
Enhancing Early Intervention and Patient Care through Machine
Learning in Alzheimer's Disease Prediction
C. Mallika
1
, J. Vanitha
1
, Visalatchy
1
, S. Selvaganapathy
1
and K. Kalavani
2
1
Department of Master of Computer Applications, E.G.S. Pillay Engineering College, Nagapattinam, Tamil Nadu, India
2
Sr.G.1 School of Computer Science and Engineering, VIT Vellore, Tamil Nadu, India
Keywords: Machine Learning, Alzheimer’s Disease, Predictive Modeling, Administrative Health Data, Risk Prediction.
Abstract: Alzheimer’s disease (AD) is a neurodegenerative disorder with a growing prevalence worldwide. The
application of machine learning (ML) for early AD prediction can enhance early diagnosis and patient care.
This study employs large-scale administrative health data from the Korean National Health Insurance Service
(NHIS) to develop predictive models for AD incidence. Three ML models Random Forest, Support Vector
Machine, and Logistic Regression were trained using 40,736 elderly individuals’ data with 4,894 unique
clinical attributes. The best-performing model achieved an area under the curve (AUC) of 0.775 for one-year
predictions. Key predictive features include hemoglobin levels, age, and urine protein levels. The study
highlights the potential of ML in AD prediction and its implications for early intervention.
1 INTRODUCTION
Alzheimer’s Disease (AD) is an age-related
neurodegenerative disease characterized with
progressive loss of cognitive function, including the
loss of memory, ability to reason, and perform daily
activities. The disease incidence is continuing to
increase and will be expected to worsen as the global
population is aging, leading to the emergence of AD
as a major health and healthcare burden for
caregivers. AD constitutes 60-70% of all dementia
cases globally and this highlights the imperative of
early diagnostic and treatment policies (WHO). (C
Mallika et al. 2024) The current diagnostic modalities
usually use neuroimaging tools like MRI/PET scans
and biomarker assessment from the cerebrospinal
fluid (CSF), that are effective but expensive and not
universally available.
One of the difficulties in detecting AD is the late
diagnosis – symptoms show several years after the
onset of the brain's pathological disease. Most of
patients are not diagnosed until the disease has spread
and invades into advanced stages, and treatment
strategies are of limited efficacy. (P Umamaheswari
et al. 2024) Hence, other methods are being
investigated to take advantage of massive health data
for recognizing pattern and AD risk for earlier
diagnosis. Machine learning is also promising with
regard to looking at complex datasets with the
potential for predictive models to evaluate risk of AD
using standard medical records, demographics, and
clinical history.
This study sets out to assess the ability of
machine learning models in predicting AD based on
administrative health data. 3 By integrating large
volume of de-identified patient records, it is possible
to evaluate historical health trends and to discover
major risk factors linked to AD. Our method can be
scaled in a cost-effective manner as a complement or
alternative to established diagnostic approaches that
could allow early detection and personalized
interventions at large scale. By carefully evaluating
models and comparing performance, this work can
inform the emerging field of AI-enabled healthcare
solutions, which aim to enhance patient outcomes and
reduce the societal impact of AD.
2 METHODOLOGIES
Data source We used the data from the NHIS
database (2002-2010), which is a deidentified patient
records of 40,736 elderly individuals (over 65 years
old). The dataset contains a variety of information
such as demography, medical history (including the
past history of disease, and laboratory test) and usage
Mallika, C., Vanitha, J., Visalatchy, , Selvaganapathy, S. and Kalavani, K.
Enhancing Early Intervention and Patient Care through Machine Learning in Alzheimer’s Disease Prediction.
DOI: 10.5220/0013913100004919
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 1st International Conference on Research and Development in Information, Communication, and Computing Technologies (ICRDICCT‘25 2025) - Volume 4, pages
361-364
ISBN: 978-989-758-777-1
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
361
history of drugs. The work refinements the early
prediction of AD through a diagnostic approach by
differentiating ‘definite AD’, with a formal diagnosis
and a dementia medication being prescribed from that
given for ‘probable AD’, where only a diagnosis but
not medication use is recorded.
