
A Hybrid Machine Learning Approach for Early Risk Prediction of
Preterm Birth Using Contraction Pattern
M. B. Patil, V. V. Bag and Kiran Kashinath Gawade
Department of Computer Science & Engineering, N. K. Orchid College of Engineering and Technology, Solapur,
Maharashtra, Tamil Nadu, India
Keywords: Preterm Birth Prediction, Machine Learning, Support Vector Machine, Random Forest, XGBoost, Uterine
Contractions, Maternal, Infant Health.
Abstract: Preterm birth means delivery of baby before 37th week of gestation which can cause severe life challenges
both to the mother and the baby. The condition has been linked to a range of prolonged complications such
as respiratory distress, infection and congenital malformations. Estimating the risk of preterm birth accurately
is a formidable challenge in the practice of obstetrics given the many causative risk-factors. However,
classifying a pregnancy as high-risk enables early medical interventions to enhance neonatal outcomes. This
study investigates the machine learning algorithms prediction (Support Vector Machine (SVM), Random
Forest, and XGBoost) of risk of preterm birth. Models were trained on a representative subset of maternal and
clinical factors and validated on accuracy, F1-score, recall, and precision. Here are some of the advantages of
machine learning in healthcare been discovered. Preterm birth is the most predictable event. The best model
was the stacking SVM with XGBoost and Random Forest. Using various algorithms in a stacking model, the
prediction accuracy was increased overall. The model allows the combination of models and therefore
improves predictability compared to the use of a single algorithm. These results reinforce a growing role for
machine learning in obstetrics through better risk assessing, predictive accuracy, and dealing with uncertainty.
Finally, this research contributes to the development of predictive models which can be used by health care
providers to allow for early interventions and improve maternal and new born health.
1 INTRODUCTION
Another major global health issue is preterm birth, or
delivery between 20 and 36 weeks of pregnancy,
which contributes to around 10 percent of all births
globally. Neonatal sepsis Neonatal sepsis is heard to
be one of the leading causes of neonatal mortality and
morbidity, consequently making these affected new-
borns at risk of facing long term health complications
6. Interest in preterm birth prediction has increased
in obstetric studies because of the severe risk it poses
to fetal and maternal health. Accurate identification
of high-risk pregnancies may allow early intervention
to prevent adverse pregnancy outcomes and improve
neonatal management. Most traditional models of
prediction rely on existing clinical risk factors:
maternal age, history of preterm labor, pre-existing
medical conditions, etc. Nevertheless, these
approaches fail to contribute the true complexity to
preterm labor with its multivariate nature that
provided the motivation to move to more advanced
predictive methods. Machine learning has also proven
to be a valuable methodology for medical diagnostics,
primarily due to its potential to deal with large
amounts of data and to detect hidden patterns. In
theory, machine learning could improve the accuracy
and efficiency of prediction of preterm delivery in
obstetrics, permitting more accurate determination by
clinicians.
The research employs three popular machine
learning models SVM, Random Forest, and XGBoost
to predict preterm birth risk using contraction-based
features. Data includes significant uterine contraction
parameters such as contraction count, duration,
standard deviation (STD), entropy, and contraction
interval. These have been chosen to detect the
changing patterns of uterine movement that are
characterized by preterm labor. Using the
investigation of these features, the research works to
detect predictor patterns that contribute to improved
prediction of risk. Apart from the evaluation of
individual machine learning models, the study in this
318
Patil, M. B., Bag, V. V. and Gawade, K. K.
A Hybrid Machine Learning Approach for Early Risk Prediction of Preterm Birth Using Contraction Pattern.
DOI: 10.5220/0013897400004919
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 1st International Conference on Research and Development in Information, Communication, and Computing Technologies (ICRDICCT‘25 2025) - Volume 3, pages
318-327
ISBN: 978-989-758-777-1
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

research employs a stacking model with the addition
of Support Vector Machine (SVM), Random Forest,
and XGBoost. Stacking is an ensemble learning
where multiple base models are learned separately
and their predictions are combined using a meta-
model, thereby improving accuracy and robustness.
The method employs the strength of different
algorithms while making up for their respective
weaknesses. Although machine learning applications
to predicting preterm birth are still in early
development, current research targets primarily
clinical and demographic data rather than
physiological signals, e.g., uterine contractions. This
study seeks to close this gap by evaluating machine
learning models trained on extracted features from
contractions. The chosen algorithms provide different
strengths: Support Vector Machine (SVM) is
particularly good at processing high-dimensional data
and nonlinear relationships through the aid of kernel
functions; Random Forest provides high accuracy and
resistance to overfitting through the aggregation of
multiple decision trees; and XGBoost provides
improved interpretability, thereby enabling improved
understanding of feature contributions to predictions.
The research compares the models on the basis of
important performance parameters like accuracy,
precision, recall, and F1-score. Among the standalone
models, the Random Forest algorithm is the most
accurate, followed by Support Vector Machine
(SVM) and XGBoost. The stacking model also
improves the accuracy of prediction by fusing the
predictions of all three models, thus also showing the
power of ensemble learning in improving diagnostic
accuracy. The results suggest the promise of machine
learning to enhance preterm birth prediction as a more
refined tool to support healthcare professionals in
making early diagnosis and intervention. With the
combination of machine learning and obstetric care,
this study adds to the body of evidence on preterm
birth prediction. The application of contraction-
related features allows for new insight into the pattern
of uterine activity, making the risk assessment more
effective. Although machine learning has evolved
significantly, the gap in studies conducted for
employing such methods for preterm birth prediction,
especially when contracting-related data is utilized,
still remains. This study aims to close the gap by
comparing the performance of various machine
learning models for this purpose, ultimately resulting
in better maternal and neonatal health.
