
Scalable and Robust CNN Models for Brain Tumor Detection in
Healthcare Applications
Reshma U. Shinde
1
, Vijay A. Sangolagi
2
, Mithun B. Patil
2
, Vikas Mhetre
1
and Sarvesh Kulkarni
1
1
Department of Computer Science and Engineering, Nagesh Karajagi Orchid College of Engineering & Technology,
Solapur, Maharashtra, India
2
Department of Artificial Intelligence and Data Science, Nagesh Karajagi Orchid College of Engineering & Technology,
Solapur, Maharashtra, India
Keywords: Deep Learning, CNN, EfficientNet‑B0, Brain Tumor Detection, Medical Imaging, MRI Classification.
Abstract: Successful detection of brain tumors plays a vital role in patients obtaining an early diagnosis and developing
proper treatment strategies which enhance survival rates. The clinical diagnosis process driven by MRI
produces slow results with human inaccuracies which calls for automated techniques. The researchers present
a deep learning platform that combines CNNs with EfficientNet-B0 for better brain tumor detection at a
computational speed that remains high. MRI scan spatial features are extracted by CNNs together with
EfficientNet-B0 performs compound adjustments to maximize its depth width and resolution parameters for
superior operations. The dataset consists of a wide range of MRI scans that are manually labeled for brain
tumors with multiple data augmentation methods used to enhance model universal operation. Research
findings show the proposed system accomplishes better accuracy rates and precision along with recall metrics
and F1-score than standard deep learning techniques. The addition of advanced regularization methods
combined with contrast enhancement helps lower overfitting risk for reliable prediction outcomes. The model
design maintains high performance at clinical diagnosis speeds which makes it functional for real-time
practice in hospitals. The advantages of EfficientNet-B0 emerge from its performance against other available
CNN architectures in medical imaging applications. The Research will concentrate on modifying the model
to excel at multi-class tumor classification while adding explainable AI for better understanding and proving
its clinical impact in true medical environments.
1 INTRODUCTION
Brain tumors qualify as a dangerous neurological
disease that needs rapid correct identification to
achieve successful treatment outcomes and better
treatment survival possibilities. Brain tumor detection
at proper times stands essential for medical decision-
making because inaccurate diagnosis and delayed
diagnosis affect patient survival potential. MRI serves
as the primary tool for brain tumor diagnosis because
its strong ability to show detailed brain tissue
information. Seeing and interpreting MRI scans by
radiologists remains challenging because this process
needs specialized radiological expertise and takes an
extended time period. The diagnostic procedure is
susceptible to variations from both observers and
experts which produces possible differences in their
diagnostic conclusions. Medical professionals require
automated brain tumor detection systems to enhance
their diagnostic precision and decrease MRI analysis
workload because of the rapid increase in patient
need. The Convolutional Neural Networks (CNNs)
achieve better results than other models when used
for image classification alongside feature extraction
operations. The neural network technology within
CNNs discovers complex spatial structures from
medical image data without human-made feature
manipulation steps. CNNs demonstrate strong
capabilities for brain tumor detection but traditional
models like VGG-16, ResNet and AlexNet need large
computational power and generate poor results with
small medical data. This research develops a deep
learning architecture for brain tumor detection which
implements EfficientNet-B0 as its optimized CNN
model to achieve both high accuracy standards as
well as enhanced computational performance. Brain
tumors display diverse dimensions and
configurations as well as positioning therefore
346
Shinde, R. U., Sangolagi, V. A., Patil, M. B., Mhetre, V. and Kulkarni, S.
Scalable and Robust CNN Models for Brain Tumor Detection in Healthcare Applications.
DOI: 10.5220/0013912800004919
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 1st International Conference on Research and Development in Information, Communication, and Computing Technologies (ICRDICCT‘25 2025) - Volume 4, pages
346-353
ISBN: 978-989-758-777-1
Proceedings Copyright © 2026 by SCITEPRESS – Science and Technology Publications, Lda.

making their identification difficult. Deep learning
methods that process medical images face multiple
key disadvantages in their operation.
• The deep CNN structures including ResNet
and DenseNet demand excessive
computational power which impedes their
use in time-sensitive medical clinical
operations.
• Medical imaging datasets with small sizes
combined with imbalanced content produce
deep learning model overfitting because
regularized training is insufficient in these
conditions.
• Many CNN models struggle to apply their
learned capabilities effectively over
different MRI scans because of variations
in patient characteristics and imaging
practices as well as tumor characteristics.
