
Abiogenesis and the Key Processes Involved in the Emergence of
Biochemicals
Bhuwan Singh Raj
Department of Zoology, Government Pataleshwar College Masturi, Bilaspur, Chhattisgarh, India
Keywords: Abiogenesis, Prebiotic Chemistry, Autocatalysis, Protocells, Origin of Life.
Abstract: Abiogenesis the origin of life from non-living matter remains a foundational yet unresolved question in
science, requiring interdisciplinary integration across chemistry, geology, and biology. This thesis
synthesizes recent advances in understanding the chemical, energetic, and environmental conditions that
may have enabled the transition from abiotic molecules to self-sustaining, replicative systems. The study
explores key processes including prebiotic synthesis of organic molecules, emergence and preservation of
molecular homochirality, autocatalytic networks (e.g., comproportionation-based autocatalysis), and
environmental catalysis within saline and hydrothermal contexts. It further examines the role of solar and
radioactive energy in driving chemical complexity, the emergence of genetic molecules (RNA/DNA), and
the compartmentalization into protocells that facilitated early molecular evolution. Particular emphasis is
placed on the integration of genetic and metabolic subsystems as a critical threshold toward cellular life.
Through a comprehensive review of experimental and theoretical studies, the thesis highlights how
synergistic interactions between catalytic surfaces, energy fluxes, and molecular self-organization could
have culminated in life’s emergence. The implications extend beyond Earth, offering a framework for
evaluating life’s potential in extraterrestrial environments.
1 INTRODUCTION
Abiogenesis, defined as the natural process by which
life emerges from non-living matter, represents one
of the most intriguing and fundamentally important
questions in the natural sciences. Although the Earth
is approximately 4.54 billion years old, evidence
suggests that life already existed by around 3.5–3.7
billion years ago, as inferred from fossilized
microbial mats (stromatolites) and isotopic
signatures in ancient rocks. Understanding how non-
living, abiotic chemical systems on the early Earth
transitioned into self-sustaining and replicating
systems remains a challenge that intersects
chemistry, geoscience, biology, and astrophysics.
Historically, scientists have developed multiple
hypotheses to account for the complexities of
abiogenesis. These range from primordial “warm
little pond” scenarios (loosely following Darwin’s
speculation) to deep-sea hydrothermal vent
hypotheses. Across these different models, a
common thread is the sequential emergence of
biochemical complexity, progressing from simple
organic precursors to more complex molecules such
as nucleotides, amino acids, and lipids. Eventually,
these building blocks must assemble into
protocellular structures possessing at least
rudimentary metabolic and genetic capabilities.
The central processes of abiogenesis can be broadly
categorized into:
• Prebiotic Synthesis of Organic Molecules:
The formation of fundamental organic
precursors (e.g., amino acids, nucleobases,
sugars) under plausible early Earth conditions.
• Environmental Conditions and Catalysis:
The role of mineral surfaces, hydrothermal
vents, and saline environments in catalyzing
the formation and stability of these
biomolecules.
• Energy Sources for Prebiotic Synthesis: The
utilization of solar, geothermal, and
radioactive energy sources to drive chemical
evolution.
• Molecular Evolution and Self-Replication:
The emergence of autocatalytic networks,
self-replicating molecules (RNA, DNA), and
compartmentalized structures (protocells).
662
Raj, B. S.
Abiogenesis and the Key Processes Involved in the Emergence of Biochemicals.
DOI: 10.5220/0013903600004919
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 1st International Conference on Research and Development in Information, Communication, and Computing Technologies (ICRDICCT‘25 2025) - Volume 3, pages
662-670
ISBN: 978-989-758-777-1
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

• Integration of Genetic and Metabolic
Systems: The co-evolutionary relationship
between early genetic information carriers
and primitive metabolic pathways that set the
stage for modern biochemistry.
1.1 Research Objectives
• To investigate plausible chemical pathways
for the abiotic synthesis of life’s key
biomolecules (e.g., amino acids, nucleotides,
lipids) under early Earth conditions.
• To examine the role of catalytic
environments such as mineral surfaces and
hydrothermal vents in facilitating molecular
assembly, autocatalysis, and
compartmentalization.
• To explore the integration of genetic and
metabolic systems, focusing on how
molecular replication and energy conversion
co-evolved in protocellular contexts.
2 METHODOLOGY
This study employs a literature-based synthesis of
experimental and theoretical research. It reviews
prebiotic chemistry experiments (e.g., Miller–Urey,
mineral catalysis), geochemical models of early
Earth (e.g., hydrothermal systems), and molecular
evolution theories (e.g., RNA world, autocatalytic
networks). Emphasis is placed on interdisciplinary
data integration to assess mechanisms enabling life’s
emergence from non-living matter.