2 RELATED WORKS
Prediction of preterm birth remains an obstetric
medicine enigma and there have been multiple studies
aimed to innovate improving diagnostic accuracy.
With the growth of machine learning in the area of
research, we have observed improvement in the
quality of prediction models and decision-making
assistance for doctors. Prior studies have extensively
explored various machine learning methods for the
prediction of high-risk pregnancy.
Liu et al. (2024) demonstrated the utility of a
machine learning predictive model for preterm birth
risk prediction, incorporating clinical parameters
within a nomogram for improved accuracy. Their
research is in line with Xu, Zhang, and Zhang (2020)
1, who also demonstrated that hybrid machine
learning models incorporating electronic health
records could be especially effective. Likewise,
Goodwin, Maher, and Callaghan (2020) examined
predictive models based on electronic health record
data, and thus further adds to the importance of big
data in obstetric analytics.
Support Vector Machines (SVM), Random
Forest, and XGBoost, as machine learning methods,
have attracted great attention for their capabilities to
process high-dimensional data and model complex
relationships. Włodarczyk et al. (2021) conducted a
comprehensive examination of machine learning
techniques focused on predicting preterm birth,
highlighting the relevance of ensemble learning
approaches. This study extends these findings to
bring contraction-based features into predictive
models, an activity that the literature has addressed
only minimally. These parameters consist primarily
of changes in patterns or signals within and around
the uterus, as suggested in (Kavitha, S. N, and Asha.
V.2024) The inclusion of uterine contraction
parameters, as suggested in (Villar, J and
Papageorghiou, A. T. 2014). Even though individual
machine learning models have been beneficial,
ensemble methods (for example, stacking) have been
shown to be more effective, especially when it comes
to improving prediction power. Combining
algorithms has the potential to enhance the risk
assessment as shown in a recent publication (Kavitha
and Asha, 2024), which has been mainly supported in
the present study. The stacking model applied in this
study capitalizes on the advantages of SVM,
Random Forest, and XGBoost and provides a more
stable predictive model. Furthermore, the robustness
of hybrid SVM models for predicting preterm birth
was highlighted by Santoso and Wulandari, 2018,
A Hybrid Machine Learning Approach for Early Risk Prediction of Preterm Birth Using Contraction Pattern
319
thus underlining the performance of ensemble
methods.
However, there are still challenges in
implementing the use of machine learning to predict
preterm birth. Literature reviews, including those of
Manogaran and Lopez (2017) and Liu and Salinas
(2017) have shown that substantial and
heterogeneous datasets are critical for improving
model generalizability. Similar to our results,
Dekker and Sibai (2020) and Menon and Torloni
(2011) aimed at the utilization of biomarker
information to predict preterm birth, however, they
suggested that the inclusion of proteomic and clinical
data would lead to the improved performance of the
prediction model.
Another important consideration refers to the
influence of maternal demography and the
environment, as discussed by Ananth and Vintzileos
(2006) and Villar and Papageorghiou (2014). The
inclusion of these variables within machine learning
models can potentially provide more holistic
predictive models. Goldenberg et al. (2008) also
highlighted the preterm birth as having a
multifactorial etiology, hence reinforcing the call for
interdisciplinary methodology that includes machine
learning and standard obstetric evaluation. Though
there have been developments in machine learning for
predicting preterm birth, there remain certain
challenges. Many of the models that have been
proposed suffer from a lack of generalizability,
feature choice, or explainability, preventing their
clinical practice application. Also, real-time
personalized prediction models using multi-modal
data remain at an embryonic stage. Even though the
use of hybrid has been made in a few works, ensemble
learning approaches' investigations remain thin.
Utilizing SVM, Random Forest & XGBoost: A
machine learning platform was constructed to provide
improvements in accuracy as well as interpretability
in these two areas of opportunities. A strong feature
selection technique, smooth multi-modal data fusion,
explainable AI and real-time risk estimation are
advancements proposed in this work. We use
stacking models to improve the performance
efficiency, and it is also clinically relevant for real-
world applications.
3 METHODOLOGY
This study explores the use of three machine learning
algorithms for preterm birth risk prediction including
the Support Vector Machine (SVM), XGBoost, and
Random Forest algorithms. These algorithms were
selected for their ability to solve complex healthcare
industry problems focused on risk management.
The system was structured with the necessary
modules such as data pre-processing, feature
extraction, training, evaluation of model,
deployment, etc. so that accurate and uniform
predictions could be made. By refining and
organizing the data systematically a significant
pattern can be recognized, which increases the
accuracy of risk prediction. The ensemble methods of
Support Vector Machine (SVM), Extreme Gradient
Boosting (XGBoost) and Random Forest are to
obtain the highest predictive performance and support
clinical decision.
