
Enhancing Blood Cancer Diognosis with Data Driven Techniques
Balaji J., Sanjai M., Hemadharshini V., Gunavathi R. and Gobisha K.
Department of Information Technology, Nandha College of Technology, Erode, Tamil Nadu, India
Keywords: Blood Cancer Classification, Deep Learning, resnet50 Model, Blood Cancer Detection.
Abstract: Blood is needed by the human body to carry essential nutrients, oxygen and immune cells. Leukemia and
lymphoma are two deliverables that can be traced to abnormalities in blood cells. Blood cancers which include
a range from leukemia to lymphomas and plasma cell diseases are among the most difficult to diagnose, yet
early detection is critical for effective treatment and positive clinical outcomes. In this scenario, the invention
of an integrated automated blood cancer detection system is essential. This pipeline classifies the blood sample
pictures and classifies between Benign, Early, Pre and Pro cancer types using a pre- trained ResNet50 model.
Images are pre-processed for it to comply with its input requirements, and then the model learns crucial
aspects to predict accurately. The model produced the type of cancer and a confidence score, which is an
indication of how likely the prediction is correct. The system developed using Keras as its deep learning
framework and Streamlit as its user interface, provides a reliable and portable tool that effectively automates
the picture processing procedure to aid with blood cancer identification. The implementation of these
consequences allows doctors in clinics to diagnose faster and more accurately by providing consistent and
accurate, classifications, while reducing manual work.
1 INTRODUCTION
Blood fills a major role in the human body,
delivering oxygen, nutrients, and immune cells
throughout organs and tissues. But in several blood
cancers, such as leukemia, lymphoma and myeloma,
mutations or uncontrolled growth in blood cells can
occur. Early detection of these tumors is needed for
effective treatment and improved survival rates.
Diagnosing blood cancer, a labor-intensive process
that is prone to human error, requires blood samples
to be painstakingly reviewed under microscope. The
rapid developments in the field of machine learning
and image analysis have made it increasingly
feasible to combine the automated identification and
categorization of blood malignancies.
By reducing the requirement for manual tasks and
facilitating better diagnostics, these technologies
could help with the fastest, efficient, and accurate
blood sample analysis. This method aims to assist
medical professionals by providing a more accurate,
more automated solution for detecting and classifying
blood malignancies, through the use of deep learning
models to classify images of blood samples. This will
eventually result in better diagnostic outcomes and
less costly treatments.
1.1 Blood Cancer Classification
Blood cancer classification is the process of
recognizing different types of blood cancer through
the study of blood characteristics. Blood
malignancies, including leukemia, lymphoma, and
myeloma, can have a myriad of presentations and
specific pathologic features. Classification is the
identification of those tumors which is based on the
abnormalities present in blood cells, including size,
shape, organization, etc. This step is critical in
determining the most appropriate treatment plan, as
well as assessing the severity of disease. Prompt and
accurate categorization allows healthcare
professionals to make informed decisions about the
most effective therapies, which greatly increases the
chance of well-timed success in any course of
therapy. Use of classification also ensures
identification of the stage or development of the
cancer which is important for long-term care and
judgment.
1.2 Deep Learning
Deep Learning is a powerful machine learning
technique back up by the architecture of multi-layered
738
J., B., M., S., V., H., R., G. and K., G.
Enhancing Blood Cancer Diognosis with Data Driven Techniques.
DOI: 10.5220/0013872000004919
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 1st International Conference on Research and Development in Information, Communication, and Computing Technologies (ICRDICCT‘25 2025) - Volume 1, pages
738-743
ISBN: 978-989-758-777-1
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

neural networks to allowing system to learn by itself
and improve from data without given explicit
instruction. The features can be derived from
unprocessed input, for instance, text or pictures,
which eliminates the need for human feature
selection. Deep learning excels in uncovering
intricate interdependencies within volumes of data
that would inevitably elude conventional approaches.
As an example, for blood cancer diagnosis, deep
learning models such as convolutional neural
networks (CNNs) are allowed to learn how to analyze
images of blood samples and to detect subtle patterns
indicative of cancer. These models are trained on
high volumes of expert-labeled data and due to this,
they evolve over a period of time and can identify and
classify blood cancer types with high accuracy with
minimal human intervention.