2.1 Prebiotic Synthesis of Organic
Molecules
2.1.1 General Considerations on Prebiotic
Synthesis
The formation of biologically relevant molecules
amino acids, nucleotides, sugars, and fatty acids is
crucial to abiogenesis. Historically, the famous
Miller-Urey experiment (1953) demonstrated that
amino acids could form under reducing atmospheric
conditions involving methane, ammonia, water, and
hydrogen, with electrical discharges simulating
lightning. Since then, the field has advanced
considerably, investigating a broader range of
environments hydrothermal vents, tidal pools, hot
springs, and more. These environments potentially
provided varying redox conditions and catalytic
surfaces favorable for producing monomers
necessary for life (Ershov, 2022; Seitz, Geisberger,
West, & Huber, 2024).
Central to these efforts is the recognition that
early Earth’s atmospheric composition likely
differed from the strongly reducing mixtures used by
Miller and Urey. Modern reconstructions suggest a
more neutral or weakly reducing atmosphere
dominated by CO₂, N₂, H₂O, and trace amounts of
other gases. Under these conditions, alternative
energy sources (e.g., UV radiation, geothermal heat,
or radioactive decay) could have supplemented or
replaced electrical discharges (Lu, Wang, Li, &
Yang, 2014; Ershov, 2022). Regardless of
atmospheric composition, the principle remains that,
given sufficient energy and the right starting
materials, organic compounds can be abiotically
synthesized. Figure 1 Shows the Evolution of
Abiogenesis Research
Figure 1: Evolution of Abiogenesis Research.
2.2 Homochirality Formation
A peculiar hallmark of life’s chemistry is
homochirality amino acids in living organisms
overwhelmingly exhibit the L-configuration,
whereas sugars in nucleic acids are almost
exclusively D-forms. This homochirality is far from
trivial, since abiotic chemical reactions tend to
produce racemic (50:50) mixtures of chiral
molecules (Toxvaerd, 2018, 2019).
2.2.1 Significance of Homochirality
The functional significance of homochirality lies in
the specificity and efficiency of biochemical
reactions. Proteins composed of L-amino acids fold
in precise, reproducible ways, enabling specific
catalytic functions. Similarly, nucleic acids
composed of D-sugars maintain consistent helical
geometries (e.g., the double helix of DNA), crucial
for replication fidelity. Even small deviations from
homochiral compositions may disrupt enzymatic
activity or structural stability.
Abiogenesis and the Key Processes Involved in the Emergence of Biochemicals
663

2.2.2 Mechanisms for Chiral Selection
Multiple theories have been advanced to explain
how one enantiomeric form came to dominate early
Earth. One possibility is that slight chiral
asymmetries introduced by polarized light or
asymmetric mineral surfaces were amplified through
autocatalytic processes or crystallization
phenomena. Toxvaerd (2018, 2019) argues that
proteins may have spontaneously selected a single
enantiomer (L-amino acids), which subsequently
influenced the chirality of carbohydrates and other
biological pathways. This interplay of chiral
amplification and selective stabilization could have
led to the nearly exclusive prevalence of one
enantiomeric form by the time life’s core metabolic
and genetic machineries were established.
2.2.3 Preservation of Homochirality
Once homochirality emerged, it needed to be
preserved in prebiotic environments. Homochirality
confers a competitive advantage in forming stable,
functional macromolecular assemblies (Toxvaerd,
2019). Thus, protocells or reaction networks
employing homochiral polymers likely outcompeted
less homochiral rivals. Over time, this selective
advantage could have locked the biosphere into the
L-amino acid/D-sugar configuration we observe
today.
2.3 Autocatalysis and Self-Sustaining
Chemical Reactions
A cornerstone of life’s emergence is the concept of
autocatalysis, in which the product of a reaction
catalyzes that same reaction, creating a self-
amplifying cycle. This capacity for self-
reinforcement underpins many theoretical models of
the origin of life, such as Stuart Kauffman’s
collectively autocatalytic sets and Manfred Eigen’s
hypercycle.
2.3.1 Comproportionation-based
Autocatalytic Cycles (CompACs)
Recent work by Peng, Adam, Fahrenbach, and
Kaçar (2023) emphasizes Comproportionation-based
Autocatalytic Cycles (CompACs) as a plausible
mechanism for self-sustaining chemical networks in
prebiotic systems. Comproportionation refers to a
reaction wherein two reactants of different oxidation
states combine to form a product of intermediate
oxidation state. When such a reaction is linked to
autocatalysis, the system can escalate in complexity,
ultimately producing life-like chemical dynamics.
These CompACs serve as chemical amplifiers:
once they form, they generate further copies of
themselves, thereby increasing the local
concentration of particular intermediates. This
phenomenon is critical for explaining how relatively
sparse resources on the early Earth could have
coalesced into robust, self-propagating networks an
essential criterion for the formation of primitive
metabolic and genetic systems (Peng et al., 2023).
CompACs in Prebiotic Chemistry Shown in Figure 2
Figure 2: CompACs in Prebiotic Chemistry.
2.3.2 From Autocatalysis to Primitive
Metabolism
Autocatalytic reactions, by their nature, could evolve
into more complex reaction chains proto-
metabolisms especially when coupled with other
chemical steps (Seitz et al., 2024). As complexity
increased, autocatalytic networks might have refined
themselves into metabolic cycles that efficiently
captured and stored energy. This progression is
reminiscent of how modern metabolic pathways
(e.g., the Calvin cycle or the Krebs cycle) consist of
multiple autocatalytic or near-autocatalytic steps.