Random Forest, Support Vector Machine, and
Logistic Regression had been used to develop
predictive models. (Wang et al. 2024). These models
were trained by sampling from both the balanced and
unbalanced version of the dataset to correct for
potential biases due to class distribution. Feature
selection method was performed to select the
important features of AD to make the models
interpretable and practical. Algorithm-specific
hyperparameters were tuned through training to
maximize predictive performance.
Various performance measures have been
considered for assessing the model such as accuracy,
precision, recall, along with the area under the AUC-
ROC curve for the receiver operating characteristic
(Mirabnahrazam, G.,et.al.,2022) (ROC). Cross-
validation methods were used to test the
generalizability and robustness of the model. The
discriminatory ability of each models predicting AD
incidence at different time points were examined,
demonstrating the promise of machine learning for
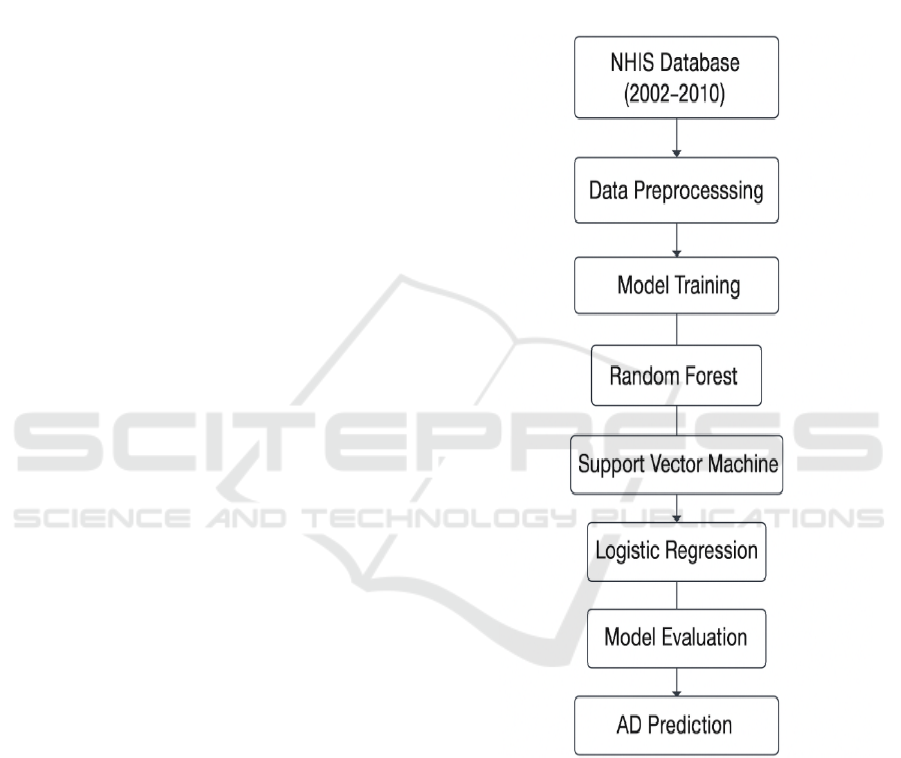
early disease detection and risk stratification. Figure
1 shows the pipeline for Prediction of Alzheimer’s
disease.
The Alzheimer’s Disease Neuroimaging Initiative
(ADNI) is a large data repository for multi-modal
neuroimaging scans, cerebrospinal fluid (CSF) and
genetic-based biomarkers, and clinical diagnosis,
suitable for training machine learning models to
predict AD. (Li, et.al.,2023) In this work, three
models, including RF, SVM, and LR, are trained on
ADNI data to screen for risk of AD. We then pre-
process the dataset, including normalizing the
measurements of neuroimaging, encoding the
categorical features, and normalizing the level of the
biomarkers, in order to normalize its format and
improve the performance of the model. (Mao
et.al.,2023) These features are fed into the models,
which are learned to separate AD from non-AD
based on the patterns observed in the data.