1.3 Resnet50 Model
The ResNet50 Model is a well-known model type of
CNN a deep learning framework effective for
computer vision tasks, particularly in image
classification applications. Each layer of the model
has 50 layers to help it learn hierarchies of patterns,
allowing it to understand and capture complex
components in photographs. One of the major
aspects of ResNet50 is its use of residual connections,
which allow the network to reduce degradation in
performance as it deepens. Due to its ability to
maintain great accuracy despite having deep
architectures, ResNet50 is extremely useful for tasks
that involve large and complex datasets. The
ResNet50 model works exceptionally well in
classifying Such medical pictures including blood
samples by identifying the distinguishing
characteristics like size, shape, and texture of blood
cells.! Its ability to analyze huge amounts of data and
spot complex patterns makes it an ideal candidate for
automating blood cancer classification.
1.4 Blood Cancer Detection
Blood cancer detection focuses on studying abnormal
activity of blood cells in order to diagnosis of various
blood cancers. The process involves both traditional
diagnostic techniques such as manual inspection of
blood samples, as well as modern computer tools
such as deep learning for automatic segmentation.
The common way to detect blood cancer is through
finding deviations from normal blood cell properties,
such as atypical cell sizes, shapes or architectures that
may indicate the presence of malignancy.
Determining the most effective treatment strategies
and enhancing patient outcomes depend on the fast
and precise identification of blood malignancies. The
detection process may be improved and expedited
with the use of sophisticated models and algorithms,
giving medical practitioners quicker, more precise
findings that facilitate wise clinical judgment.
2 LITERATURE REVIEW
According to Md. Aslam Mollah et al, In order for a
sensor to be able to measure the salinity of seawater,
it has to have high sensitivity, structural simplicity,
and durability, according to the study published in
theJournal of Molecular Biology. In this work, an
ultrahigh sensitivity PCF salinity sensor was
proposed based on a sagnac interferometer (SI). The
finite element method (FEM) is used to investigate
the propagation properties of the suggested PCF. A
sensitivity of up to 37,500 nm/RIU and 7.5 nm/%
was achieved throughout the salinity of 0% to 100%
obtained. The PCF proposed here achieves the
highest resolutions of 2.66 × 10−06 RIU and 1.33 ×
10−02% as well as good linearities with the length of
2.20 cm is inferior to PCF with other lengths value,
up to 0.9924. Due to its remarkable results, this
proposed sensor has the ability to detect salinity in
seawater. Salt levels matter because they greatly
affect activities beneath the sea and ocean species.
But the whoifiée indicator of salinity is the electric
conductivity of the chlorite ions. But this
measurement is affected by the interference from
other contamination ions. Research has been attracted
due to the various benefits of fiber optic salinity
sensors such as their programmable birefringence,
small structure, remote sensing, tunable dispersion
and immunity to electromagnetic interference (EMI).
Here, a PCF salinity sensor based on the SI
phenomenon is studied numerically. Every one of the
PCF air gaps is believed to be filled with mixed salt
concentrations of the seawater (M.R.B.A. Faysal, et.,
al. 2020).
Most surface plasmon resonance (SPR)- based
photonic crystal fiber (PCF) sensors have been
reported for detecting the analyte refractive index
(RI) values between 1.33 and 1.41 (i.e.: human blood,
body fluid), whereas rising state-of the-art detection
techniques requires a more improved platform for the
accurate detection of HMIs. Here we propose a way
to mitigate this effect through a transformation of the
structure, which can be achieved using the existing
fabrication processes that the sensor architecture is
built from. We present our sensor that uses an air-
core PCF, and unlike existing PCF-based sensors,
Enhancing Blood Cancer Diognosis with Data Driven Techniques
739

the analyte enter the core of the PCF via vertical side
opening channel to measure RI of analytes higher
than that of the background of PCF. We employ a
chemically stable plasmonic (gold) material, and
since the plasmonic material is not directly contacted
by the analyte, the interference effect is minimized.
For the analyte RI of 1.42, the sensitivity, and
resolution of the spectrum have been found to be
11,700 nm/RIU and 8.55 × 10−6 RIU, respectively.