Although contemporary metabolisms are facilitated
by highly evolved enzymes, the principle of
autocatalysis remains integral to their operation.
Figure 3 Shows the Evolution of Autocatalytic
Reactions in Metabolism.
Figure 3: Evolution of Autocatalytic Reactions in
Metabolism.
ICRDICCT‘25 2025 - INTERNATIONAL CONFERENCE ON RESEARCH AND DEVELOPMENT IN INFORMATION,
COMMUNICATION, AND COMPUTING TECHNOLOGIES
664
3 ENVIRONMENTAL
CONDITIONS AND
CATALYSIS
3.1 Saline Environments and
Hydrothermal Vents
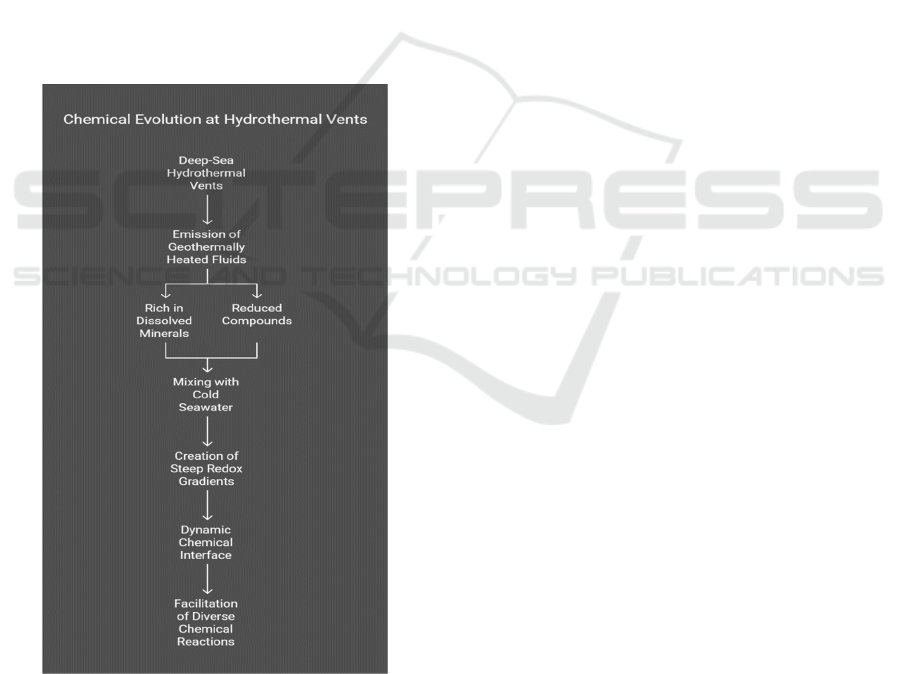
Extensive research points to saline environments as
hotbeds for early chemical evolution. Among such
environments, deep-sea hydrothermal vents stand
out for their unique chemical and physical
properties. These vents, often located at mid-ocean
ridges or seamounts, emit geothermally heated fluids
rich in dissolved minerals and reduced compounds
(e.g., hydrogen sulfide, hydrogen gas). When these
fluids mix with the cold, oxygen-poor seawater,
steep redox and pH gradients are generated, creating
a dynamic interface conducive to a wide range of
chemical reactions (Toxvaerd, 2019). Figure 4
Shows the Chemical Evolution at Hydrothermal
Vents.
Figure 4: Chemical Evolution at Hydrothermal Vents.
3.1.1 Role of Hydrothermal Vent Chemistry
Hydrothermal vents offer several advantages for
prebiotic chemistry:
• Thermal and Chemical Gradients:
Temperature and chemical gradients near
vents can drive endergonic (energy-
consuming) reactions that would otherwise be
unfavorable.
• Metal-Rich Environments: Iron, nickel, and
other transition metals can act as catalysts or
cofactors, facilitating the synthesis of
complex organics (Seitz et al., 2024).
• Protective Niches: Mineral deposits and
porous vent chimneys provide structured
environments where molecules can become
concentrated, shielded from dilution, and
stabilized by local mineral surfaces.
3.1.2 In Situ Formation and Preservation of
Organic Molecules
In line with Toxvaerd (2019), the high salinity and
mineral diversity of hydrothermal vents could have
incubated organic molecules, accelerating
polymerization reactions that might be slow or
negligible in open-ocean settings. Moreover, vent
systems often contain micro-environments with
distinct pH regimes from strongly alkaline to
moderately acidic enabling different chemical
processes to proceed in close proximity. These
micro-environments could sequentially foster
diverse reactions, from amino acid synthesis to the
formation of early peptides or RNA oligomers.