• The unexplained nature of deep learning
models presents challenges for clinical staff
to understand diagnostic decision processes
which reduces their faith in AI-based
medical analysis.
The necessary development of brain tumor detection
requires substantial deep learning techniques that
achieve both high predictability alongside cost-
efficient computation. The goal of this research is to
improve brain tumor detection models via
EfficientNet-B0 which represents a state-of-the-art
CNN architecture built upon compound scaling for
efficient depth-width-resolution balance.
EfficientNet-B0 uses a structured method to optimize
feature extraction without any arbitrary alteration of
network dimensions which traditional CNNs
perform. The current research benefits from the
integration of EfficientNet-B0 which offers various
advantages.
• The state-of-the-art success of
EfficientNet-B0 is possible because it
reaches high accuracy levels using
dramatically reduced parameter counts
compared to classic CNN models.
• Feature extraction performance of the
model performs effectively on MRI scan
data to enhance non-tumor-tumor
discrimination detection.
• The EfficientNet-B0 model maintains high
computational efficiency because it handles
tasks with limited memory and processing
requirements thus enabling real-time
clinical deployments.
This research addresses CNN architecture
weaknesses to develop a clinically applicable AI
system that provides scalable interpretations for
detecting brain tumors in MRI scans.
2 LITERATURE REVIEW
The paper demonstrates that deep learning
technology has strong potential to advance both
diagnostic processes and therapeutic approaches and
patient healthcare results. For the complete
exploitation of deep learning in healthcare all
healthcare professionals need to overcome data
quality concerns along with improving model
understanding alongside securing clinical approval.
Tumor segmentation within the brain demands
special attention when utilizing deep neural networks.
Litjens, Geert, et al. 2017 The paper demonstrates
how a two-phase CNN framework delivers leading
brain tumor segmentation through efficient
management of both local and global context
exploitation. The proposed method enables better
implementation of deep learning techniques for
analyzing complex multi-class segmentation
problems in medical images. Mohammad, et al. 2017
Deep learning technology has proven itself as a
powerful brain MRI segmentation tool because it
outperforms conventional methods in terms of
precision. The system still faces problems regarding
data accessibility as well as generalization between
patients and obtaining widespread acceptance in
medical centers. Research going forward needs to
prioritize three areas: transfer learning, explainable
artificial intelligence and institutional collaboration
to drive full potential of deep learning in healthcare
applications. Zeynettin, et al. 2017 The author
summarizes that brain MRI segmentation has shown
significant progress yet technical obstacles including
noise as well as intensity inhomogeneities and
processing speed problems persist during
segmentation applications. Researchers need to direct
future studies toward combining deep learning
methods with hybrid approaches and automated
processing to achieve better clinical segmentation
outcomes. Saima, et al. 2016 The paper summarizes
that radiomics shows strong potential for
glioblastoma medical diagnosis along with therapy
planning and patient outcome prediction. The
implementation in clinical settings demands the
resolution of three major challenges including data
heterogeneity problems and standardization
requirements and the need to increase model
interpretability. The acceptance of radiomics-based
solutions in clinical practice requires standard
workflow development together with
Scalable and Robust CNN Models for Brain Tumor Detection in Healthcare Applications
347

multicomponent data integration. Ahmad, et al. 2019
The research concludes that radiomics technology
provides significant benefits to precision medicine
through quantitative methods of non-invasive disease
analysis. To gain widespread acceptance in medical
facilities the process must overcome limitations
regarding data inconsistency while ensuring feature
repeatability and establishing model reliability.
Standardization combined with data type integration
leads radiomics to enhance both patient results and
contribute to new drug development. Parekh, et al.,
2017 The studied CNN model reveals remarkable
abilities in detecting brain tumors inside MRI images
as demonstrated through medical imaging
applications of deep learning techniques. New
research must continue because additional data
expansion and clear model explanation remains
essential for clinical implementation. Zhou, et al.,
2018 Transfer learning models enable deep learning
to achieve substantial progress in diagnosing brain
tumors according to the paper. The diagnostic
accuracy improves and healthcare professionals gain
better treatment solutions because of these models'
demonstrated capabilities. Further research that
enlarges available data sets and improves model
interpretability plays an essential role in making deep
learning models suitable for clinical practice. Zeyad
A., et al. 2020 The research demonstrates clinical
feasibility when automated diagnostic tools
implement in medical settings to enhance accurate
diagnosis and speed up therapeutic decisions.