However, our proposed sensor still has the potential
to catch active samples of these chemical and
biological liquids. Various organizations have
released several surface plasmon resonance (SPR)
sensors in the past few years that detect analytes with
a range of 1.33 to 1.41. Micro- fluidic slotted sensors,
internal and external metal- coated PCF-based
sensors, nanowire-based sensors, and D-shaped
configuration-based sensors (A.K. Paul, et., al. 2020)
were derived from it, resulting in five types of PCF-
based SPR sensors.
Mohammad Al Mahfuz et al. In this work, we
have proposed a dual-core photonic crystal fiber (DC-
PCF)-based surface plasmon resonance (SPR)
biocompatible sensor for the refractive index (RI)
sensing of bio-organic molecules and biochemical
analytes in the visible to near-infrared (0.5 to 2 µm)
region. The sensor construction is easy with two
hexagonal ring lattices all with round air aperture.
The use of plasmonic material and an analyte
detecting layer on the outer surface of the fiber allows
practical applications to be made. Gold (Au) with 30
nm of thickness, a noble plasmonic material, is
utilized to excite the plasmons on the surface. It is
also suggested that a thin layer (~5 nm) of TiO2
(titanium oxide) acts as an interlayer cementing the
Au and the silica glass. Based on the mode solver the
finite element method (FEM) is utilized to investigate
the sensor response. Using both amplitude and
wavelength interrogation methods, numerical
findings reveal an optimal wavelength sensitivity
(WS) of as high as 28000 nm/RIU, an optimal
amplitude sensitivity (AS) of 6829 RIU−1, an optimal
amplitude resolution (AR) of 5x10−6 RIU, and a
wavelength resolution (WR) of 3.57x10−6 RIU for
the proposed sensor. Moreover, such a PCF-SPR
sensor, having 2800 RIU−1 as the highest FOMpeak
value, is currently the starkest sensor (M. Al Mahfuz,
et., al 2020).
In this work a novel technique named as 2-D
photonic crystal waveguid (PCW) based cell
detection has been proposed by Abinash Panda et al.
for identifying the nature of either normal or
malignant cells. The proposed metamaterial is
designed with 5 × 5 silicon-based rods on a square
lattice with a central defect and air as the background.
To correctly sense, we classify two sets of live cells:
Group I: Malignant Cell (YD-10B); Group II:
Normal Cell (INOK). Properly adjusting the plane
wave expansion (PWE) method, the electric field
distribution and the peak reflected wavelength have
been achieved in the designed PCW structure.
Accurate identification of normal and malignant cells,
a large number of structural parameters, including
but not limited to lattice spacing, circular rod
diameter, and backdrop material type, are needed.
This MATLAB simulation indicates that yellow
color (i.e. reflected wavelength) belongs to cancerous
cells and orange color (i.e. reflected wavelength)
corresponds to healthy cells. Similarly, the negative
dispersion coefficient, scattering loss, and the
nonlinear coefficient of the proposed structure are
precisely evaluated on normal and malignant cells
separately. Moreover, this proposed sensor has a high
sensitivity of 2360.12 nm/RIU, low resolution of 1.78
× 10−6, and high-quality factor (as high as 99.765)
when differentiating normal and malignant cells (P.P.
Devi and A. Panda, 2020).
Chunlian Cen et al. Here, we propose to use
critical coupling and impedance matching theory to
computationally simulate the perfect absorption of
monolayer graphene. We studied a perfect single-
band absorption of the monolayer graphene by using
the important coupling effect and impedance
matching. Errors from the data fittings remained
within 10%, leading to a high quality factor (Q-factor
= 664.2) absorption spectrum with the absorbance
~100% in the near-infrared range. The position of the
absorption spectrum can be adjusted by changing the
ratio of the air hole radii of the elliptic cylinder to
structural period. The attained S = 342.7 nm/RIU
(refractive index unit) and FOM = 199.2 (figure of
merit) could be achieved by an absorber, which shows
great potential for biosensor technology
development. We hope that our research can serve as
an interesting application for graphene photonics and
optoelectronics. Plasmon metamaterials have
recently been a hot topic due to their unique EM
(electromagnetic) control capability. As such, it is
currently the most studied material due to its optical
and physical properties. The scope of
electromagnetic metamaterials has gone from being
limited to microwave frequency range to terahertz,
infrared, and almost the entire visible light
electromagnetic spectrum as illustrated by the study
(Jiang L et., al.2020) Metamaterial absorbers, on the
other hand, have been shown to improve absorption
in solar, microwave, infrared, and optical systems.
