depending on patient history and clinical
records, while skin and blood cancer diagnosis
depends on image pattern recognition. Also, most
Rani, S. R., Praveen, T., Srihitha, G., Narayana, K. T. S. and Harika, N.
Cancer Care Nexus.
DOI: 10.5220/0013917900004919
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 1st International Conference on Research and Development in Information, Communication, and Computing Technologies (ICRDICCT‘25 2025) - Volume 4, pages
623-630
ISBN: 978-989-758-777-1
Proceedings Copyright © 2026 by SCITEPRESS – Science and Technology Publications, Lda.
623

regions, especially in developing nations, do not have
trained oncologists, radiologists, and pathologists,
leading to hurdles in early diagnosis. AI-based
diagnostic tools are an apparent solution with
automated, effective, and affordable screening,
facilitating bridging of the gap in healthcare
accessibility and early cancer detection.
Cancer Care Nexus circumvents the limitations of
conventional cancer diagnosis by coupling
sophisticated machine learning models into a single-
platform, end-to-end diagnostic solution. For breast
and lung cancer diagnosis, Random Forest models
consume patient medical history, symptoms, and
demographics and yield a non-invasive, affordable,
and scalable solution for early-stage diagnosis. For
skin and blood cancer diagnosis, Convolutional
Neural Networks (CNNs) analyze high-resolution
medical images, including dermatological scans and
microscopic blood smear images, and detect subtle
patterns and abnormalities to improve diagnostic
accuracy over conventional manual inspection. The
system provides a single, user-friendly interface that
integrates both text- and image-based models,
allowing healthcare practitioners to enter patient data
and receive real-time diagnostic predictions. This
reduces the need for multiple independent tools,
accelerating the diagnostic process and enhancing
accessibility, efficiency, and accuracy in cancer
screening and early detection.
The use of Cancer Care Nexus has a number of
benefits, and it is a revolutionary device for cancer
screening. AI- driven models provide high diagnostic
accuracy, improving the chances of early-stage
detection, which raises treatment success rates and
reduces mortality significantly. The platform
combines breast, lung, skin, and blood cancer
screening in one system, eliminating the use of
individual screening devices and making the process
cost-effective and efficient. By reducing dependence
on expensive imaging techniques and specialist
professionals, Cancer Care Nexus enhances
accessibility, particularly in developing nations, and
can be integrated into telemedicine services to
enhance healthcare reach. Its explain- able and
automated results allow non-specialists, including
primary care doctors and community health workers,
to con- duct preliminary screenings. Scalable in
nature, the platform allows for future integration of
new cancer types and improved AI models, ensuring
continuous improvement with medical research
developments. AI-driven automation also speeds up
diagnosis while minimizing operational costs,
making cancer screening cost-effective and
accessible. The system has robust data security
features, complying with HIPAA and GDPR
standards to secure encrypted patient information.
Moreover, its explainable AI models pinpoint the
most significant factors driving predictions, ensuring
transparency, clinical relevance, and trustworthiness,
making Cancer Care Nexus a reliable decision-
support system for doctors.
Khalid et al. suggested a deep learning-powered
breast cancer detection model from computerized
mammograms by utilizing feature selection methods
like low-variance feature elimination, univariate
feature selection, and recursive feature elimination
for improved accuracy. Their research employed a
dataset of 3,002 mammography images from 1,501
participants obtained from February 2007 to May
2015, testing six models of classification random
forest, decision tree, k- nearest neighbors, logistic
regression, support vector classifier, and linear
support vector classifier B. N. Kumar et al. The
outcome indicated high accuracy using less
computational power, and hence the model is
effective in the early detection of breast cancer.
Combining MRI and CNN-based classification V.
Sureshkumaret al, Khalid et al. presented an elastic
solution that optimizes diagnostic processes while
tackling computational issues. Their work adds to AI-
assisted cancer diagnostics, paving the way for en-
hanced predictive analytics and broader dataset
generalization in the future.