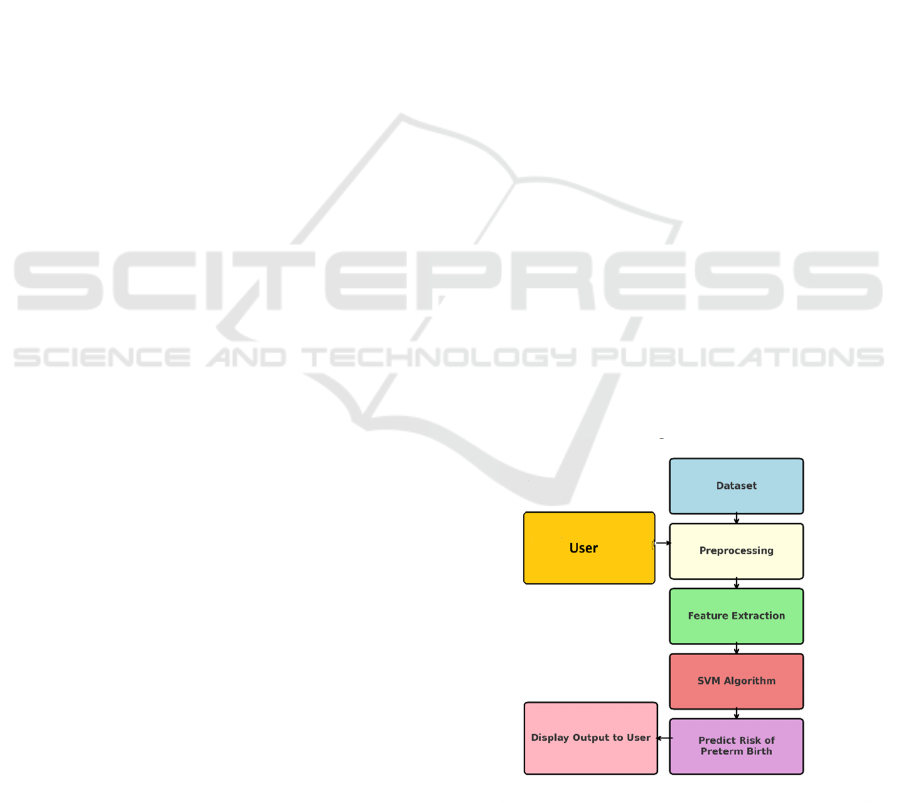
3.1 Algorithm Details
The suggested model leverages an ensemble of
machine learning methods in assessing the risk of
preterm birth. Data preprocessing, feature selection,
model training, testing, and validation are all part of
the process. The learnt model takes user-input data
and returns a risk prediction, thus offering an
effective tool for early intervention in maternal care.
• Support Vector Machine (SVM): Support
Vector Machine (SVM) is a robust supervised
learning algorithm used extensively for
classification and regression tasks. It works in
n-dimensional space, identifying the optimal
hyperplane that can discern data points into
two classes as optimally as possible and thus
makes it particularly suitable for predictive
healthcare applications. SVM Architecture
Shown in the Figure 1.
Figure 1: SVM architecture.
•
XGBoost: XGBoost is a gradient-boosting
method that builds multiple decision trees to
ICRDICCT‘25 2025 - INTERNATIONAL CONFERENCE ON RESEARCH AND DEVELOPMENT IN INFORMATION,
COMMUNICATION, AND COMPUTING TECHNOLOGIES
320
enhance predictive power. XGBoost is a tree
model where nodes are equivalent to
decision rules for feature values, and leaf
nodes are equivalent to class labels
(classification) or real values (regression).
XGBoost is efficient, scalable, and can deal
with missing values. Figure 2 shows the
XGBoost Architecture.
Figure 2: XGBoost architecture.
• Random Forest: Random Forest is an
ensemble learning method that
enhances the accuracy of
predictions by voting from an ensemble of
many decision trees. Random Forest is
different from individual
trees because it avoids overfitting through tr
aining on randomly selected subsets of data
and
features. Random Forest predicts by majorit
y voting (classification) or averaging
(regression) and is thus a robust model
for health risk assessment. Random Forest
Architectures Shown in the Figure 3.
Figure 3: Random forest architecture.
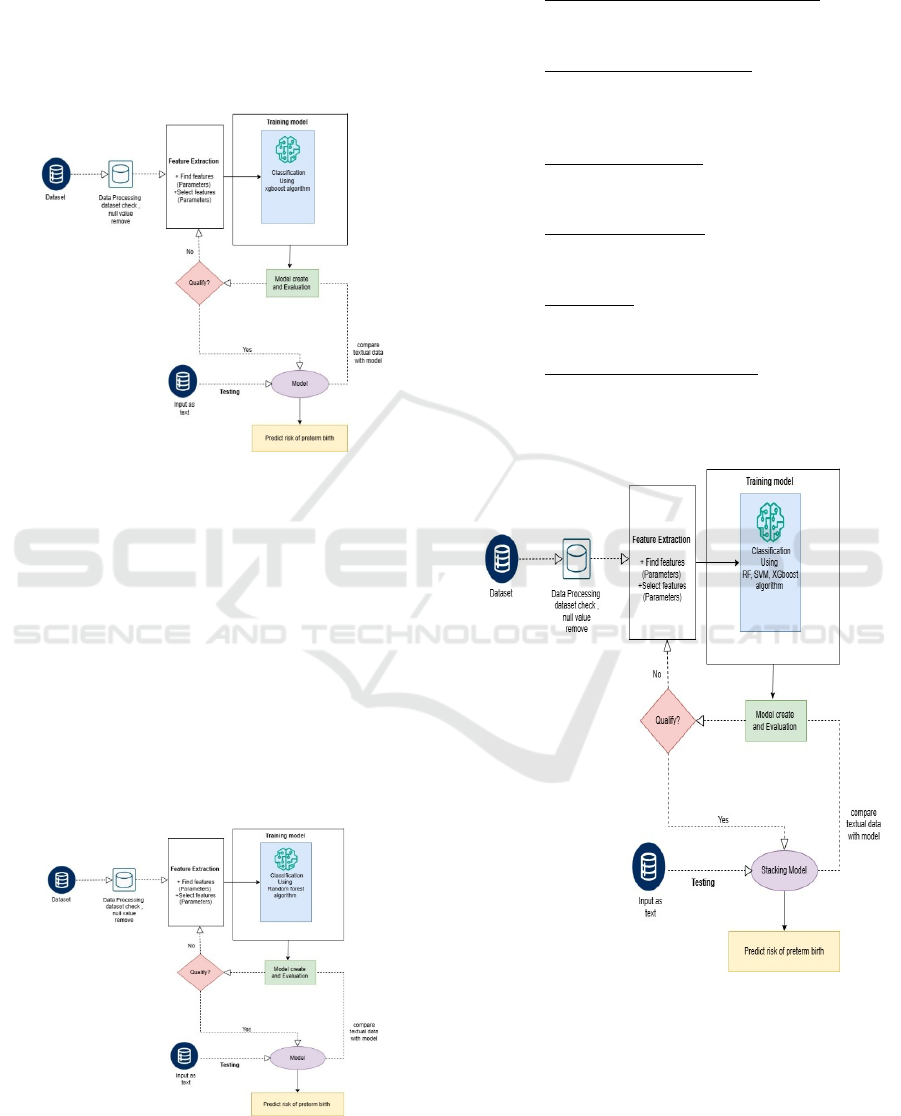
Stacking Model Architecture
The architecture consists of:
• Preprocessing and Data Ingestion – Cleans
raw data, handles missing values, and
derives significant features.