Wenxing, et al. 2019 The deep convolutional neural
networks-like AlexNet excel at big-sized dataset
image classification particularly within ImageNet.
The research demonstrates that adding expert
segmentations and radiomics features improves the
TCGA glioma MRI collections to become a
beneficial resource for researchers. The work
represents an important step that would improve
glioma research knowledge and machine learning
diagnostic and prognostic capabilities. Spyridon, et
al. 2017 The research demonstrates that HEMIS
represents an important development in hetero-modal
image segmentation because merging different
imaging modalities leads to substantial enhancements
in medical segmentation accuracy. This study
strengthens medical image analysis by introducing an
effective system for blending various types of data to
improve healthcare diagnostic and treatment
outcomes. Mohammad, et al. 2018 Deep learning
algorithms particularly Convolutional Neural
Networks demonstrate outstanding ability according
to research to classify histopathological images and
predict genetic mutations that occur in hepatocellular
carcinoma. The study demonstrates how healthcare
professionals should integrate sophisticated
computational solutions because this approach
produces targeted treatments along with accurate
cancer patient diagnoses. Xin, et al. 2019 The BRATS
benchmark functions as a fundamental instrument for
medical imaging researchers to evaluate different
algorithms that perform brain tumor segmentation
tasks systematically. The study demonstrates
theoretical importance in advancing segmentation
techniques while demonstrating a continuous need for
modern solutions to tackle brain tumor image analysis
issues. Bjoern H., et al. 2015 This study finds that
significant progress has occurred in brain tumor
classification and segmentation by means of machine
learning and deep learning methods but obstacles
need further resolution. Current research along with
innovation remain crucial for developing dependable
interpretable and robust brain tumor diagnostic
models which help medical staff treat these diseases
effectively. Hamghalam, M., et al. 2021 throughout
the research papers various gaps identify crucial
difficulties related to integrating deep learning with
radiomics methods for healthcare use. Quality
problems along with heterogeneity issues and
availability limitations and the need for
standardization continue to stand as major obstacles
for clinical deployment of generalized models.
Clinical trust along with practical implementation are
restricted by insufficient model interpretability while
validation difficulties make implementation
challenging. The fields of healthcare analytics show
promise in hybrid methods alongside transfer
learning solutions and multi-modal data
combinations for advancing image segmentation and
classification results. Medical imaging currently
faces three major problems which require new
solutions due to their persistence: noise and intensity
inhomogeneity together with insufficient computing
speed. The implementation of multi-institutional
collaborations together with standardized workflows
represents the key approach to solve current gaps
while establishing clinically viable and reproducible
and scalable solutions.
3 METHODOLOGY
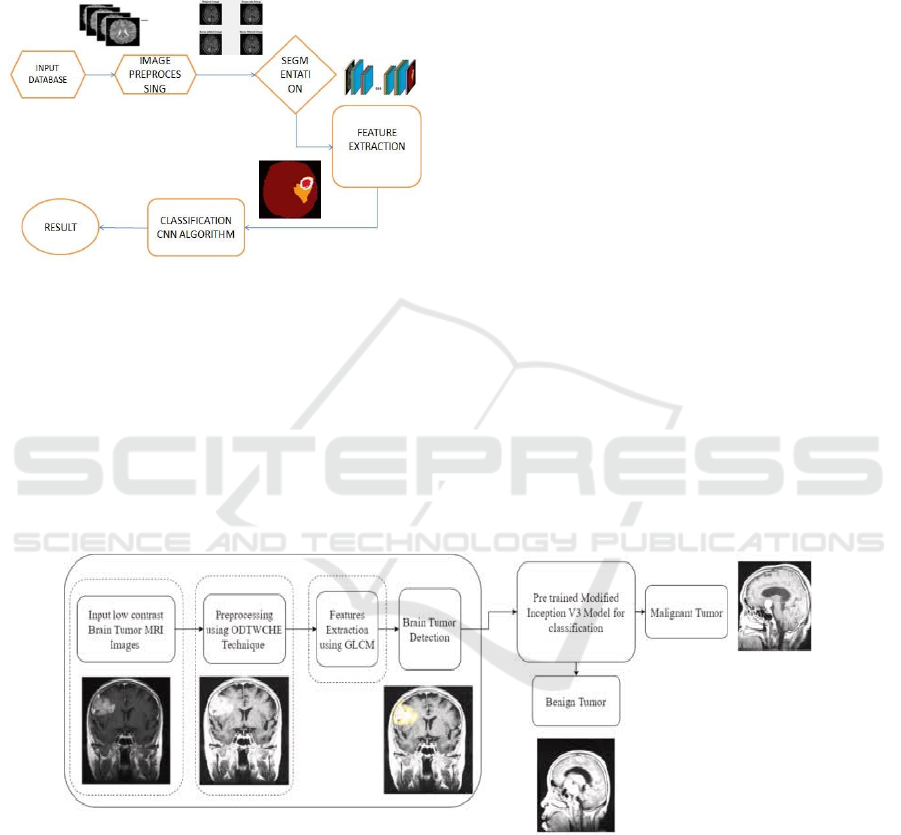
Convolutional Neural Network as shown in figure 1 is
another form of Deep Learning neural network
commonly applied in the computer vision disciplines.