ICRDICCT‘25 2025 - INTERNATIONAL CONFERENCE ON RESEARCH AND DEVELOPMENT IN INFORMATION,
COMMUNICATION, AND COMPUTING TECHNOLOGIES
740
3 EXISTING SYSTEM
To realize early detection of blood cancer, the
current paper proposes twin-core photonic crystal
fiber (TC-PCF), which involves the refractive index
(RI) of healthy and cancerous blood cells. Normal
and cancerous cells are thought to be 30 to 70 percent
liquid, according to what is known. Because the
middle air hole is longer than the other two, these
samples should be infiltrated at that spot. Fig. 1:
Schematic setup of the known TC-PCF for the normal
and malignant cells, using a two-dimensional finite
element method (FEM) to monitor the variations of
the transmitted spectrum and coupling length while
considering the refractive index (RI). As per the
transmitted spectrum shift, the proposed sensor may
show a high sensitivity (8571.43 nm/RIU). Due to the
straightforward detection strategy, the suggested TC-
PCF sensor can potentially be applied to detection of
blood cancer in a convenient and cost-effective way.
4 PROPOSED SYSTEM
This technique combines Convolutional Neural
Networks (CNN) with ResNet architecture to enhance
the analysis of blood cell images for improved
accuracy in identifying anomalies, including blood
cancers. The integration of residual learning into
ResNet ensures efficient information flow by using
shortcut connections to avoid problems such as
disappearing gradients. The classification is made
through multiple convolutional layers that help
extract features such as size, form and texture that
help distinguish between healthy blood cells and
malignant ones. Using pooling and fully connected
layers, those features are then merged to obtain a
proper classification. Honed for processing
efficiency, the system handles large, diverse datasets
and is flexible enough to perform a variety of
analytical tasks. Providing a consistent, automated
way to analyse medical images, it has the potential to
be used in multiple disease settings because of its
scalability. Automated blood smears analysis has
improved significantly with this state-of-the-art
technique, increasing diagnostic fidelity and enabling
accurate analysis, diagnosis, and prediction.
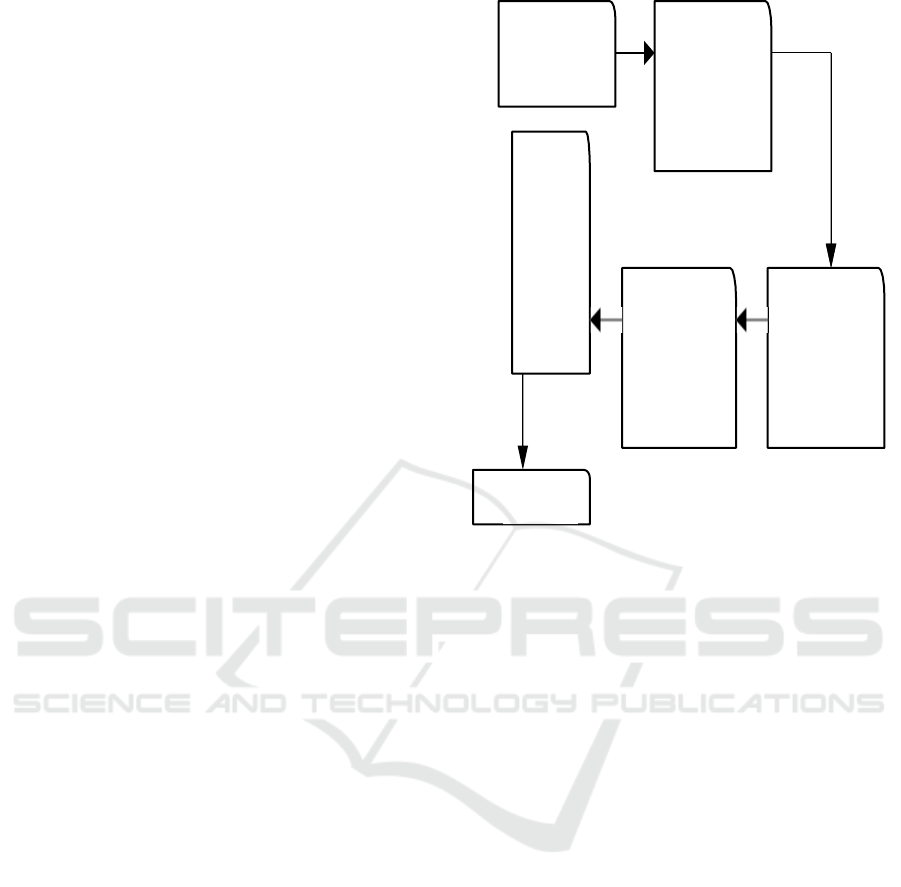
Figure 1: System Flow Diagram.