• Stacking Model Training – It trains SVM,
XGBoost, and Random Forest models and
combines their predictions using a meta-
model.
• Model Evaluation – Evaluates model
performance based on accuracy, precision,
recall, and F1-score.
• Model Deployment – Deploys the trained
model into a production environment for
real-world applications.
• Prediction – Utilizes current patient data to
establish the possibility of preterm birth
through utilization of discovered patterns.
• Action/Alert Mechanism – Works by
triggering automatic action or alerts for
high-risk conditions, thereby providing
immediate medical action. Figure 4 shows
the Stacking Model Architecture.
Figure 4: Stacking model architecture.
3.2 Data Preprocessing
In order to efficiently train models, the dataset has
been preprocessed for missing values handling,
feature tuning, extraction of useful data, and splitting
it in a proper manner.
A Hybrid Machine Learning Approach for Early Risk Prediction of Preterm Birth Using Contraction Pattern
321
3.2.1 Handling Missing Values
Missing values in data points may contribute
significantly to how a model works; therefore, a
systematic solution was implemented. Where missing
values were discovered, statistical operations
involving deletion, imputation, or consistency checks
were employed. For this particular case, there was no
missing data.
3.2.2 Feature Importance Analysis
Preterm Detection Dataset
Top contributing features:
Count Contraction (34.5%)
Length of Contraction (33.2%)
Entropy (24.7%)
Contraction Times (7.1%)
Contraction-related metrics (count, duration, and
entropy) are the most important.
Standard deviation (STD) has minimal impact.
Pregnancy Risk Prediction Dataset
Top contributing features:
Systolic Blood Pressure (27.6%)
Heart Rate (16.5%)
Diastolic Blood Pressure (14.1%)
Body Temperature (13.9%)
Age (11.1%)
Blood pressure and heart rate play significant
roles in assessing pregnancy risks.
BMI and blood glucose contribute less but still
impact predictions.
3.2.3 Splitting Dataset for Training and
Testing
The data is split into 80% training and 20% testing to
allow for maximum model learning and to avoid bias,
in order to get a fair performance evaluation. This
enables the model to learn from a large chunk of the
data
3.3 Model Training and Evaluation
Two different models are developed:
3.3.1 Detection of Preterm Birth Analytic
Model
This model is specifically formulated to detect the
incidence of preterm deliveries by stacking ensemble
approach with a combination of Support Vector
Machine (SVM), XGBoost, and Random Forest
models. By leveraging the power of every algorithm,
the model greatly enhances the prediction accuracy.
After the training process, the model is retained for
future prediction, thus ensuring efficient and effective
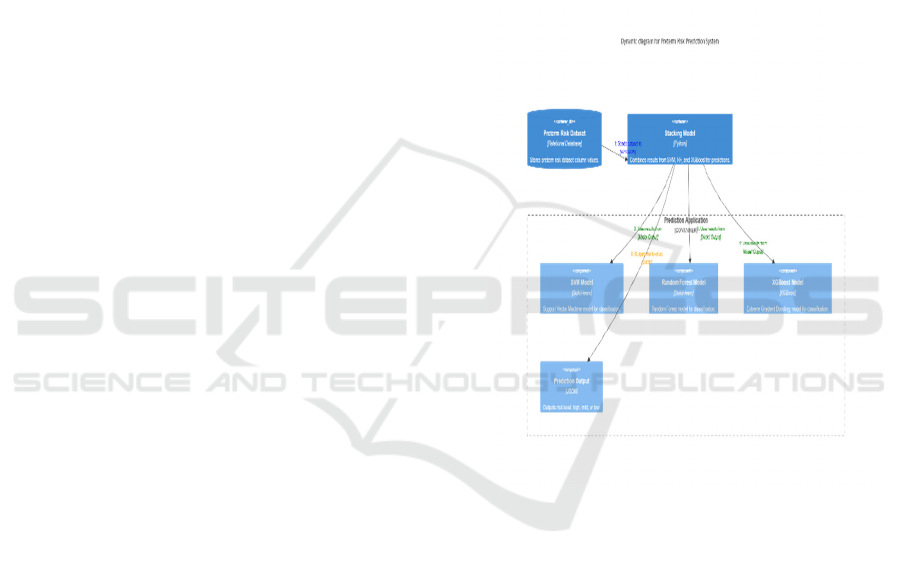
detection of preterm delivery cases. Figure 5 Shows
the C4 Container and Component Diagram for
Prediction of Preterm Birth.
Figure 5: C4 container and component diagram for
prediction of preterm birth.
3.4 Preterm Birth Risk Prediction
Model
This model estimates preterm birth risk by classifying
pregnancies into varying degrees of risk. Stacking
ensemble with the application of SVM, Random
Forest, and XGBoost enhances performance. 80:20
training and testing ensures it generalizes. Model
performance is tested after training with accuracy
measures and classification reports to ensure its
performance in predicting preterm birth risk. C4
Container and Component Diagram for Risk
Prediction of Preterm Birth Shown in the Figure 6.