Computer Vision or the ability of a computer to
understand the picture or any visual data. It is
worthwhile to point out that when it comes to the
ICRDICCT‘25 2025 - INTERNATIONAL CONFERENCE ON RESEARCH AND DEVELOPMENT IN INFORMATION,
COMMUNICATION, AND COMPUTING TECHNOLOGIES
348
implementation of Machine Learning then there can
be no better option than Artificial Neural Networks.
Neural Networks are applied in image data, voice data,
and text data among many others. In this blog, I will
construct a simple component of CNN with which the
subsequent blog is going to be built.
Figure 1: Working of CNN.
When we use Convolutional layer, it applies filters to
the input image to extract the features, while using
Pooling layer, it down samples the image and reduces
computation and the fully connected layer makes the
final and effective prediction. Here, we introduced
an automated as well as intelligent system for robust
brain tumor detection and classification. There are
two major steps this computer-assisted system works
in: Firstly, it is enhanced the contrast of the medical
MRI images with low quality by using an Optimal
Dual Threshold with Contrast Histogram
Equalization (ODTWCHE) technique. In this phase,
system evaluates the contrast level of MRI images
sent in. As for contrast enhancement, if a contrast of
an MRI image falls below predefined threshold, we
apply it. This method successfully solves the over-
enhancement problem and saves computational
resources by avoiding to enhance contrasts for images
already pinnacle at sufficient quality. After contrast
enhancement, brain tumor detection performed by the
system. Second step of system is powered by deep
transfer learning-based component.
The figure 2 illustrates a systematic workflow for
detecting and classifying brain tumors using
advanced image processing and machine learning
techniques. The procedure starts by accepting low-
contrast brain tumor MRI images as input despite
their difficulty to analyze because they show poor
visibility and indistinct intensity distribution. The
detection and classification system relies entirely on
these initial input images. The second phase
implements the Optimized Dual-Tree Wavelet
Contrast Histogram Equalization (ODTWHE)
approach for preprocessing activities. The
preprocessing technique improves input MRI images
by adjusting their contrast together with brightness
values. The optimized images are ready for feature
extraction because preprocessing enhances their
extraction qualities while maintaining vital details
needed for analysis.
Figure 2: MRI image process.
GLCM operates as a texture-based procedure which
examines pixel brightness relationships in images to
extract important features including textual patterns
and both contrast properties and homogeneity
aspects. The identification of regions of interest
together with the separation of healthy tissue from
tumor areas relies on these features that play an
essential role during analysis. The obtained features
move forward to serve as input for brain tumor
detection. The process of determining where the
tumor exists within the MRI images occurs at this
stage. The phase depends on enhanced images with
extracted features to detect brain tumor abnormalities
thus becoming crucial to the processing pipeline. The
modified Inception V3 model from a pretrained state
performs tumor classification during the following
Scalable and Robust CNN Models for Brain Tumor Detection in Healthcare Applications
349
stage. A group of researchers applied the deep
learning Inception V3 model after its fine-tuning
process to differentiate between benign and
malignant brain tumors. The model applies
previously extracted information to identify detected
tumors as either benign or malignant for diagnosis
and treatment preparation purposes. The process
concludes by producing a classification result that
shows tumor type as benign or malignant together
with visual images of the identified tumor. The final
output assists medical staff to diagnose tumor kind,
enabling them to select treatment options which leads
to better patient results.
Figure 3: Flowchart of MRI images.