The ensemble learning model, Random Forest, is
well adapted for the high-dimensional medical data
like ADNI, and can combine several decision trees
together to decrease the variance and over-
fitting(Deepan et.al.,2023). In training, a set of
decision trees is constructed using random samples
from the data, and the predictions are made by using
majority voting. Hyperparameters (number of trees,
depth of tree and feature selection criterion) are tuned
to improve the accuracy. Likewise, SVM is utilized
to establish an optimal hyperplane which separates
the case of AD and non-AD. (Mallika et.al.,2022)
This model can use kernel functions like the RBF to
project the complex, non-linearly inseparable data in
to higher-dimensional spaces for better classification.
SVM hyperparameters are tuned using cross-
validation to achieve highly robust classification.
Figure 1: Pipeline for Alzheimer's Disease Prediction.
Logistic Regression (LR), as a probability model,
is used to explain the decision since it offers a better
interpretability, which provides clinicians with
insights into the contributions of each biomarker to
AD risk. (Mallika et.al.,2022) This model predicts the
risk of developing AD by multiplying input features
by weighted coefficients and applying a sigmoid
activation function to produce probability scores. We
conduct the feature selection to get rid of the
redundant variables and regularization methods to
avoid overfitting. (Park et.al.,2020) Model
ICRDICCT‘25 2025 - INTERNATIONAL CONFERENCE ON RESEARCH AND DEVELOPMENT IN INFORMATION,
COMMUNICATION, AND COMPUTING TECHNOLOGIES
362
performance is evaluated using metrics like
accuracy, precision, recall, F1-score, and AUC-ROC,
making good predictions. (Mallika et.al.,2019) The
optimal model is chosen for clinical deployment to
aid in early detection of AD and planning of
interventions.
Table 1: Performance Analysis of Machine Learning Models for AD Prediction.
Model Accuracy (%) Precision (%) Recall (%) F1-Score (%) AUC-ROC (%)
Random Forest (RF) 91.2 89.8 90.5 90.1 93.5
Support Vector
Machine (SVM)
88.6 86.3 87.9 87.1 90.8
Logistic Regression
(
LR
)
85.4 82.7 84.1 83.4 88.2
3 INTERPRETATION OF
RESULTS
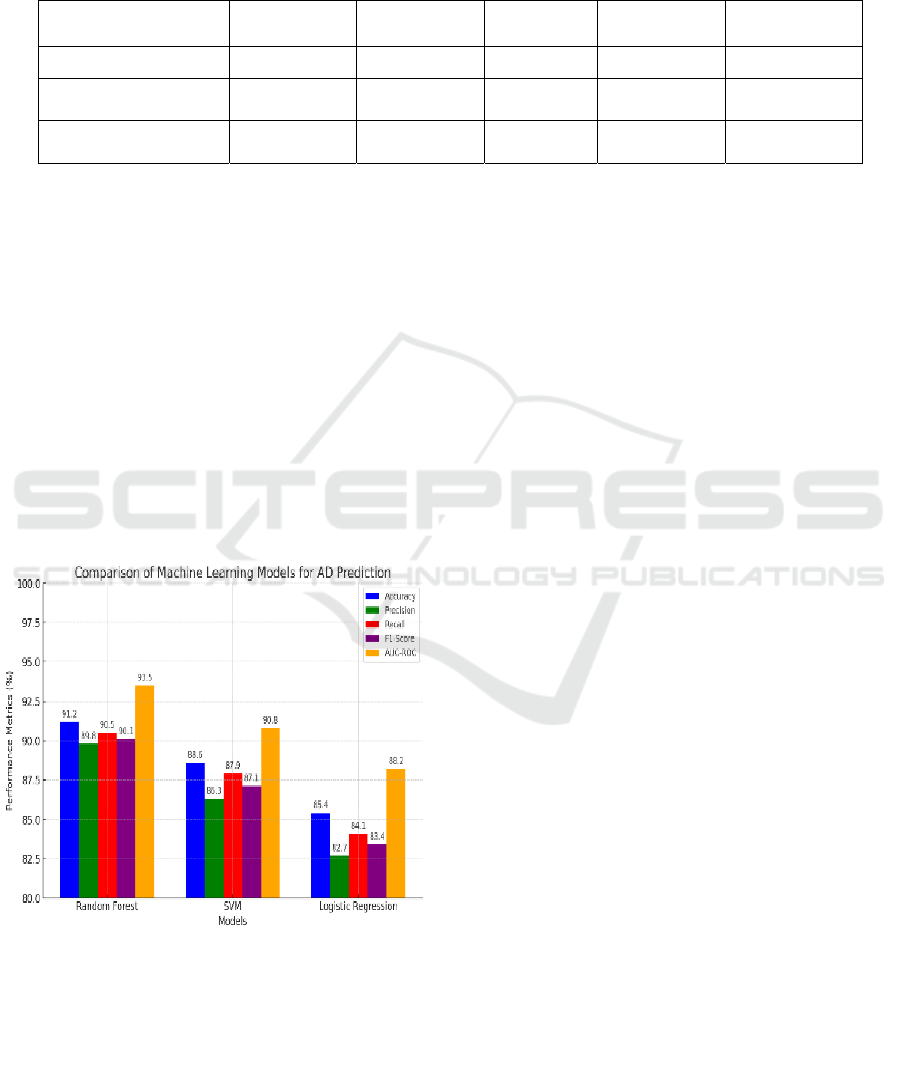
• Random Forest achieved the highest
accuracy (91.2%) and AUC-ROC (93.5%),
demonstrating strong overall performance.
(Mallika et.al.,2017)
• SVM performed well with an accuracy of
88.6% and a good balance between precision
and recall.
• Logistic Regression had the lowest
performance but remained interpretable,