ICRDICCT‘25 2025 - INTERNATIONAL CONFERENCE ON RESEARCH AND DEVELOPMENT IN INFORMATION,
COMMUNICATION, AND COMPUTING TECHNOLOGIES
322

Figure 6: C4 container and component diagram for risk
prediction of preterm birth.
The performance of the models is gauged through
the use of confusion matrices, accuracy values, and
classification reports. These evaluation metrics
provide valuable insights into the general
performance of the models, including their ability to
classify instances appropriately (accuracy), identify
actual preterm birth instances (recall), and attain
predictive consistency (precision).
4 RESULTS AND EVALUATION
This research will focus on designing and applying
machine learning models that can accurately predict
the risk of preterm birth using ensemble learning
techniques. The two models were designed to screen
for preterm birth prevalence and preterm birth risk in
pregnancy respectively. We trained our models with
two internal sets of processed maternal health
features, with noise from the data removed and the
most informative features retained for model
training.
A stacking ensemble approach utilizing an
XGBoost, Support Vector Machine (SVM), and
Random Forest model was employed in order to
increase predictive capability. Models are compared
based on accuracy, precision, recall, and F1-score as
per the traditional machine learning performance
measures. It proved the importance of feature
selection and data preprocessing for enhancing model
performance as the ensemble learning technique was
the accurate predictor and also the efficient one.
4.1 Performance Metrics
Precision: Precision is a positive prediction accuracy
measure and is computed by TP / (TP + FP). High
precision indicates good prediction of preterm birth
cases with few false positives.
𝑃𝑟𝑒𝑐𝑖𝑠𝑖𝑜𝑛
(1)
Recall (Sensitivity): Recall measures the model's
capacity to identify all the true preterm birth cases. It
is determined as TP / (TP + FN), i.e., the number of
true positive cases out of all true positive cases.
𝑅𝑒𝑐𝑎𝑙𝑙
(
2
)
F1-Score: F1-score is the harmonic mean of
precision and recall, which is the average of the two
metrics in imbalanced class conditions. F1-score
ranges from 0 to 1, the greater the value, the better the
performance of the model in detecting preterm birth
risks.
𝐹1 𝑆𝑐𝑜𝑟𝑒 2
(3)
4.2 Model Evaluation Results
The performance of various machine learning models
was evaluated using important metrics such as
precision, recall, accuracy, and F1-score.
Two models were assessed:
• Preterm Birth Occurrence Detection
Model Performance
• Preterm Birth Risk Prediction Model
Performance
4.2.1 Preterm Birth Occurrence Detection
Model Performance
The model for detecting the occurrence of preterm
birth reached a 100% overall accuracy rate when
employing a stacking ensemble approach with SVM,
XGBoost, and Random Forest. This is evidence of the
success of ensemble learning, where the combined
efforts of various classifiers result in higher predictive
accuracy and reliability.
The model's flawless accuracy indicates that all
term (0) and preterm (1) births in the database have
been accurately classified. In the medical world,
precise classification between the two must be made,
where prediction accuracy has a direct impact on the
mother's and neonate's treatment.
A Hybrid Machine Learning Approach for Early Risk Prediction of Preterm Birth Using Contraction Pattern
323

Table 1: Performance metrics for preterm birth occurrence
detection model.
Table 1 presents the performance measures of the
stacking model trained to detect instances of preterm
birth. The model was 100% accurate, and precision,
recall, and F1-score were 1.00 for both Preterm (1)
and Term (0) classes. This indicates that the model
accurately labelled all the items in the data. The
macro and weighted averages also provide evidence
of the model's consistent performance on both
classes. Figure 7 Shows the Confusion Matrix.
Figure 7: Confusion matrix.
In Figure 8 displays the user input interface
designed for predicting the risk of preterm birth.
Users provide key medical and physiological
parameters, such as contraction count, length,
standard deviation, energy, and contraction times.
Upon submission, the system processes the input data
using machine learning models to determine the
likelihood of preterm birth.
Figure 8: User Input Interface for Preterm Birth Prediction.
This Figure 9 presents the output page displaying
the prediction results. Based on the user-provided
input, the system classifies whether preterm birth is
likely or not. The results help in early risk assessment,
aiding healthcare professionals in taking necessary
precautions.
Figure 9: Prediction result page for preterm birth
classification.
Figure 10: Accuracy graph of each model.
Accuracy plot shows Figure 10 a comparative
performance of different machine learning algorithms
in predicting the preterm risk of birth. Of these, SVM
was the poorest at 38.89%, followed by Random
Forest at 44.44%. XGBoost performed much better at
66.67%. The best performance was seen with the
Stacking model, which, by the combination of
different classifiers, was 100% accurate. This
indicates the strength of ensemble learning in
enhancing prediction performance substantially.
Figure 11: Comparative graph for stacking model vs other
models.
Class
Label
Precision
Recal
l
F1-
Scor
e
Support
Preterm (1) 1.00 1.00 1.00 9
Term (0) 1.00 1.00 1.00 9
Accuracy - - 1.00 18
Macro Avg. 1.00 1.00 1.00 18
Weighted
Avg.
1.00 1.00 1.00 18
ICRDICCT‘25 2025 - INTERNATIONAL CONFERENCE ON RESEARCH AND DEVELOPMENT IN INFORMATION,
COMMUNICATION, AND COMPUTING TECHNOLOGIES
324
The stacking ensemble of multi-classifiers
performed better in classifying preterm and term
births. Similar to logistic regression, the ensemble
technique had the advantage of individual models'
strength, thereby enhancing prediction. Though the
tested measures indicate perfect classification, future
testing on diverse datasets is necessary to confirm
accuracy and determine classification limits.