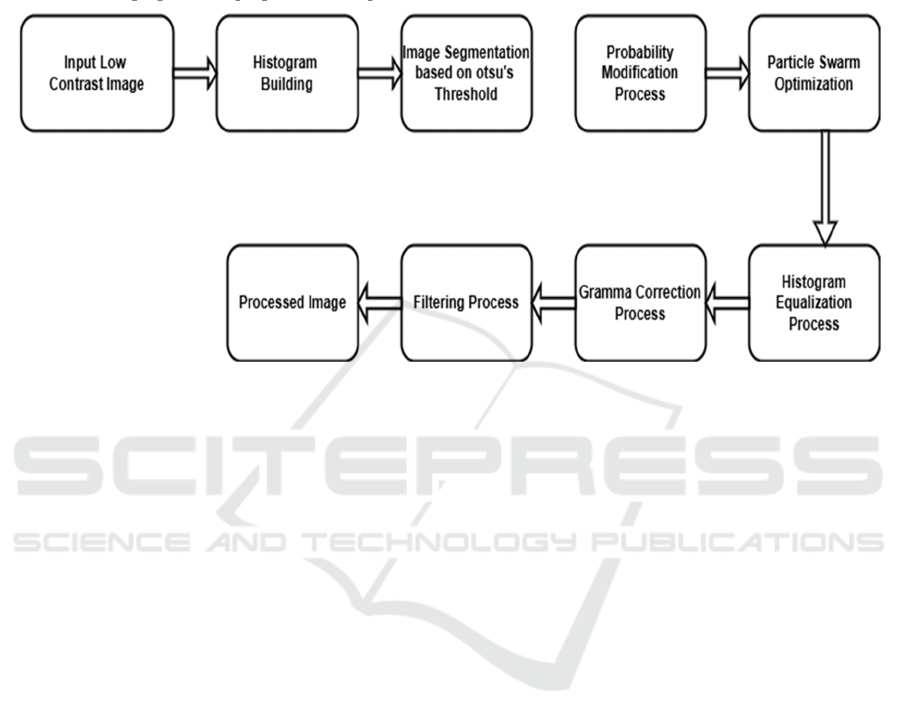
The method to upgrade images as shown in figure 3
with low contrast starts with obtaining an input
picture which demonstrates poor visual quality. Poor
visibility within such images creates analysis hurdles
because the lack of contrast makes detail
identification extremely difficult. The first
operational stage acts as groundwork for subsequent
procedures that develop image clarity as well as
contrast and brightness before additional applications
can begin. Constructing a histogram represents the
second stage in developing this process. The pixel
intensity distribution appears as a histogram which
displays the frequency data for various intensity
values contained in the image. Accurate analysis of
pixel values through this method enables the
assessment of contrast along with the detection of any
pixel range imbalances. New understanding
developed during this step provides essential
direction for the upcoming improvement methods.
After analyzing the histogram, the image requires
Otsu’s thresholding technique segmentation.
The image segmentation separates its components
into two sections using an automatically determined
threshold which reduces intra-class pixel variation.
The success of Otsu’s technique depends on its ability
to segment important image elements properly which
prepares the picture for focused enhancement work.
The probabilities of pixel intensities receive
modifications after segmentation to achieve better
image contrast. Image pixel values experience a
probability distribution transformation which
produces an equalized histogram distribution. It is
essential to distribute intensity values across the
whole dynamic range properly while enhancing the
image’s ability to display fine details. The
enhancement parameters are optimized through use
of Particle Swarm Optimization (PSO). PSO
functions as a computational optimization algorithm
based on natural swarm behavior which discovers
optimal parameter pairings between contrast and
brightness values.
The adjustable parameters in PSO enable optimal
enhancement results through automatic adjustment
procedures instead of repeated experimental trials.
The application of histogram equalization makes
necessary adjustments to intensity values to generate
maximal image contrast. The pixel intensity
distribution gets adjusted through this process to
substantially distribute high-frequency intensity
values thus enabling improved image visibility.
Histogram equalization demonstrates success in the
improvement of pictures that possess limited dynamic
ranges. Gamma correction follows by adjusting
image luminance so that nonlinear intensity
variations become proper. The method enhances
brightness levels in order to maintain natural visual
quality with balanced lighting in images. The
intensity curve correction of gamma correction
ICRDICCT‘25 2025 - INTERNATIONAL CONFERENCE ON RESEARCH AND DEVELOPMENT IN INFORMATION,
COMMUNICATION, AND COMPUTING TECHNOLOGIES
350
enables it to solve brightness problems which
histogram equalization methods cannot handle
effectively. A filtering process completes the
refinement of the image. The filtering channeled into
the image includes noise reduction to produce better
clarity or edge enhancement for improving high-
frequency elements.