3.2 Mineral Catalysis in Prebiotic
Chemistry
Mineral surfaces are frequently invoked as potential
catalysts in prebiotic chemistry. Clays, metal
sulfides, and other naturally occurring minerals
provide structured surfaces that adsorb organic
monomers and promote their polymerization. These
surfaces can also stabilize reactive intermediates that
would degrade quickly in free solution (Joshi,
Dubey, Aldersley, & Sausville, 2015; Seitz et al.,
2024).
3.2.1 Montmorillonite Clay Catalysis
Montmorillonite clays are layered silicates known to
enhance the formation of RNA oligomers from
activated nucleotides (Joshi et al., 2015). Early
experiments demonstrated that nucleotides adsorbed
onto clay surfaces can polymerize into short RNA
chains a vital step toward the origin of genetic
molecules. The clay’s layered structure traps
Abiogenesis and the Key Processes Involved in the Emergence of Biochemicals
665

reactants, promoting proximity and favorable
orientation for condensation reactions.
Additionally, montmorillonite can facilitate the
encapsulation of these oligomers into lipid vesicles,
forming rudimentary protocells. By supporting both
polymerization and compartmentalization,
montmorillonite clays serve as a multifaceted
catalyst in the broader context of abiogenesis.
3.2.2 Transition Metal Sulfides
Iron sulfides (FeS, FeS₂) and nickel sulfides (NiS)
have also been implicated in prebiotic chemistry.
Such minerals can catalyze reactions that form
amino acids, amides, and other fundamental building
blocks from simpler precursors (Seitz et al., 2024).
A proposed mechanism involves the adsorption
of carbon monoxide (CO) and ammonia (NH₃) onto
metal sulfide surfaces, followed by reductive
coupling that yields small organic molecules. Over
time, these molecules could assemble into peptides,
eventually guiding the transition to more complex
metabolic pathways, reminiscent of the iron-sulfur
clusters ubiquitous in contemporary enzymes (e.g.,
ferredoxins).
3.2.3 Synthesis Pathways on Mineral
Surfaces
The key point is that mineral-catalyzed reactions
likely lowered the activation energies for vital
chemical steps in the early Earth environment. By
binding reactive intermediates close together and
stabilizing transition states, mineral surfaces
effectively acted as primitive “enzymes” before the
advent of biological macromolecules. Such catalytic
behavior is further augmented by localized thermal,
pH, or electrochemical gradients found in geological
structures like hydrothermal vents or volcanic
sediments.
4 ENERGY SOURCES FOR
PREBIOTIC SYNTHESIS
4.1 Solar and Radioactive Energy in
Abiogenesis
Energy is indispensable for driving endergonic
synthesis processes and enabling molecular
reorganization. On early Earth, potential energy
sources included solar radiation (UV/visible light),
geothermal gradients, lightning discharges, and
radioactive decay (Lu, Wang, Li, & Yang, 2014;
Ershov, 2022). While each form of energy might
have played a role, their relative contributions
remain a point of active research.
4.1.1 Photochemistry and Semiconducting
Minerals
Solar energy, particularly UV radiation, has
sufficient energy to break chemical bonds and
generate highly reactive species. In the context of
abiogenesis, semiconducting minerals such as iron
oxides (e.g., hematite, magnetite) and titanium
dioxide (TiO₂) could act as photo-catalysts (Lu et al.,
2014). When these materials absorb photons, they
generate electron-hole pairs (photoelectrons and
positive holes).
The photoelectrons can reduce carbon dioxide or
nitrogen species into organic compounds and
ammonia, respectively, while the holes may oxidize
water or other electron donors. This photochemical
reduction-oxidation scheme could have led to the
formation of simple sugars, lipids, or even more
complex organics if stabilized by a suitable chemical
environment.
Over time, repeated photochemical cycles,
combined with adsorption on mineral surfaces,
might have accumulated sufficient concentrations of
organic molecules to kickstart more elaborate
prebiotic pathways.
4.1.2 Radioactive Decay and Radiolysis of
Water
Natural radioactivity from elements like uranium,
thorium, and potassium in the Earth’s crust can also
drive chemical transformations through ionizing
radiation (Ershov, 2022). In oceanic settings,
radiolysis of water produces radicals such as
hydrogen (H·) and hydroxyl (OH·).
These radicals can, in turn, generate hydrogen
peroxide (H₂O₂) and other oxidizing or reducing
agents, depending on local conditions. Such
products could facilitate the formation of organic
molecules from dissolved carbonates or nitrates,
complementing other energy input sources.
Moreover, certain radioactive elements might
have been more abundant or localized in
hydrothermal vent systems, further enhancing
chemical reactivity in specific microhabitats. Such
localized spikes in radiation could have been pivotal
in creating distinctive chemical niches that selected
for autocatalytic pathways or other emergent
processes critical to life’s origins.
ICRDICCT‘25 2025 - INTERNATIONAL CONFERENCE ON RESEARCH AND DEVELOPMENT IN INFORMATION,
COMMUNICATION, AND COMPUTING TECHNOLOGIES
666

5 MOLECULAR EVOLUTION
AND SELF-REPLICATION
5.1 Formatio of Self-Replicating
Molecules
A fundamental leap in abiogenesis is the evolution
of self-replicating molecules, enabling heredity and
Darwinian evolution. Modern life relies on nucleic
acids RNA and DNA for information storage and
retrieval. Hence, understanding how these polymers
emerged under prebiotic conditions remains a focus
of intense study.