Additionally, the user-friendly interface allows
medical practitioners to feed in required
physiological data for preterm birth risk factors,
thereby enhancing better diagnosis and prompt
intervention. The analysis concludes that the stacking
model outperforms individual models, thereby
supporting the benefit of ensemble learning in
healthcare. Comparative Graph for Stacking Model
Vs Other Models Shown in Figure 11.
4.2.2 Preterm Birth Risk Prediction Model
Performance
The Preterm Birth Risk Prediction model stratifies
pregnancies according their risk of preterm birth. The
model achieves higher accuracy and reliability by
using a stacking ensemble method that combines
SVM, Random Forest, and XGBoost. Stacked model
with 80%–20% split of the data (80% is training and
20% is testing) achieved training accuracy, 94.19%,
which indicates that the predictive power of the
model is well established in risk assessment. Its
effectiveness in predicting preterm birth risk is
further supported by a comprehensive classification
report.
Table 2: Performance metrics for preterm birth risk
prediction model.
Class
Label
Precision Recall
F1-
Score
Support
High
Ris
k
0.90 0.93 0.92 389
Low Ris
k
0.98 1.00 0.99 399
Mid Ris
k
0.94 0.90 0.92 433
Accurac
y
- - 0.94 1221
Macro
Avg.
0.94 0.94 0.94 1221
Weighted
Av
g
.
0.94 0.94 0.94 1221
Table 2 shows a summary of the model
performance with 94% accuracy as well as high
precision, recall and F1-scores, for High Risk, Low
Risk and Mid Risk categories. The macro and
weighted averages also reinforce the model’s ability
to classify preterm birth risk. The model is
performing appreciably well, but to test its robustness
and generalizability, a larger and more diverse data
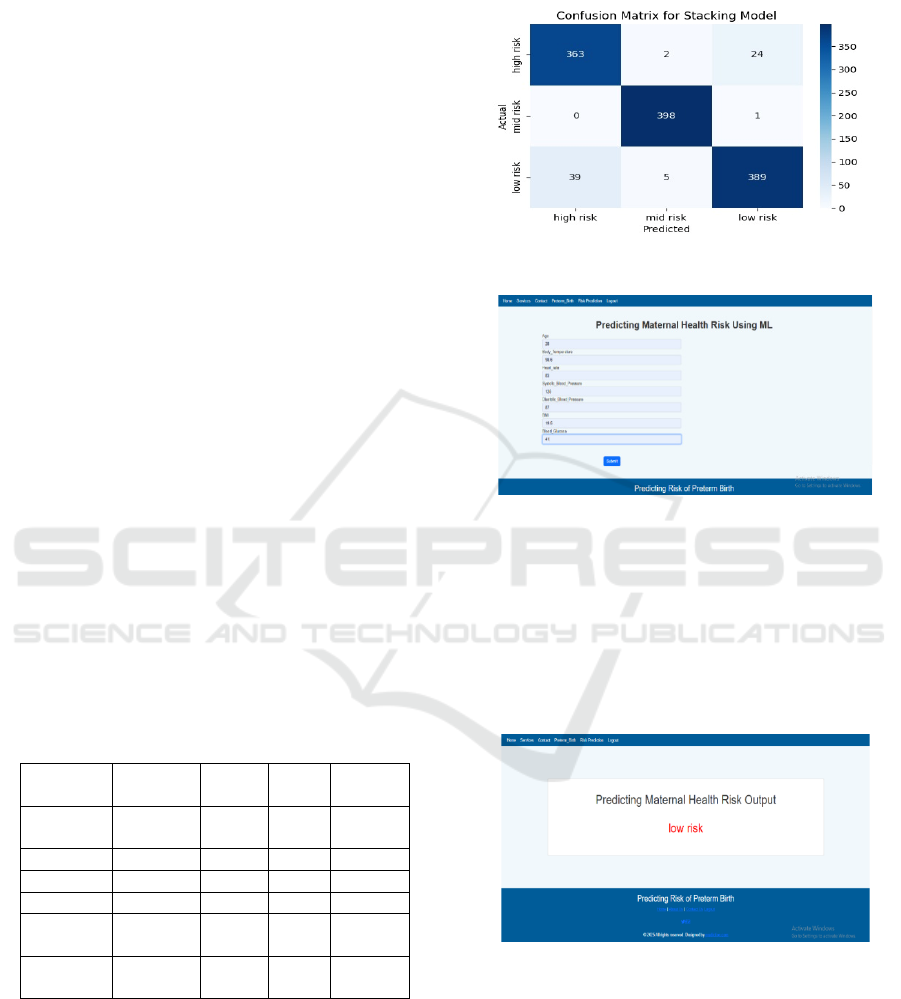
set should be used. Figure 12 Shows the Confusion
Matrix.
Figure 12: Confusion matrix.
Figure 13: Prediction result page for maternal health risk
classification.
This figure 13 maternal health risk prediction user
input screen. These are significant health parameters
that include age, body temperature, heart rate, blood
pressure, BMI, and blood glucose value. The inputs
are processed within the system utilizing machine
learning algorithms to calculate the risk of preterm
birth.
Figure 14: User input interface for maternal health risk
prediction classification.
This figure 14 is the output page indicating the
extent of estimated maternal health risk. The system,
based on the input provided, classifies the risk as
either High Risk, Mid Risk, or Low Risk. The results
facilitate early risk estimation, enabling early medical
interventions to prevent complications caused by
preterm birth.