4.1 Load Data
The Load Data Module is the first step and it is used
to collect and prepare the dataset for further
operations. This module is responsible for
recovering images, which are tagged with the type of
blood cancer or whether it is non-cancerous. To
facilitate organizing and retrieving the pictures, they
are organized systematically This module ensures
that the inputs are ready in such a fashion and
structure, that the system can use it appropriately in
the subsequent workflows, which is training the data
and testing the data.
4.2 Data Preprocessing
The data preprocessing module’s objective is to clean
and transform the raw data into a form that could be
used as input in the model. This means that all the
pictures have to be resized to a standard size, their
pixels have to be transformed to the same range, as
well as performing all the other required
manipulations to get the data compatible with the
model. The objective of this module is to obtain
better quality data so that noise and inconsistencies
are discarded, and high-level structured data is clear
and organized for the best possible outcome during
the analysis and learning stages.
LOAD
DATA
DATA
PRE-
PROCESSI
NG
MODE
L
EVAL
UATI
ON
TRAINING
AND
TESTING
FEATURE
EXTRACTI
ON
RESULT
Enhancing Blood Cancer Diognosis with Data Driven Techniques
741

4.3 Feature Extraction
This means that it is the job of the Feature Extraction
Module to find and select features of the blood sample
photos that are relevant to classification. This
module utilizes the various textures, patterns, and
shapes found in the images to recognize and classify
distinct types of blood cancer. These important
features can be automatically extracted by deep
learning technique types of computations including
convolutional neural networks (CNNs). This module
not only simplifies the data but focuses well on the
important portions of the pictures, while also helping
the model to figure, and, classify the pictures
accurately.
4.4 Training and Testing
The Module for Training and Testing incorporates the
learning process of the model. During the training
phase, the model learns to recognize patterns in the
photos based on labeled data. It adjusts its internal
parameters to minimize errors and improve
predictions. An independent set of data is used to
evaluate the model's accuracy and generalizability
after training. This ensures that the model can
accurately classify new and unseen images,
predicting how well it will perform when used on
other datasets in the future.
4.5 Model Evaluation
The fine-tuning phase of the Model Evaluation
Module determines the performance of a trained
model and tests its
accuracy. After training/testing
metrics like F1-score, accuracy, precision, recall are
used to determine
the model performance. Such
metrics assess the models ability
to classify the
benign and malignant samples and the types of blood
cancer. Make sure that the final
model is trustworthy
and gives consistent quality predictions by taking a
look at the output of the model and to find out if
improvements can be made.
5 RESULT ANALYSIS
The system's ability to detect and classify different
types of blood cancer using picture data is
demonstrated in the analysis of the project's outcome.
Performance evaluation exhibits how the ability of
the model to accurately predict benign vs malignant
samples and classify them under certain categories as
early, pre or advanced phases. Metrics of the
classification process, such as accuracy, precision,
recall, and F1-score, help ensure that the feature
extraction and model training modules have
successfully identified key patterns that help in the
human defects. The outcomes, which demonstrate
pleasingly high degrees of accuracy and consistency
in predictions, confirm the approach of analyzing
blood sample images through deep-learning
algorithms. This review illustrates how one such
initiative measured up against its goals and shows
how it might aid in diagnosis of early and precise
blood cancer type. Accuracy for the existing system
and proposed system are tabulated in table 1 and
illustrated in figure 2.
Table 1: Comparison Table.
Algorithm Accuracy
Existing system
77
Proposed system 85
Figure 2: Graph Diagram.