5.1.1 Synthesis of Nucleobases
Jeilani, Williams, Walton, and Nguyen (2016)
demonstrated potential unified reaction pathways
that could yield both RNA and DNA nucleobases
under similar prebiotic conditions. Their research
explored how purines (adenine, guanine) and
pyrimidines (cytosine, uracil, thymine) might share
common synthetic routes, challenging earlier
assumptions that RNA and DNA must have evolved
entirely separately. This raises the possibility that
primordial chemistry could have simultaneously
produced the constituents of both genetic polymers.
5.1.2 Prebiotic Polymerization of
Nucleotides
Even if nucleobases, ribose or deoxyribose sugars,
and phosphate groups were available, polymerizing
them into RNA or DNA is not trivial. The
dehydration condensation required to form
phosphodiester bonds is thermodynamically
unfavorable in aqueous environments.
Mineral-catalyzed or chemically activated
nucleotides (e.g., imidazolides) have been proposed
to overcome these barriers. Montmorillonite clays
can facilitate the polymerization of activated
nucleotides into short oligomers (Joshi et al., 2015),
providing a plausible route to early RNA strands.
Once such strands reached lengths enabling
rudimentary catalytic or replicative activities, natural
selection could have led to more sophisticated
genetic behaviors.
5.2 Compartmentalization and the
Role of Protocells
While synthesizing organic molecules is necessary,
it is insufficient to ensure stable biochemical
evolution. Compartmentalization is a critical step, as
it prevents dilution of key molecules and allows
reaction networks to be locally optimized (Urban,
2014). Early protocells likely formed from
amphiphilic molecules fatty acids, phospholipids, or
other surfactants that spontaneously organize into
bilayer membranes in aqueous media.
5.2.1 Self-Assembly of Amphiphiles
Fatty acids, especially those produced under
hydrothermal or extraterrestrial conditions (e.g., in
carbonaceous chondrite meteorites), can
spontaneously form micelles. Under appropriate pH
and ionic conditions, these micelles can transition
into vesicles closed bilayer structures encapsulating
an aqueous interior. Such vesicles can incorporate or
concentrate prebiotic catalysts, nucleic acids, or
other crucial biomolecules within their lumen
(Urban, 2014).
5.2.2 Protocell Dynamics and Growth
Protocells exhibit intriguing growth and division
behaviors even in purely abiotic contexts. If a
vesicle acquires additional amphiphiles or if
environmental changes alter the osmotic balance, the
vesicle can expand.
Upon becoming sufficiently large, shear forces
or energetic perturbations can cause a protocell to
split into smaller vesicles. This rudimentary
“division” process allows for distribution of internal
contents into daughter protocells. If those contents
include autocatalytic or replicative systems, the
protocell lineage can, in principle, replicate itself
(Urban, 2014).
5.2.3 Concentration and Catalysis Within
Protocells
By providing a semi-permeable boundary, protocells
not only concentrate reactants but also protect fragile
intermediates (e.g., RNA oligomers) from
degradation. Certain mineral particles might even be
embedded within membranes, further enhancing
catalytic capabilities.
The regulated microenvironment within
protocells thus sets the stage for more integrated
metabolic and genetic functions to develop a
precursor to fully modern cellular life (Joshi et al.,
2015).
Abiogenesis and the Key Processes Involved in the Emergence of Biochemicals
667
6 INTEGRATIONS OF GENETIC
AND METABOLIC SYSTEMS
6.1 Co-evolution of Genes and
Metabolism
One of the most critical phases in abiogenesis is the
co-evolution of primitive genetic molecules (e.g.,
RNA, DNA) and nascent metabolic pathways. Di
Rocco and Coons (2018) propose that the earliest
forms of life likely sprang from a gradual integration
of genetic information (capable of replication and
mutation) with catalytic networks that supplied the
energy and building blocks necessary for growth. As
genetic elements encoded specific enzymatic
functions, metabolic systems became more refined
and efficient, supporting further genetic complexity.