Kabiraj et al.suggested a breast cancer risk
prediction model based on ensemble machine
learning methods, namely Random Forest and
Extreme Gradient Boosting (XGBoost). Their
research used a breast cancer dataset of 275 instances
with 12 features to compare the predictive
performance of these algorithms. The findings
exhibited a 74.73% accuracy with Random Forest and
73.63% with XGBoost, indicating the promising
potential of ensemble learning techniques in cancer
risk prediction A. Jafari, et al. Their study is
consistent with several studies focusing on the use of
machine learning for breast cancer detection based on
patient information and risk factors including family
history, physical inactivity, psycho- logical stress,
and differences in breast size. The application of
machine learning in medical diagnosis has been
effective in detecting patterns that might go
unnoticed under conventional techniques, thereby
rendering these models highly useful for the early
detection of diseases. The research adds to the
increasing use of AI-based cancer diagnosis,
driving the construction of stronger predictive models
with a view to improving clinical decision-making
and patient outcomes.
ICRDICCT‘25 2025 - INTERNATIONAL CONFERENCE ON RESEARCH AND DEVELOPMENT IN INFORMATION,
COMMUNICATION, AND COMPUTING TECHNOLOGIES
624

B. S et al . suggested a machine learning-based
lung cancer detection system to support radiologists
in enhancing diagnostic accuracy and patient survival
rates. The authors investigated several classification
methods, such as Support Vector Machine (SVM), K-
Nearest Neighbor (KNN), Decision Tree, Logistic
Regression, Na¨ıve Bayes, and Random Forest, to
identify lung cancer S. P. Maurya et al. The system
utilized a multi-stage classification method, including
data enhancement and segmentation by thresholding
and marker-controlled watershed techniques R.
Javed,et al. The research proved that machine
learning greatly enhances the detection of lung
cancer, with the Random Forest algorithm having the
best accuracy of 88.5%. The results are consistent
with studies showing the efficiency of AI-based
diagnostic tools in medical imaging, especially in the
detection of early-stage lung cancer P. Chaturvedi et
al. Through the use of machine learning for
automated classification, this method lightens the
workload of radiologists and increases diagnostic
accuracy. The research is an addition to current
development of AI-driven healthcare solutions,
advancing the development of more accurate and
accessible lung cancer detection.
Agarwal et al.suggested a machine learning
approach for the detection of lung cancer with an aim
to minimize human error and maximize diagnostic
accuracy with the help of automation. The research
work used four machines learning algorithms
Random Forest, Logistic Regression, Support Vector
Machine, and Decision Tree S. P. Maurya et al,
applied on a dataset of lung cancer in Google Colab,
which offers a cloud platform that includes GPU
support. The efficiency of these algorithms was
measured across four main parameters: accuracy,
recall, harmonic mean, and precision P. Chaturvedi
et al.. The research emphasized the role of automated
detection systems in reducing diagnostic errors and
improving early detection of cancer. Their results
confirm the findings of ongoing studies showing the
capability of AI-based models in enhancing lung
cancer diagnosis by facilitating quicker and more
accurate screening. The comparative study of several
algorithms helps achieve progress in machine
learning technology in medical diagnosis and assists
in developing cancer detection systems that are
effective and scalable and are fit for medical use.
Shehta et al. suggested a deep learning-based
method for the diagnosis of blood cancer, focusing on
early detection to enhance treatment success rates and
minimize mortality. Their research compared various
deep learning architectures, such as ResNetRS50,
RegNetX016, AlexNet, ConvNext, EfficientNet,
Inception V3, Xception, and VGG19, to determine
the best model for efficient and accurate prediction of
blood cancer. Of these, ResNetRS50 showed higher
accuracy and speed with low error rates, and is a
potential device for early detection of cancer. Their
work aligns with continued attempts to apply deep
learning for medical diagnosis since AI-based models
continue to refine cancer screening through increased
detection precision and less reliance on human
examination. Using deep convolutional neural
networks, their work helps develop computerized and
scalable methods for blood cancer diagnosis that
support the utilization of deep learning in enhancing
clinical outcomes and early intervention plans.