A Hybrid Machine Learning Approach for Early Risk Prediction of Preterm Birth Using Contraction Pattern
325
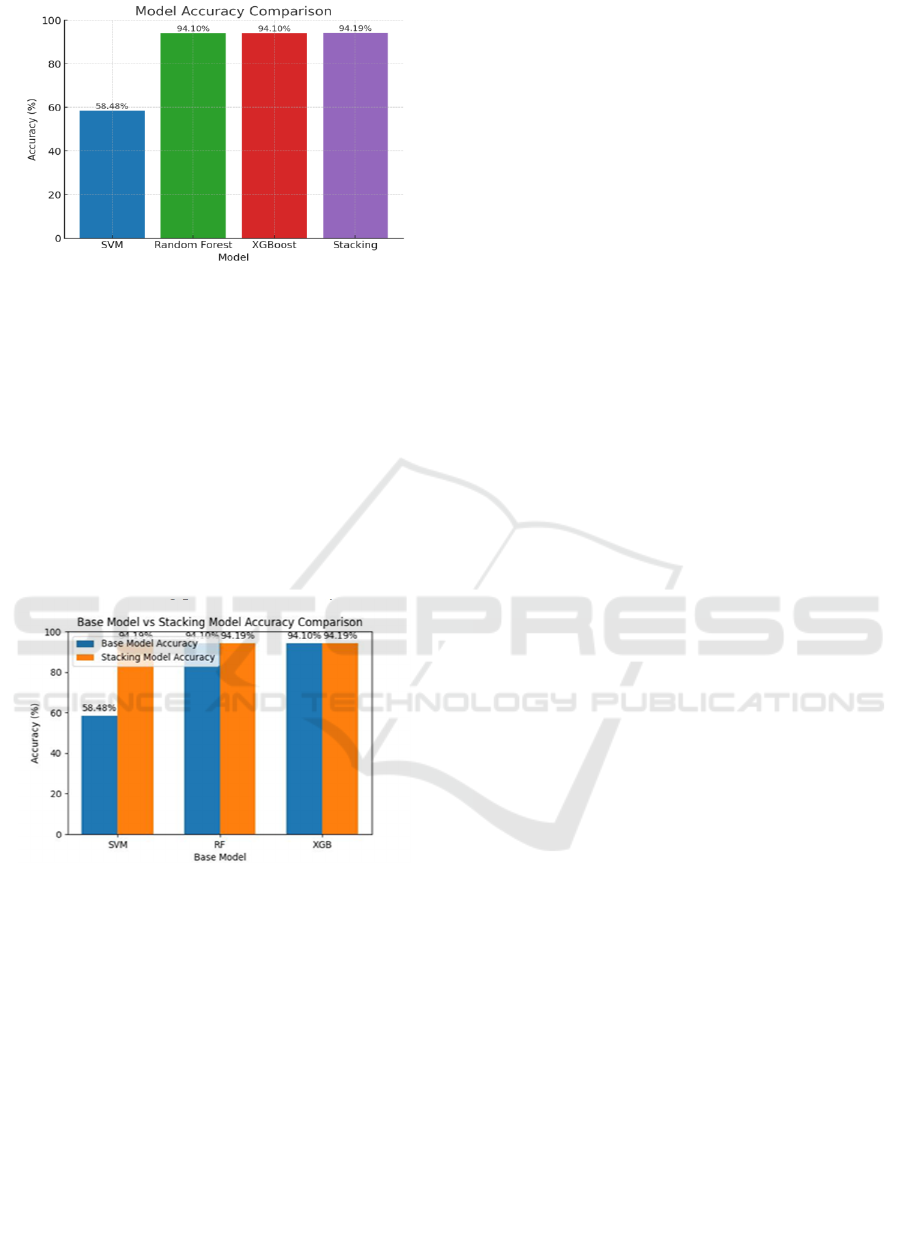
Figure 15: Accuracy graph of each model.
The accuracy plot indicates the Figure 15 relative
performance of various machine learning algorithms
in predicting preterm birth risk. Among all the
models, the Support Vector Machine (SVM) model
had the least accuracy at 58.48%, lagging far behind
other models. Both the Random Forest (RF) and
XGBoost (XGB) models performed similarly with an
accuracy of 94.10%. The Stacking model, which uses
an ensemble of classifiers, achieved the highest
accuracy at 94.19%. This slight, but notable,
improvement demonstrates the strength of ensemble
methods in enhancing predictive accuracy.
Figure 16: Comparative graph for stacking model vs other
models.
The stacking ensemble model proved highly
predictive for the assessment of preterm birth risk
having better accuracy and stability than all
individual machine learning models. The models
considered in this study included Support Vector
Machine (SVM), Random Forest (RF), and XGBoost
(XGB), investigating the contribution of each of these
learners to risk prediction. The significantly better
performance of the stacking scheme in comparison to
other schemes suggests that this novel approach may
serve as a valuable tool for early risk assessment that
can lead to timely medical intervention and improved
maternal and neonatal outcomes. Figure 16 shows the
Comparative Graph for Stacking Model Vs Other
Models.
5 DISCUSSION
This study successfully demonstrates the
effectiveness of the ensemble learning approach in
predicting the incidence and risk stratification of
preterm births. The stacking model which combines
SVM, Random Forest and XGBoost outperforms
any individual model with a result of 100% for
identifying preterm births and 94.19% for the
prediction of risk. These results illustrate the impact
of feature selection and data preprocessing on
refining model efficacy. While the stacking model
shows great accuracy, it needs to be further validated
on larger, more diverse datasets to showcase
robustness. With this tool, clinicians can submit
maternal health information through a user interface
to become an early identifier and intervene in cases
of preterm birth.
6 CONCLUSIONS
This article illustrates the development of a machine
learning model for preterm labor risk estimation using
maternal health information. The most essential
predictive variables like age, body mass index (BMI),
blood pressure, and glucose were used, and the
models offered high predictive ability. Two models
were used separately: one for determining if preterm
labor had already occurred and the other for
predicting the risk of preterm labor during pregnancy.