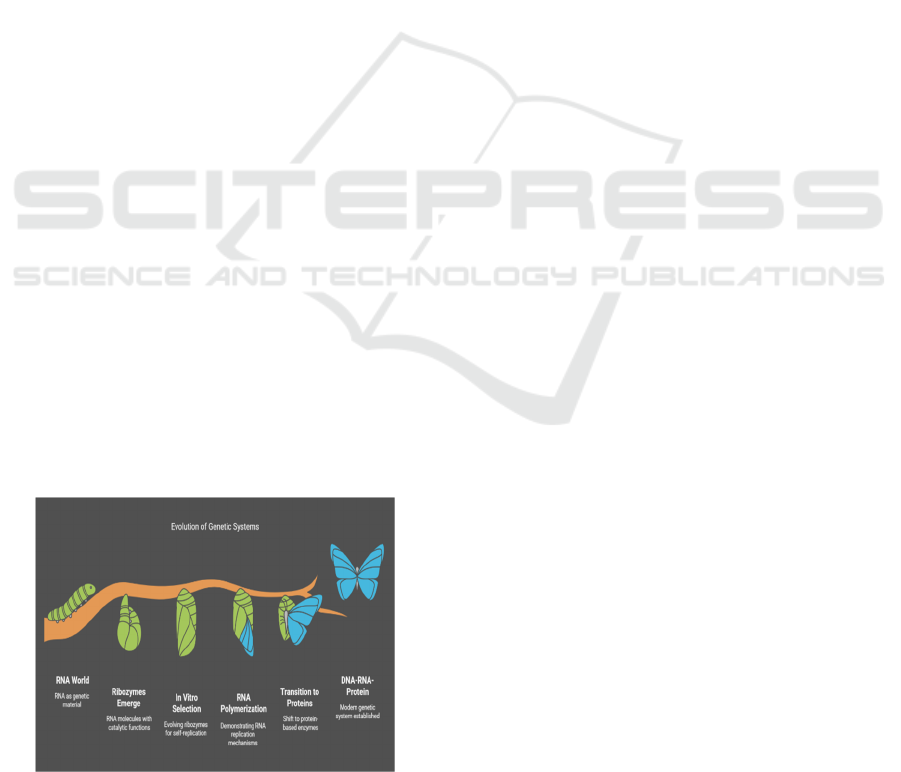
6.1.1 RNA as Both Catalyst and Template
The “RNA world” hypothesis posits that RNA could
have served as both the genetic repository and the
catalyst for metabolic reactions before the evolution
of DNA and protein enzymes. Ribozymes RNA
molecules with catalytic capacities offer direct
empirical support for this hypothesis. Through in
vitro selection, researchers have evolved ribozymes
capable of polymerizing RNA, demonstrating a
mechanism for self-replication at the molecular
level.
While these laboratory ribozymes are not yet as
efficient as protein-based polymerases, they
underscore the plausibility of RNA-centric genetic
and metabolic systems. Over evolutionary
timescales, this RNA-based metabolism might have
been gradually supplanted by protein enzymes,
which are more diverse and catalytically efficient,
leading to the modern DNA–RNA–protein world.
Figure 5 Shows the Evolution of Genetic Systems.
Figure 5: Evolution of Genetic Systems.
6.1.2 Emergence of DNA
DNA’s superior chemical stability owing to the lack
of a 2′-hydroxyl group and often double-stranded
conformation makes it a more secure archive for
genetic information. Researchers hypothesize that
enzymes resembling modern ribonucleotide
reductases emerged to convert ribonucleotides into
deoxyribonucleotides (Jeilani et al., 2016). Once a
cell or protocell possessed such reductase activity,
DNA’s improved fidelity and stability would confer
a significant selective advantage, leading to a
genomic “upgrade” from RNA to DNA.
6.2 From Metabolic ‘Protocells’ to
Modern Cells
A progressive viewpoint sees protocells initially
lacking robust genetic information acquiring or
evolving RNA-based genetic systems. As these
systems matured, they coordinated with metabolic
cycles to enhance resource acquisition, energy
conversion, and polymer synthesis. Over time, more
complex feedback loops between genetic material
and metabolic processes crystallized into the
fundamental architecture of cellular life, including:
• Highly regulated membranes with
embedded transport proteins.
• Comprehensive metabolic pathways
(glycolysis, photosynthesis, or
chemosynthesis).
• Information flow from DNA to RNA to
proteins (the central dogma of modern
biology).
The final result is life as we know it—complex,
self-replicating systems that respond to
environmental challenges and opportunities.
7 OPEN QUESTIONS AND
FUTURE DIRECTIONS
While substantial progress has been made in
understanding abiogenesis, numerous unanswered
questions remain:
• Exact Environmental Conditions: Our
knowledge of the early Earth’s atmosphere,
ocean chemistry, and temperature gradients is
evolving. Small changes in assumptions (e.g.,
pH or gas composition) can greatly influence
which prebiotic pathways are viable (Lu et
al., 2014).
ICRDICCT‘25 2025 - INTERNATIONAL CONFERENCE ON RESEARCH AND DEVELOPMENT IN INFORMATION,
COMMUNICATION, AND COMPUTING TECHNOLOGIES
668

• Order and Timing of Key Events: It
remains debated whether metabolic networks
predate genetic systems (“metabolism first”)
or vice versa (“genetics first”). It is possible
that partial, rudimentary versions of both
emerged in tandem, reinforcing each other’s
development (Di Rocco & Coons, 2018).
• Alternatives to RNA: Although RNA stands
out as a prime candidate for the first genetic
polymer, alternative nucleic-acid analogs
(like PNA, TNA, or GNA) cannot be ruled
out. Evidence suggests that these analogs
may polymerize more readily or be more
stable under certain conditions, which might
have preceded or co-existed with RNA
(Jeilani et al., 2016).