Hemalatha et al. suggested an artificial neural
network (ANN)-based method for the diagnosis of
blood cancer based on sensor-generated
physiological data. Their research em- ployed a
sensor network to record important health parameters,
such as cardiac and respiratory rates, body
temperature, and blood pressure, which were then
classified by an ANN . The model had a 92.1%
diagnostic accuracy, showing that ANN-based
systems can successfully diagnose blood cancer and
learn to perform better as additional data are
introduced. The current research fits within the recent
stream of studies exploring AI-assisted cancer
detection and the contribution of neural networks
toward improving diagnostic accuracy at a reduced
cost and dependency on comprehensive clinical
testing. Through the use of sensor data and automated
classification, this research adds to the creation of
cost-effective, scalable, and real-time solutions for
early detection of blood cancer, providing a potential
alternative for more accessible and faster diagnosis in
medical environments.
Akinrinade et al. suggested a deep learning
approach for skin cancer detection, focusing on early
diagnosis to enhance patient outcomes, especially in
underserved areas. Their research mitigated issues
like class imbalance and dataset constraints by
employing methods such as transfer learning, data
augmentation, and Generative Adversarial Networks
(GANs) to boost model performance. The study used
convolutional neural networks (CNNs) to scan
dermoscopic images and utilized texture-based
features to discriminate between malignant and
benign lesions. It also experimented with sampling
strategies and loss functions to enhance imbalanced
dataset classification accuracy O. Akinrinade et al. It
compared ensemble and hybrid models and identified
the most efficient method of early detection of skin
cancer. Their results are consistent with the recent
progress in AI-based healthcare, illustrating that deep
Cancer Care Nexus
625
learning methods can highly improve skin cancer
diagnosis accuracy and accessibility. By
implementing such models on digital health
platforms, the research helps advance the
development of scalable and automated solutions for
early cancer screening, diminishing reliance on
conventional diagnostic approaches.
Kandhro et al. suggested an advanced deep
learning technique for detecting skin cancer
through upgrading the VGG19 pre-trained model
using max pooling and dense layers. Different pre-
trained models, such as VGG19, ResNet152v2,
InceptionResNetV2, DenseNet201, ResNet50, and
InceptionV3 were employed in their study to derive
features from a skin lesion dataset that included
malignant and benign samples. These features were
then categorized based on machine learning
algorithms including Linear Support Vector Machine
(SVM), k-Nearest Neighbors (KNN), Decision Tree
(DT), and Logistic Regression (LR). The research
proved that the use of the enriched VGG19 (E-
VGG19) model A. Kandhro, e t a l with
conventional classifiers greatly enhanced
classification accuracy. Performance was assessed
through recall, F1 score, precision, sensitivity, and
accuracy, proving the efficacy of hybrid methods in
enhancing skin cancer diagnosis. Their results help
in the continued evolution of AI-driven automated
diagnostic systems, offering clinicians more precise
and effective means of early skin cancer detection,
ultimately enhancing patient outcomes.
2 METHODOLOGY
2.1 Dataset Details
The Cancer Care Nexus system uses a
heterogeneous and well-organized dataset for multi-
cancer detection, including blood, skin, lung, and
breast cancer. Each dataset is designed to fit the
respective machine learning models applied for
detection, providing high accuracy and reliability in
classification. In the case of blood cancer detection,
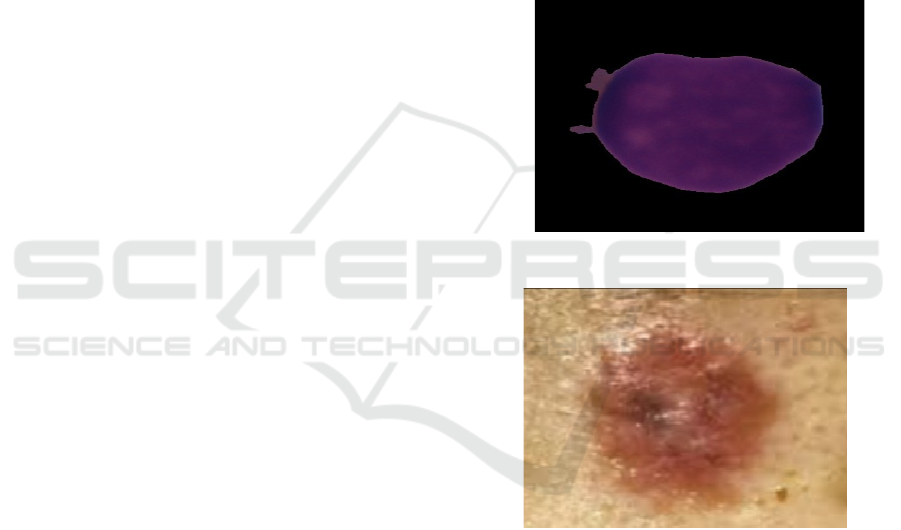
a dataset of 1,659 cancer and 3,389 normal images
in .bmp format (each 450 × 450 pixels) is utilized as
shown in Figure 1.They is high- resolution
microscopic images that facilitate feature extraction
using deep learning for detecting irregular blood cell
shapes. Likewise, in the skin cancer dataset, there are
569 cancer and 235 normal images in .jpg format
(each 194 × 259 pixels) so that Convolutional
Neural Networks (CNNs) can classify malignant and
benign skin lesions with precision as shown in Figure
2.