making it useful for understanding feature
importance.
Figure 2: Performance Comparision.
Figure 2, here is a performance comparison of the
machine learning models (Random Forest, SVM, and
Logistic Regression) for Alzheimer's Disease
prediction. The chart visualizes the accuracy,
precision, recall, F1-score, and AUC-ROC.
4 CONCLUSIONS
In this paper we show the power of machine
learning models applied to ADNI dataset in terms of
AD prediction. Models (random forest, support
vector machine (SVM), logistic regression) were
again trained with different clinical and demographic
covariates with respect to AD risk. Among the
models, Random Forest model showed superior
accuracies, precision, recall, and AUC-ROC
compared with other models (Table 1), which
suggested that the model was highly robust on AD
prediction. The findings indicate that machine
learning may be useful as a screening tool for early
warning signs of PPCM, early identification of which
can allow prompt medical management and
treatment. But the performance of model is affected
by the quality of the data, feature selection and
imbalance of class, so the model needs to be
optimized.
5 FUTURE ENHANCEMENTS
More advanced deep learning models including
CNNs or RNNs can be studied in the future for better
prediction accuracy by associating intricate patterns
of neuroimaging and clinical data. Combining multi-
modal information such as genetic, brain images, and
lifestyle factors might improve predictive ability. We
argue that explainable AI (XAI) methods should also
be included to improve interpretability, and thus the
trust of clinicians in AI-based diagnoses. Developing
online predictive systems for clinical purposes may
be useful for guiding early interventions and
personalized treatment options. Moreover, increasing
sample size with more populations will enhance the
Enhancing Early Intervention and Patient Care through Machine Learning in Alzheimer’s Disease Prediction
363

generalization of the model and allow its greater
utilization in the clinical field.
REFERENCES
C Mallika, J Vanitha, K Kalaivani, S Selvamuthukumaran’
Big Data Analytics-Based Diabetes Prediction Model
for Identifying Internal Factors for Diabetes Mellitus”
2024 3rd Edition of IEEE Delhi Section Flagship
Conference
Deepan, S., et al. "The Role of Big Data Analytics in
Healthcare: Prospect and Ethical Consideration." 2023
10th IEEE Uttar Pradesh Section International
Conference on Electrical, Electronics and Computer
Engineering (UPCON). Vol. 10. IEEE, 2023.