• Catalytic Efficiency and Fidelity: How did
early enzymatic activities maintain sufficient
fidelity for evolutionary progress without the
refined proofreading mechanisms seen in
modern DNA replication?
• Emergence of Homochirality: While
models of chiral amplification exist,
experimental demonstrations that replicate
the full transition from near-racemic mixtures
to predominantly homochiral biomolecules in
plausible conditions remain a work in
progress (Toxvaerd, 2018, 2019).
• Extraterrestrial Influences: Some theories
propose that organic precursors arrived via
meteoritic or cometary infall. If so, the early
Earth received a “head start” on complex
chemistry (Ershov, 2022). How significant
was this exogenous delivery compared to in
situ synthesis?
Addressing these questions will require an
interdisciplinary approach, combining geochemical
modeling, laboratory simulations of prebiotic
environments, and computational explorations of
vast chemical reaction networks. Additionally,
exploring extreme Earth environments (e.g., deep-
sea vents, hot springs, hyper-saline lakes) continues
to provide insights into how life can persist and
perhaps how it originated in habitats similar to those
of the early Earth.
8 CONCLUSIONS
Abiogenesis is a multifaceted process, not a singular
event. Its study spans the formation of small organic
molecules to the rise of self-replicating,
compartmentalized systems capable of Darwinian
evolution. Key themes identified in this discourse
include:
• Prebiotic Synthesis of Organic Molecules:
Early Earth conditions coupled with catalytic
minerals and various energy sources
produced amino acids, nucleobases, and other
critical monomers.
• Autocatalysis and Homochirality: Self-
sustaining reaction cycles (e.g., CompACs)
and the preferential establishment of one
molecular handedness likely set the
biochemical stage for higher complexity.
• Environmental Catalysts: Hydrothermal
vents, saline environments, and mineral
surfaces provided unique conditions for
concentrating reactants, stabilizing
intermediates, and accelerating key reactions.
• Energy Inputs: Solar radiation, geothermal,
and radioactive decay each supplied the
energetic “push” necessary for forming
increasingly complex and ordered structures.
• Molecular Evolution and Replication: The
emergence of self-replicating polymers (e.g.,
RNA, DNA) was a watershed moment,
enabling heredity and cumulative
evolutionary change.
• Compartmentalization: Protocells formed
from amphiphilic molecules—helping to
localize chemical networks, increase
efficiency, and protect nascent genetic and
metabolic machineries.
• Integration of Genetic and Metabolic
Systems: Early metabolic pathways and
genetic elements likely co-evolved, leading to
the intricate interplay that characterizes
modern cells.
Through these processes, life transitioned from
simple chemistry to complex, evolving biochemical
systems. The references cited illustrate the breadth
of experimental and theoretical research dedicated to
unveiling how matter organized itself into the living
forms that eventually spread across our planet.
Although myriad details await further clarification,
the converging picture is that abiogenesis was driven
by a synergy of geological, chemical, and physical
processes operating on a young Earth replete with
reactive environments and potent energy sources.
The implications of these findings extend far
beyond Earth: if these processes are not Earth-
specific but universal, then life may be a common
outcome wherever compatible environments exist.
Future investigations ranging from laboratory-based
origin-of-life simulations to the in-depth analysis of
other planetary bodies will continue to refine our
Abiogenesis and the Key Processes Involved in the Emergence of Biochemicals
669
understanding of how life can originate and evolve.
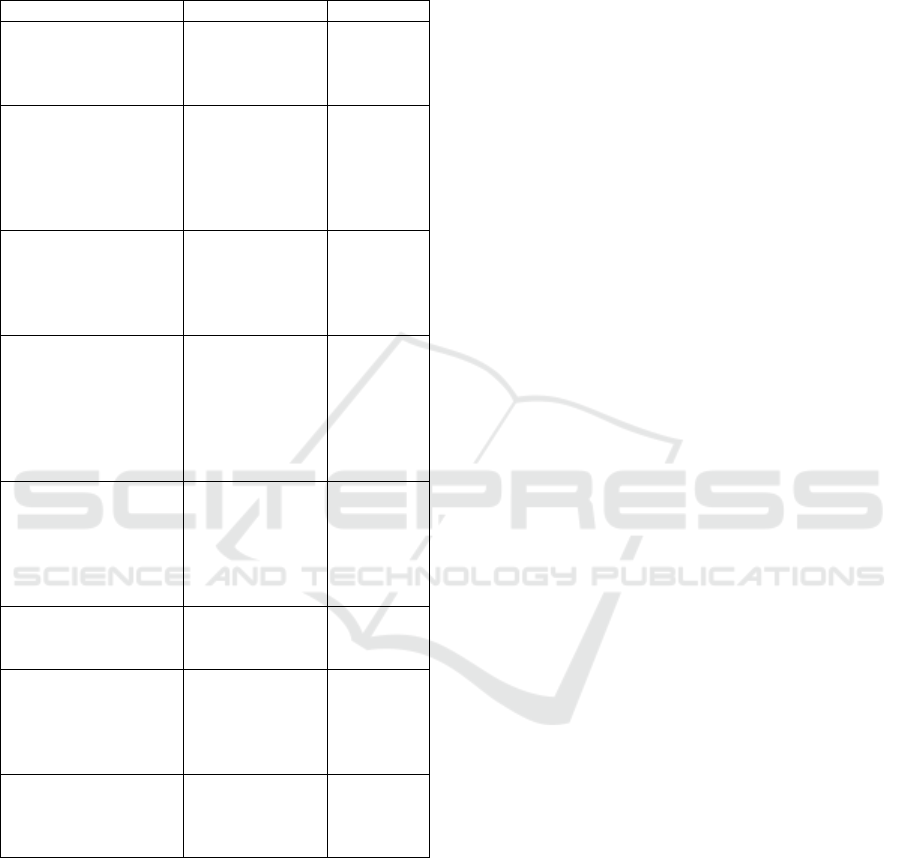
Summary Table of Key Processes in Abiogenesis
Shown in Table 1.