For the diagnosis of lung cancer, a structured
dataset of
310 × 16 (.csv format) is employed with
patient data, clinical features, and diagnostic labels.
The breast cancer dataset also has a similar structured
form, 570 × 6 (.csv format), enabling the use of
machine learning classifiers like Random Forest and
Logistic Regression for cancer detection at an early
stage.
This collection of datasets provides a complete
multi-modal analysis, combining text-based and
image-based machine learning models for efficient
and scalable cancer diagnosis to assist healthcare
professionals in making quicker and more accurate
predictions.
Figure 1: Blood cancer.
Figure 2: Skin cancer.
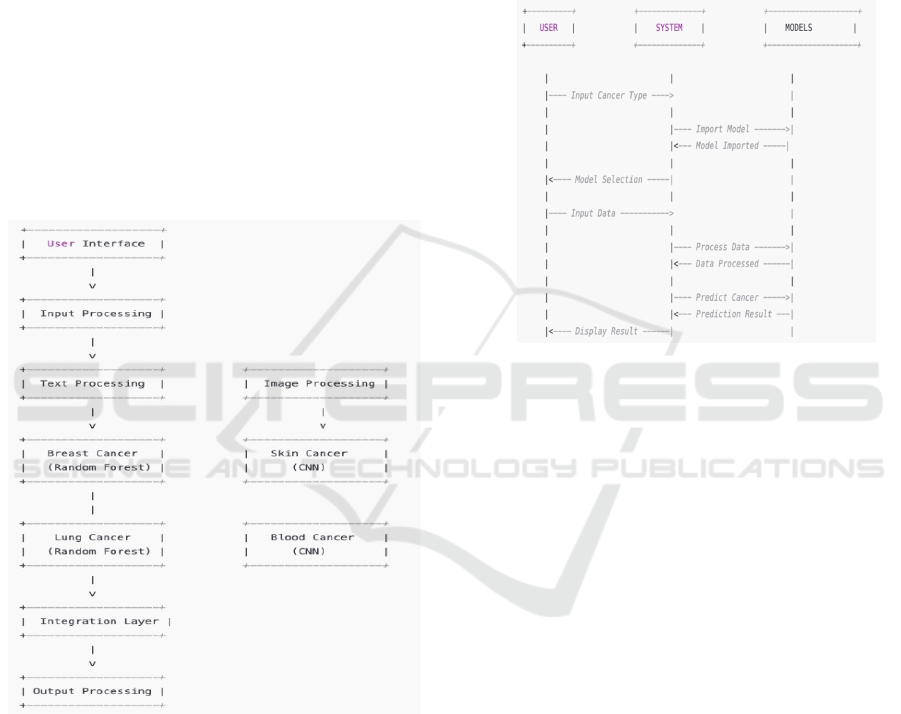
2.1.1 User Interaction & Data Input
The Cancer Care Nexus starts with user interaction,
whereby the healthcare providers choose the cancer
to be diagnosed breast, lung, skin, or blood using a
friendly interface (UI). Based on the choice, the
system calls for text-based input (breast and lung
cancer) or image-based input (skin and blood cancer).
The UI makes sure that the data is captured in the right
format, reminding users if some information is
incomplete or invalid. Valid user input is ensured
through proper validation, and this avoids errors,
allowing high-quality data to enter the system. The
ICRDICCT‘25 2025 - INTERNATIONAL CONFERENCE ON RESEARCH AND DEVELOPMENT IN INFORMATION,
COMMUNICATION, AND COMPUTING TECHNOLOGIES
626

easy-to-use interface shown in Figure 3 makes
navigation easier, enabling healthcare professionals to
upload patient information and view diagnostic results
in real time. The input is then chan- neled to the
preprocessing module, where data is cleaned and
standardized. This step is essential in minimizing
human error, providing consistency, and facilitating
smooth processing for the following detection
models.