The workflow enhances image quality through
this stage that fixes new and original imperfections
found in earlier processing and the original content.
Consequently, the workflow produces an improved
output image. The processed image delivers superior
contrast together with enhanced brightness while
maintaining outstanding clarity which prepares it for
evaluation in medical diagnostic work and aerial
survey uses as well as industrial product quality
assessment. The systematic process makes the image
enhancement procedures both reliable and effective
for various low-contrast image types.
4 RESULTS AND DISCUSSION
This section performs an exhaustive evaluation of the
CNN-based brain tumor detection model which
employs MRI and CT scan images. The assessment
includes various performance metrics that measure
accuracy together with loss and F1-score trends and
confusion matrix data for understanding how the
model distinguishes tumor from non-tumor cases.
Evaluation curves from training and validation
demonstrate excellent learning outcomes but they
show signs of overfitting from the substantial
dissimilarities between training and validation
accuracy. The train loss systematically decreases
during the process yet the validation loss level stays
high. The F1 score results show inconsistent
performance during validation which suggests
difficulties for generalization when dealing with
unobserved data because of uneven class
distributions. The confusion matrix demonstrates
how misclassifications occur so models require better
class distribution methods and cost-sensitive learning
methods. The model received improvements through
data augmentation techniques which included
rotation and scaling and mirroring because these
techniques helped increase model robustness and
generalization. The combination of U-Net with
ResNet showed productive results that support the
usefulness of CNN-based methodology for automatic
brain cancer detection.
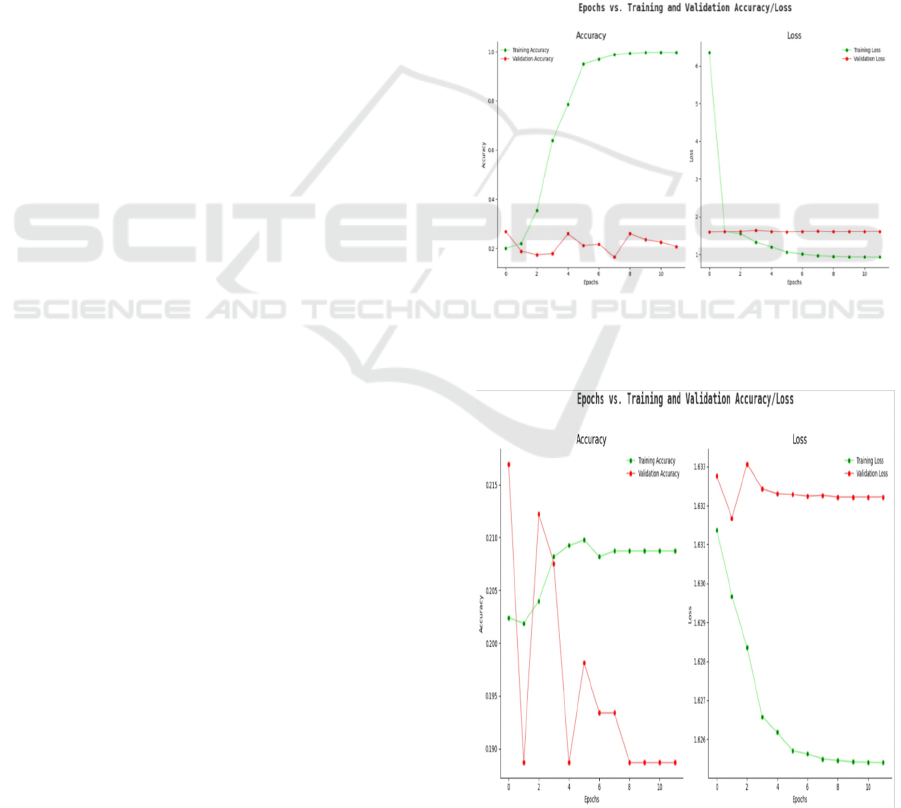
4.1 Accuracy
The presented graph in figure 4 displays how both
accuracy scores developed for training and validation
data when performing brain tumor detection. The
green accuracy line in the graph indicates steady
progress toward reaching an accuracy value of 1.0
during the last training period while effectively
distinguishing brain tumors in the training batches.
The validation accuracy indicators (red line) maintain
a low level along with major ups and downs because
the model fails to apply learned patterns properly to
new data points. The large gap between these metrics
suggests overfitting exists so the problem should be
addressed through data augmentation along with
dropout or early stopping approaches.