Table 1: Summary Table of Key Processes in Abiogenesis.
Process Descri
p
tion References
Homochirality
Formation
Selection and
preservation of
enantiomeric
b
iomolecules
Toxvaerd
(2018,
2019)
Autocatalysis
Self-sustaining
chemical cycles
enabling
complex
molecular
structures
Peng et al.
(2023)
Saline Environments
Hydrothermal
vents as
incubators for
organic
synthesis
Toxvaerd
(2019)
Mineral Catalysis
Clays (e.g.,
montmorillonite)
and metal
sulfides
catalyzing
biomolecular
s
y
nthesis
Joshi et al.
(2015);
Seitz et al.
(2024)
Solar and Radioactive
Energy
Energy sources
(UV radiation,
radiolysis)
facilitating
prebiotic
synthesis
Lu et al.
(2014);
Ershov
(2022)
Self-Replicating
Molecules
Prebiotic
formation of
RNA and DNA
Jeilani et
al. (2016)
Compartmentalization
Formation of
protocells
enabling
biochemical
re
g
ulation
Urban
(2014)
Co-evolution of
Genes & Metabolism
Integration of
genetic and
metabolic
systems
Di Rocco
& Coons
(2018)
REFERENCES
Di Rocco, R. J., & Coons, E. E. (2018). Abiogenesis: The
emergence of life from non-living matter. In
Consilience, Truth and the Mind of God: Science,
Philosophy and Theology in the Search for Ultimate
Meaning.
Ershov, B. G. (2022). Important role of seawater radiolysis
of the world ocean in the chemical evolution of the
early Earth. Radiation Physics and Chemistry, 194,
109946.https://doi.org/10.1016/j.radphyschem.2022.1
09946
Jeilani, Y. A., Williams, P. N., Walton, S., & Nguyen, M.
T. (2016). Unified reaction pathways for the prebiotic
formation of RNA and DNA nucleobases. Physical
Chemistry Chemical Physics, 18(47), 32137–32145.
https://doi.org/10.1039/C6CP06626D
Joshi, P. C., Dubey, K., Aldersley, M. F., & Sausville, M.
(2015). Clay catalyzed RNA synthesis under Martian
conditions: Application for Mars return samples.
BiochemicalandBiophysicalResearchCommunications,
462(2),144150.https://doi.org/10.1016/j.bbrc.2015.04.
052
Lu, A. H., Wang, X., Li, Y., & Yang, X. X. (2014).
Mineral photoelectrons and their implications for the
origin and early evolution of life on Earth. Science
China Earth Sciences, 57(6), 1106–1116.
https://doi.org/10.1007/s11430-013-4768-4
Peng, Z., Adam, Z. R., Fahrenbach, A. C., & Kaçar, B.
(2023). Assessment of stoichiometric autocatalysis
across element groups. Journal of the American
ChemicalSociety,145(10),45714585.https://doi.org/10.
1021/jacs.2c12892
Seitz, C., Geisberger, T., West, A. R., & Huber, C. (2024).
From zero to hero: The cyanide-free formation of
amino acids and amides from acetylene, ammonia and
carbon monoxide in aqueous environments in a
simulated Hadean scenario. Life, 14(1), 35.
https://doi.org/10.3390/life14010035
Toxvaerd, S. (2018). The start of abiogenesis: Preservation
of homochirality in proteins as a necessary and
sufficient condition for the establishment of
metabolism. Journal of Theoretical Biology, 450, 120–
126. https://doi.org/10.1016/j.jtbi.2018.04.029
Toxvaerd, S. (2019). A prerequisite for life. Journal of
TheoreticalBiology,467,15.https://doi.org/10.1016/j.jtb
i.2019.01.003
Urban, P. L. (2014). Compartmentalised chemistry: From
studies on the origin of life to engineered biochemical
systems. New Journal of Chemistry, 38(11), 5133–
5140.https://doi.org/10.1039/C4NJ01164E
ICRDICCT‘25 2025 - INTERNATIONAL CONFERENCE ON RESEARCH AND DEVELOPMENT IN INFORMATION,
COMMUNICATION, AND COMPUTING TECHNOLOGIES
670