Figure 3: User interface.
2.1.2 Input Preprocessing
After the input data is received, the
preprocessing module cleans and optimizes it
to ensure proper classification. In the case of
text data (lung and breast cancer), the system
performs text normalization by eliminating
inconsistencies like special characters, stop
words, and formatting errors. The system then
tokenizes the text, dividing it into meaningful
parts for machine learning analysis. For visual
data (blood and skin cancer), preprocessing
includes scaling, normalizing, and image
augmentation to improve the performance of the
model. Normalization of images provides
consistency in brightness, contrast, and scale,
while image augmentation operations (including
rotation and flipping) facilitate model
generalization. These preprocessing techniques
remove noise, improve pertinent features, and
ensure the data is in a structured form prior to
input into the detection models. By pre-
processing data, the system reduces human
interaction to a great extent, enhancing
efficiency and ensuring high accuracy in
diagnosis.
2.1.3 Models Used
The Cancer Care Nexus framework integrates
different models, each defined for a specific type of
cancer to offer accurate and rapid diagnosis. A
Convolutional Neural Network (CNN) is utilized for
image classification by the skin cancer detection
module. The model contains three convolutional
layers with 32, 64, and 128 filters (3×3 kernels), each
followed by MaxPooling (2×2) to reduce spatial
dimensions. After feature extraction, the model
flattens data into a 1D vector of size 36,992 that is
input to a dense layer with 512 units and a dropout
layer (0.5) to prevent overfitting. The final sigmoid-
activated dense layer enables binary classification
to produce a total of 18,033,177 trainable parameters.
For blood cancer detection, a more complex CNN
architecture is employed, which consists of six
convolutional layers having successively large filter
sizes (32, 64, 128, and 256 filters). Each
convolutional layer is followed by batch
normalization to normalize training and MaxPooling
(2×2) for dimensionality reduction.
The network comprises fully connected layers of
1,024 and 512 neurons, two dropout layers, and a
sigmoid classification layer for binary output. This
design results in 23,252,929 trainable parameters and
hence makes the model effective to detect intricate
patterns in blood smear images. To detect lung and
breast cancer, random forest classifier is employed
which is designed specifically for dealing with
structured data. Instead of images, these models
operate on numeric and categorical values of data
present in tabular data sets in order to detect patterns
in patients’ history. The Random Forest algorithm
builds numerous decision trees and averages multiple
outputs, increasing classification robustness and
reducing overfitting. By combining CNN-based
image processing for skin and blood cancer and
Random Forest-based structured data processing for
lung and breast cancer, the Cancer Care Nexus system
provides an end-to-end, multi-modal cancer detection
solution. The hybrid approach enhances the
classification’s diagnostic accuracy, providing
uniform classification for various cancers, and
optimizing computational efficiency for real-world
medical use.
2.1.4 Cancer Detection Model Execution
Following preprocessing, the system sends the data
to the corresponding cancer detection model
depending on the chosen type of cancer. In the case of
breast and lung cancer, the system uses a Random
Forest algorithm, which is very effec- tive for
Cancer Care Nexus
627
structured text data analysis in predictive
classification. For skin and blood cancer, the system
uses Convolutional Neural Networks (CNNs), which
are particularly useful in image detection and
detection of abnormalities in medical scans. All the
models were trained on extensive datasets to
identify unique cancer characteristics with high
precision and recall rates. The models process the
input data and output classification results, as shown
in figure 5 forecasting whether the sample is
cancerous or not. The output also comprises
confidence scores, which express the model’s
confidence level in its prediction. Through the use
of sophisticated AI models, Cancer Care Nexus
improves accuracy in diagnosis, lessening reliance
on conventional, time-consuming screening
techniques.
2.1.5 Integration & Result Processing
Figure 4: Proposed architecture.