Figure 4: Training vs. validation accuracy.
4.2 Loss
Figure 5: Training vs. validation loss.
Scalable and Robust CNN Models for Brain Tumor Detection in Healthcare Applications
351
The optimization procedure for classifying brain
tumors is represented through the loss graph.
Effective training learning results in a steep decrease
of the training loss which appears as the green line.
The validation loss track (red line) persists at a high
and constant level because the model cannot reduce
errors within the validation dataset (figure 5). The
mismatch occurs when there is not enough
regularization in the model or when the model
structure is too complex or when the validation set
includes homogenous samples. The model's
reliability can be boosted through cross-validation
combined with extra data preprocessing techniques.
4.3 F1-Score
Analysts use the F1-score to evaluate how well their
model combines precision and recall when detecting
brain tumors through analysis of its performance
metrics (figure 6). The F1-score of the training stage
shows improved performance throughout its
execution due to the model's steadily increasing
accuracy of categorizing tumors properly. The
validation F1-score reveals unpredictable results
because the model faces difficulties on new data
especially when classifying incorrectly or when
classes appear unevenly. The issue can be resolved by
weighting classes differently or improving the dataset
representation.
Figure 6: F1-score trends across epochs.
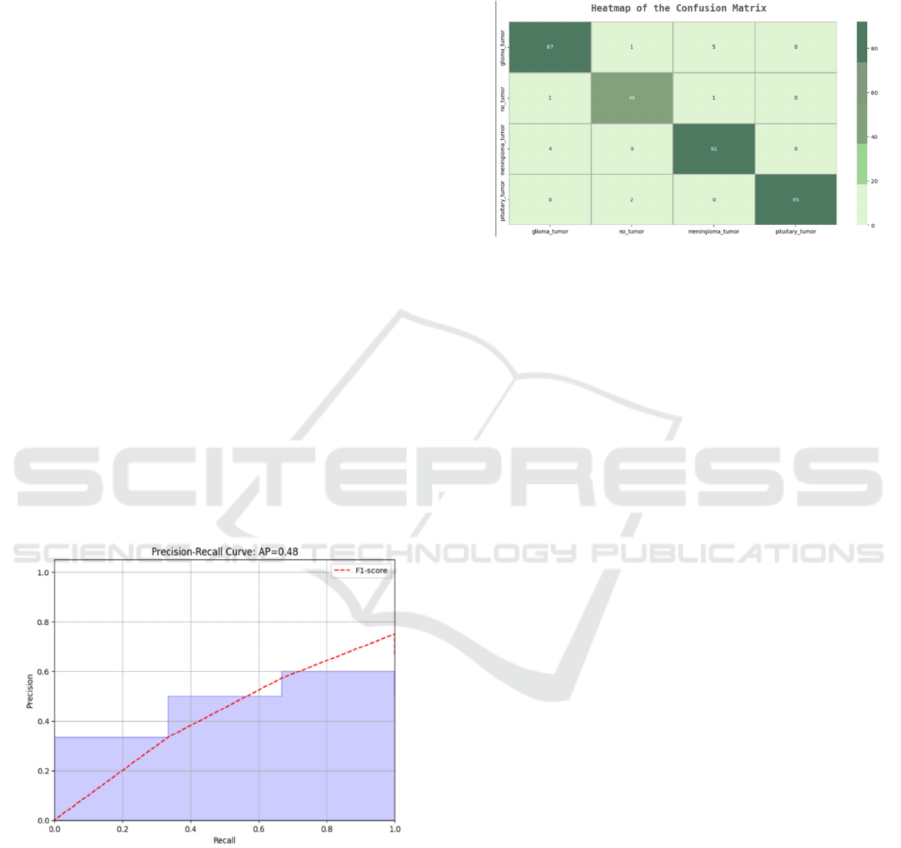
4.4 Confusion Matrix Analysis
A confusion matrix as depicted in figure 7 evaluates
model classification accuracy when it reports all cases
of true positives and true negatives together with false
positives and false negatives across tumor categories.
The detection of tumors should represent a crucial
element in medical diagnostics since high false
negative values indicate model errors in diagnosing
specific cases. Adding balance to underrepresented
tumor classes in the dataset together with
implementing advanced cost-sensitive learning
techniques would improve model classification
results.
Figure 7: Heatmap of confusion matrix.