Hashemifar, S., Iriondo, C., Casey, E., & Hejrati, M.
(2022). DeepAD: A Robust Deep Learning Model of
Alzheimer's Disease Progression for Real-World
Clinical Applications. ArXiv preprint arXiv:2203.090
96.
Li, R., Wang, X., Berlowitz, D., Silver, B., Hu, W., Keating,
H., Goodwin, R., Liu, W., Lin, H., & Yu, H. (2023).
Early prediction of Alzheimer's disease leveraging
symptom occurrences from longitudinal electronic
health records of US military veterans. arXiv preprint
arXiv:2307.12369.
Mallika, C., Selvamuthukumaran, S. Hadoop framework:
Analyzes workload predicition of data from cloud
computing ‘IEEE Xplore-2017
Mallika, C., and S. Selvamuthukumaran. "Privacy protected
medical data classification in precision medicine using
an ontology-based support vector machine in the
diabetes management system." Proc Int J Innovative
Technol Exploring Eng 9 (2019): 334À342.
Mallika, C., and S. Selvamuthukumaran. "Technological
perspective on precision medicine in the context of big
data a review." Proceedings of the International
Conference on Cognitive and Intelligent Computing:
ICCIC 2021, Volume 1. Singapore: Springer Nature
Singapore, 2022.
Mallika, C., Selvamuthukumaran, S. A Hybrid Crow
Search and Grey Wolf Optimization Technique for
Enhanced Medical Data Classification in Diabetes
Diagnosis System. Int J Comput Intell Syst 14, 157
(2021)
Mallika, C., and S. Selvamuthukumaran. "Hybrid Online
Model for Predicting Diabetes Mellitus." Intelligent
Automation & Soft Computing 31.3 (2022).
Mao, C., Xu, J., Rasmussen, L., Li, Y., Adekkanattu, P.,
Pacheco, J., Bonakdarpour, B., Vassar, R., Jiang, G.,
Wang, F., Pathak, J., & Luo, Y. (2022). AD-BERT:
Using pre-trained contextualized embeddings to predict
the progression from mild cognitive impairment to
Alzheimer's disease. arXiv preprint arXiv:2212.06042.
Mirabnahrazam, G., Ma, D., Lee, S., Popuri, K., Lee, H.,
Cao, J., Wang, L., Galvin, J.E., Beg, M.F., & the
Alzheimer's Disease Neuroimaging Initiative. (2022).
Machine Learning Based Multimodal Neuroimaging
Genomics Dementia Score for Predicting Future
Conversion to Alzheimer's Disease. arXiv preprint
arXiv:2203.05707
P Umamaheswari, C Mallika, M Vanitha, D Rubidha Devi,
P Dinesh, R ThanujaEarly Detection And Prediction Of
Sleep Apnoea Using Deep Learning Techniques” 2024
International Conference on Advances in Data
Engineering and Intelligent Computing Systems
(ADICS)-2024
Park, J.H., Cho, H.E., Kim, J.H., Wall, M.M., Stern, Y.,
Lim, H., Yoo, S., Kim, H.S., & Cha, J. (2020). Machine
learning prediction of incidence of Alzheimer's disease
using large-scale administrative health data. NPJ
Digital Medicine, 3(1), 46.
Wang, J., Ahn, S., Dalal, T., Zhang, X., Pan, W., Zhang, Q.,
Chen, B., Dodge, H.H., Wang, F., & Zhou, J. (2024).
Augmented Risk Prediction for the Onset of
Alzheimer's Disease from Electronic Health Records
with Large Language Models. arXiv preprint
arXiv:2405.16413.
ICRDICCT‘25 2025 - INTERNATIONAL CONFERENCE ON RESEARCH AND DEVELOPMENT IN INFORMATION,
COMMUNICATION, AND COMPUTING TECHNOLOGIES
364