After the predictions are made by the cancer detection
models, the Integration Layer integrates and
processes these outputs, which is done such that the
data flows smoothly across various cancer detection
modules. The system logically integrates and formats
output, organizing them for clear presentation. It
computes confidence scores to enable healthcare
workers to gauge the validity of the diagnosis. The
integration layer guarantees the detection process to
be efficient and without errors, so that the end results
are free from inconsistencies. It also merges multi-
type cancer diagnoses into a single unified answer as
shown in architecture in figure 4 which is essential for
patients with risk for multiple cancers. The
integration layer is critical to simplify, since
healthcare workers are no longer required to manually
decipher results from more than one system. Rather,
all the diagnostic findings are displayed in one screen
to give a unified and precise medical diagnosis.
Figure 5: Proposed modules.
3 OUTPUT GENERATION &
PRESENTATION
After the results are processed, the output processing
mod- ule formats the diagnostic report for
presentation in the user interface (UI). The results
comprise cancer type classification, probability
scores, and image-based diagnosis supporting
visualizations. To make the information readable, the
module structures and presents the information in a
visual and intuitive fashion, facilitating easier
interpretation by medical specialists. For borderline
predictions, the system can recommend additional
medical assessment, allowing clinicians to make
better-informed decisions. The module also features a
clinical recommendation area, providing suggestions
for the next steps depending on AI analysis. The
output processing guarantees the final results are
trustworthy, properly structured, and readable, aiding
healthcare professionals in providing timely and
accurate diagnoses. This step closes the gap between
AI- derived insights and actual clinical decision-
making.
ICRDICCT‘25 2025 - INTERNATIONAL CONFERENCE ON RESEARCH AND DEVELOPMENT IN INFORMATION,
COMMUNICATION, AND COMPUTING TECHNOLOGIES
628
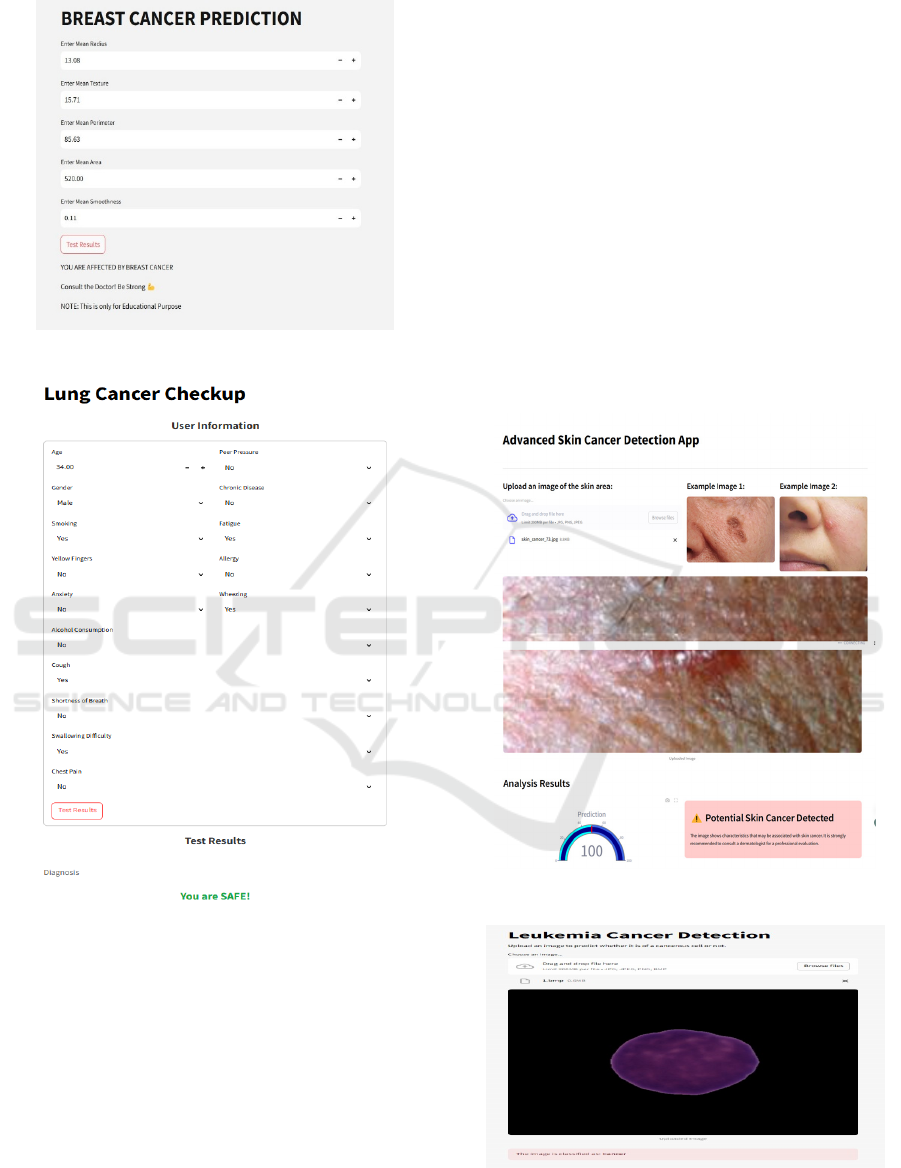
Figure 6: Breast cancer predition.