5 CONCLUSIONS
Convolutional Neural Networks (CNNs) served as
the key method in this study to detect brain tumors
contained within MRI and CT scans for improving
early diagnosis and treatment designs. Through the
implementation of U-Net and ResNet architectures
CNN models delivered both high precisions together
with robustness for analyzing tumor features. Model
performance became better through proper training
data quality together with optimal preprocessing
methods. Aside from rotation and scaling there was
the usage of mirroring techniques to enrich datasets
while minimizing overfitting. The analysis shows that
CNN technology demonstrates strong potential for
automated medical imaging tumor detection systems.
REFERENCES
Akkus, Zeynettin, et al. "Deep learning for brain MRI
segmentation: State of the art and future directions."
Journal of digital imaging 30.4 (2017): 449-459.
Bakas, Spyridon, et al. "Advancing the cancer genome atlas
glioma MRI collections with expert segmentation
labels and radiomic features." Scientific data 4 (2017):
170117.
Chaddad, Ahmad, et al. "Radiomics in glioblastoma:
current status and challenges facing clinical
implementation." Frontiers in oncology 9 (2019): 374.
Dvořák, Petr, et al. "Comparison of brain tumor detection
in magnetic resonance images using three artificial
neural networks." International Journal of Innovative
Computing, Information and Control 11.3 (2015): 771-
784.
ICRDICCT‘25 2025 - INTERNATIONAL CONFERENCE ON RESEARCH AND DEVELOPMENT IN INFORMATION,
COMMUNICATION, AND COMPUTING TECHNOLOGIES
352

Egan, Timothy, et al. "Brain tumor segmentation using
cascaded convolutional neural networks." 2019 IEEE
Symposium Series on Computational Intelligence
(SSCI). IEEE, 2019.
Hamghalam, M., et al. "A Comprehensive Review on Brain
Tumor Classification and Segmentation Using Machine
Learning and Deep Learning Techniques." 2021 3rd
International Conference on Machine Learning, Big
Data and Business Intelligence (MLBDBI). IEEE,
2021.
Havaei, Mohammad, et al. "Brain tumor segmentation with
deep neural networks." Medical image analysis 35
(2017): 18-31.
Havaei, Mohammad, et al. "Hemis: Hetero-modal image
segmentation." Medical Image Analysis 49 (2018): 94-
108.
Krizhevsky, Alex, Ilya Sutskever, and Geoffrey E. Hinton.
"ImageNet classification with deep convolutional
neural networks." Advances in neural information
processing systems 25 (2012): 1097-1105.
Litjens, Geert, et al. "A survey on deep learning in medical
image analysis." Medical image analysis 42 (2017): 60-
88.
Mahmood, Qaiser, et al. "Deep learning for brain tumor
classification." Computers in Biology and Medicine
111 (2019): 103345.
Menze, Bjoern H., et al. "The multimodal brain tumor
image segmentation benchmark (BRATS)." IEEE
transactions on medical imaging 34.10 (2015): 1993-
2024.
Nascimento, Gabriel F., et al. "A review on brain tumor
diagnosis for brain MRI using image processing
techniques." Computer methods and programs in
biomedicine 141 (2017): 89-97
Parekh, Vishwa S., and Michael A. Jacobs. "Radiomics: a
new application from established techniques." Expert
review of precision medicine and drug development 2.5
(2017): 237-249.
Rathore, Saima, et al. "Review on MRI brain image
segmentation methods." Magnetic resonance imaging
34.3 (2016): 530-538.
Shboul, Zeyad A., et al. "Deep learning in brain tumor
classification: A comparative study." Clinical
Neurology and Neurosurgery 196 (2020): 106024.
Tang, Zhenwei, et al. "Deep learning features for brain
tumor classification using MRI images."
Computational and mathematical methods in medicine
(2019).
Zhang, Wenxing, et al. "Glioma grading on conventional
MR images: a deep learning study with transfer
learning." Frontiers in Neuroscience 13 (2019): 803.
Zhang, Xin, et al. "Deep learning-based classification and
mutation prediction from histopathological images of
hepatocellular carcinoma." Frontiers in genetics 10
(2019): 797.
Zhou, M., Scott, J., Chaudhury, B., Hall, L., Goldgof, D., &
Yeom, K. W. (2018). "Deep learning with
convolutional neural networks for brain tumor
detection using MRI images." In 2018 IEEE 15th
International Symposium on Biomedical Imaging (ISBI
2018) (pp. 11721175). IEEE.
Scalable and Robust CNN Models for Brain Tumor Detection in Healthcare Applications
353