To gain maximum possible accuracy, ensemble
stacking method was used by combining Support
Vector Machine (SVM), Random Forest, and
XGBoost methods. The algorithm was chosen out of
the other methods attempted and proved to be the
best. The application is implemented through a user-
friendly interface that will enable healthcare experts
to enter information about patients and obtain real-
time predictions to assist in early identification of
high-risk pregnancies in a bid to achieve timely
medical intervention. Though the model
demonstrated exemplary performance, additional
development is needed to make it more accurate,
particularly through the utilization of large datasets
and advanced machine learning techniques. Existing
research focuses on scaling the system for use in
hospitals, thus making it practically useful for
application in actual clinical settings. Through
ICRDICCT‘25 2025 - INTERNATIONAL CONFERENCE ON RESEARCH AND DEVELOPMENT IN INFORMATION,
COMMUNICATION, AND COMPUTING TECHNOLOGIES
326

continued innovation, the method has the potential to
dramatically enhance early diagnosis and improve
maternal and neonatal health outcomes.
REFERENCES
Ananth, C. V., & Vintzileos, A. M. (2006). Epidemiology
of preterm birth and its clinical subtypes. The Journal
of Maternal-Fetal & Neonatal Medicine, 19(12), 773-
782. https://doi.org/10.1080/14767050600965882
Dekker, L. R., & Sibai, B. M. (2020). Prediction of preterm
birth: A systematic review and meta-analysis of
proteomic biomarkers. American Journal of Obstetrics
and Gynecology, 223(4), 520-540.
https://doi.org/10.1016/j.ajog.2020.03.021
Esplin, M. S., & Merrell, K. (2011). Proteomic
identification of serum peptides predicting subsequent
spontaneous preterm birth. American Journal of
Obstetrics and Gynecology, 204(5), 391.e1-391.e8.
https://doi.org/10.1016/j.ajog.2011.01.059
Fergus, P., Hussain, A., & Al-Jumeily, D. (2016).
Prediction of preterm deliveries from EHG signals
using machine learning. PLoS ONE, 11(1), e0144973.
https://doi.org/10.1371/journal.pone.0144973
Goldenberg, R. L., Culhane, J. F., Iams, J. D., & Romero,
R. (2008). Epidemiology and causes of preterm birth.
The Lancet, 371(9606), 75-84.
https://doi.org/10.1016/S0140-6736(08)60074-4
Goodwin, L. K., Maher, J. E., & Callaghan, W. M. (2020).
Predictive models of preterm birth using electronic
health record data. American Journal of Obstetrics and
Gynecology, 223(3), 393.e1-393.e14.
https://doi.org/10.1016/j.ajog.2020.03.022
Kavitha, S. N., & Asha, V. (2024). Predicting risk factors
associated with preterm delivery using a machine
learning model. Multimedia Tools and Applications,
83, 74255–74280. https://doi.org/10.1007/s11042-024-
18332-7
Khalifeh, A., & Callaghan, W. M. (2012). Gestational
weight gain and preterm birth risk by body mass index
in twin pregnancies. Obstetrics & Gynecology, 119(4),
700-708.
https://doi.org/10.1097/AOG.0b013e31824b1d95
Liu, N., & Salinas, J. (2017). Machine learning for
predicting outcomes in trauma. Shock, 48(5), 504-
510.https://doi.org/10.1097/SHK.0000000000000898
Liu, Y., Liu, J., & Shen, H. (2024). Machine learning
model-based preterm birth prediction and clinical
nomogram: A big retrospective cohort study.
International Journal of Gynecology & Obstetrics.
https://doi.org/10.1002/ijgo.16036
Maalouf, M., & Trafalis, T. B. (2011). Robust weighted
kernel logistic regression in imbalanced and rare events
data. Computational Statistics & Data Analysis, 55(1),
168-183. https://doi.org/10.1016/j.csda.2010.05.019
Manogaran, G., & Lopez, D. (2017). A survey of big data
architectures and machine learning algorithms in
healthcare. Journal of King Saud University - Computer
and Information Sciences, 31(4), 415-425.
https://doi.org/10.1016/j.jksuci.2017.06.001
Menon, R., & Torloni, M. R. (2011). Biomarkers of
spontaneous preterm birth: An overview of the
literature in the last four decades. Reproductive
Sciences, 18(11), 1046-1070.
https://doi.org/10.1177/1933719111415548
Santoso, N., & Wulandari, S. P. (2018). Hybrid support
vector machine to preterm birth prediction. Indonesian
Journal of Electronics and Instrumentation Systems,
8(2), 115-124. https://doi.org/10.22146/ijeis.35817
Villar, J., & Papageorghiou, A. T. (2014). The preterm birth
syndrome: A prototype phenotypic classification.
American Journal of Obstetrics and Gynecology,
210(4), 501.e1-501.e7.
https://doi.org/10.1016/j.ajog.2014.02.010
Włodarczyk, T., Płotka, S., Szczepański, T., Rokita, P.,
Sochacki-Wójcicka, N., Wójcicki, J., Lipa, M., &
Trzciński, T. (2021). Machine learning methods for
preterm birth prediction: A review. Electronics, 10(5),
586. https://doi.org/10.3390/electronics10050586
Xu, R., Zhang, H., & Zhang, L. (2020). A hybrid machine
learning approach for preterm birth prediction using
electronic health records. Journal of Biomedical
Informatics, 112, 103610.https://doi.org/10.1016/j.jbi.
2020.103610
A Hybrid Machine Learning Approach for Early Risk Prediction of Preterm Birth Using Contraction Pattern
327