Figure 7: Lung cancer predition.
4 CONCLUSION AND FUTURE
WORK
The Cancer Care Nexus system is an easy-to-use
diagnostic platform combining machine learning
algorithms for detection of breast, lung, skin, and
blood cancers. As shown in figure 6, figure 7, figure
8. It uses random forest classifiers for text cancers and
convolutional neural networks (CNNs) for image
cancers with assured reliability. A common interface
facilitates smooth interaction, with feedback from the
user helping to improve it continuously. Patient data
security features ensure privacy, and the scalability of
the system makes it suitable for real-world
applications. Future developments will involve
cutting-edge deep learning methods, multimodal
input data with text, images, and genomic
information, and subtype-specific models for cancer.
Real-time diagnostic functionality will enhance
processing speed, and personalization through patient
history and genetic information will improve
predictions. Continuous learning will allow models to
adapt, and cross modal data fusion will enhance
analysis. Privacy enhancing methods such as
federated learning will protect patient information,
while international collaborations will increase
datasets, making Cancer Care Nexus a stronger and
wiser diagnostic tool. Figure 9 shows the lung cancer
prediction.
Figure 8: Skin cancer predition.
Figure 9: Lung cancer predition.
Cancer Care Nexus
629

5 RESULTS
Cancer Care Nexus is an artificial intelligence-based
cancer detection platform that is able to detect various
types of can- cers through machine learning
algorithms. The Breast Cancer Module uses a
Random Forest Classifier for text analysis, with an
accuracy of 93%, and the Lung Cancer Module uses
the same algorithm for detecting lung cancer with
an accuracy of 95%. For picture-based detection, the
Skin Cancer Module uses a Convolutional Neural
Network (CNN) to achieve 92% accuracy, while the
Blood Cancer Module is also based on CNN and
gives an 85% accuracy. Through these domain-
specific models being integrated together, Cancer
Care Nexus guarantees an end-to-end effective
diagnostic process and early cancer detection, while
helping healthcare experts to make proper clinical
decisions.
REFERENCES
A. I. Shehta, M. Nasr, and A. E. D. M. El Ghazali,” Blood
cancer prediction model based on deep learning
technique,” Sci. Rep., vol. 15, no. 1, p. 1889, 2025.
A. Jafari, “Machine-learning methods in detecting breast
cancer and related therapeutic issues: a review,”
Computer Methods in Biomechanics and Biomedical
Engineering: Imaging & Visualization, vol. 12, no. 1, p.
2299093, 2024.
B. S, P. R, and A. B,” Lung Cancer Detection using
Machine Learning,” in Proc. Int. Conf. Appl. Artif.
Intell. Comput. (ICAAIC), Salem, India, 2022, pp. 539-
543, doi: 10.1109/ICAAIC53929.2022.9793061.
B. N. Kumar, N. C. Gowda, B. J. Ambika, H. N. Veena, B.
Ben Sujitha, and D. R. Ramani, “An efficient breast
cancer detection using machine learning classification
models,” International Journal of Online & Biomedical
Engineering, vol. 20, no. 13, 2024.
I. A. Kandhro, S. Manickam, K. Fatima, M. Uddin, U.
Malik, A. Naz, and A. Dandoush, “Performance
evaluation of E-VGG19 model: Enhancing real-time
skin cancer detection and classification,” Heliyon, vol.