6 CONCLUSIONS
In conclusion, this study provides an approach to
employ state-of-the-art machine learning algorithms
to diagnose and classify blood cancer using image
data. By processing the data in a systematic manner,
they were able to extract relevant features, train the
model and classifier such that the blood samples were
able to identify the visual patterns in the images.
Model evaluation ensures accuracy and reliability of
the model. This method shows how successful
machine learning will be impactful in the medical
industry by providing a helpful tool to assist the
diagnosis and detection of blood-related disorders.
The method is a significant advance in medical
Accuracy
85
80
75
70
Existing system Proposed
System
ICRDICCT‘25 2025 - INTERNATIONAL CONFERENCE ON RESEARCH AND DEVELOPMENT IN INFORMATION,
COMMUNICATION, AND COMPUTING TECHNOLOGIES
742

analysis as it automates the identification process
and produces reliable results.
Future Work.
To increase the generalizability of the model, this
study can be further improved in the future by
including more heterogeneous datasets including
samples from other demographics and a wider
spectrum of blood cancer types. Adding in data from
additional sources, such as genetic markers or clinical
test results, could potentially improve the predictive
power of the system. Further studies could
potentially focus on enhancing feature extraction
techniques; perhaps by utilizing highly advanced
deep learning architectures ormethods such as
transfer learning to bolster model performance. Also,
we hope to reduce the computation complexity and to
tune the model for faster training steps to scale up the
system for more applications. Another potential
future direction is to automate the process itself for
various blood-related diseases. This would make the
system more useful than just cancer detection and
ultimately facilitate more accurate and efficient
diagnosis in clinical environments.
REFERENCES
A.K. Paul, M.S. Habib, N.H. Hai, S.A. Razzak, Air-core
photonic crystal fiber-based plasmonic sensor for high
refractive index sensing, Opt. Commun. 464 (2020)
125556.
Cen C, Chen X, Zhang Y, Yang H, Yi Z, Yao W, et al. A
two-band metamaterial absorber for surface plasmon
resonance at terahertz in graphene. 2020; Physica E. 10,
117:113840.
Dai, W., Chen, F., Luo, H., Xiong, Y., Wang, X., Cheng,
Y., and Gong, R. are the authors of this work. For
enhanced microwave absorption capabilities, carbonyl
iron@void@ nitrogen-doped carbon with a yolk-shell
structure is created. J. Alloy Compd. 2020, 812,
152083.
Jiang L, Xu D, Yi Z, Chen Z, Cen C, and others. Graphene
is a high quality factor, high sensitivity metamaterial
that is the perfect absorber due to critical coupling
theory and impedance matching. Nanomaterials, 2020,
10:95
M. Al Mahfuz, M.A. Hossain, E. Haque, N.H. Hai, Y.
Namihira, and Ahmed A plasmonic RI sensor with a
dual-core photonic crystal fiber that operates in the
visible to near-infrared spectrum, In IEEE Sensors
Journal (2020),
https://doi.org/10.1109/JSEN.2020.2980327
M.R.B.A. Faysal, M.R. Hasan, M.B. Sagnac
interferometer-based photonic crystal fiber salinity
sensor with high sensitivity, Results Phys. 16 (2020)
103022,
Miao, C.; Fang, R.; Mou, H.Y.; and Xiao, W. Sandwich-
like Si@TiO2@rGO composites are easily synthesized
for application as high-performance anodes in lithium
ion batteries. J. Alloy, Compd. C 2020, 818, 152884.
[Source]
P.P. Devi and A. Panda. Refractive index-based photonic
crystal biosensor for the identification of cancerous
cells, Opt. Fiber Technol. 54 (2020) 102123,
Z. Fan, Z. Guo, X. Kong, and Z. Meng, Wide- range
refractive index sensor based on photonic crystal fiber
with phase matching between metal defect mode and
core mode, Opt. Commun. (2020) 125233.
Zhang, C.F.; Xu, D.Y.; Wu, P.H.; Chen, Z.Q.; Jian, R.H.
Graphene-Based Unpatterned Narrow Dual-Band
Monolayer Perfect Absorber with Near-Infrared
Critical Coupling. Micromachines 11, 58. 8, 2020.
Enhancing Blood Cancer Diognosis with Data Driven Techniques
743