10, no. 10, 2024.
I. A. Kandhro et al.,” Performance evaluation of E-VGG19
model: Enhancing real-time skin cancer detection and
classification,” Heliyon, vol. 10, no. 10, 2024.
K. Hemalatha et al.,” An Enhanced Analysis of Blood
Cancer Prediction Using ANN Sensor-Based Model,”
Eng. Proc., vol. 59, no. 1, p. 65, 2023.
Khalid, A.; Mehmood, A.; Alabrah, A.; Alkhamees, B.F.;
Amin, F.; AlSalman, H.; Choi, G.S. Breast Cancer
Detection and Prevention Using Machine Learning.
Diagnostics 2023, 13, 3113.
M. Adjouadi et al., “Classification of leukemia blood
samples using neural networks,” Annals of Biomedical
Engineering, vol. 38, pp. 1473- 1482, 2010.
M. K. Monika, N. A. Vignesh, C. U. Kumari, M. N. V. S.
S. Kumar, and E. L. Lydia, “Skin cancer detection and
classification using machine learning,” Materials
Today: Proceedings, vol. 33, pp. 4266-4270, 2020.
O. Akinrinade and C. Du,” Skin cancer detection using deep
machine learning techniques,” Intell.-Based Med., vol.
11, p. 100191, 2025.
O. Akinrinade and C. Du, “Skin cancer detection using deep
machine learning techniques,” Intelligence-Based
Medicine, vol. 11, p. 100191, 2025.
P. Chaturvedi, A. Jhamb, M. Vanani, and V. Nemade,
“Prediction and classification of lung cancer using
machine learning techniques,” in IOP Conference
Series: Materials Science and Engineering, vol. 1099,
no. 1, p. 012059, Mar. 2021, IOP Publishing.
R. Raina et al., “A systematic review on acute leukemia
detection using deep learning techniques,” Archives of
Computational Methods in Engineering, vol. 30, no. 1,
pp. 251-270, 2023.
R. Javed, T. Abbas, A. H. Khan, A. Daud, A. Bukhari, and
R. Alharbey, “Deep learning for lungs cancer detection:
a review,” Artificial Intelligence Review, vol. 57, no. 8,
p. 197, 2024.
S. Kabiraj et al.,” Breast cancer risk prediction using
XGBoost and random forest algorithm,” in Proc. 11th
Int. Conf. Comput., Commun. Netw. Technol.
(ICCCNT), Kharagpur, India, Jul. 2020, pp. 1-6, doi:
10.1109/ICCCNT49239.2020.9225433.
S. Agarwal, S. Thakur, and A. Chaudhary,” Prediction of
Lung Can- cer Using Machine Learning Techniques
and their Comparative Anal- ysis,” in Proc. 10th Int.
Conf. Reliability, Infocom Technol. Optim. (Trends
Future Directions) (ICRITO), Noida, India, 2022, pp. 1-
5, doi: 10.1109/ICRITO56286.2022.9965052.
S. P. Maurya, P. S. Sisodia, R. Mishra, and D. P. Singh,
“Performance of machine learning algorithms for lung
cancer prediction: a comparative approach,” Scientific
Reports, vol. 14, no. 1, p. 18562, 2024.
U. K. Lilhore, S. Simaiya, Y. K. Sharma, K. S. Kaswan, K.
B. Rao, V. M. Rao, A. Baliyan, A. Bijalwan, and R.
Alroobaea, “A precise model for skin cancer diagnosis
using hybrid U-Net and improved MobileNet-V3 with
hyperparameters optimization,” Scientific Reports, vol.
14, 2024.
V. Sureshkumar, R. S. N. Prasad, S. Balasubramaniam, D.
Jagannathan, J. Daniel, and S. Dhanasekaran, “Breast
cancer detection and analytics us- ing hybrid CNN and
extreme learning machine,” Journal of Personalized
Medicine, vol. 14, no. 8, p. 792, 2024.
ICRDICCT‘25 2025 - INTERNATIONAL CONFERENCE ON RESEARCH AND DEVELOPMENT IN INFORMATION,
COMMUNICATION, AND COMPUTING TECHNOLOGIES
630
