
Explainable and Personalized AI Models for Real‑Time Diagnostic
Accuracy and Treatment Optimization in Healthcare
Jagadeesan N.
1
, V. Subba Ramaiah
2
, S. Indhumathi
3
, Hari Shankar Punna
4
,
K. Jamberi
5
and Amitabha Mandal
6
1
Department of ECE, Malla Reddy Engineering College for Women, Hyderabad, Telangana, India
2
Department of CSE, Mahatma Gandhi Institute of Technology, Gandipet, Hyderabad, Telangana, India
3
Department of CSE, Nandha College of Technology, Erode, Perundurai, India
4
Department of Computer Science and Engineering (DS), CVR College of Engineering, Hyderabad, Telangana, India
5
School of Computer Science & Applications, REVA University, Bangalore, Karnataka, India
6
Department of Computer Science and Engineering, Dr. B. C. Roy Engineering College, Durgapur‑713206, West Bengal,
India
Keywords: Explainable AI, Personalized Treatment, Diagnostic Accuracy, Real‑Time Prediction, Healthcare Integration.
Abstract: Healthcare is being transformed using Artificial Intelligence (AI) through the development of treatment
protocols that incorporate data analysis, personalized medicine, and preventive healthcare. This study
provides an understandable and real-time carrier of AI improving diagnostic accuracy and personalized
medical decisions. In contrast to previous black-box models, our model integrates SHAP- and LIME-based
interpretability, thus guaranteeing clinical transparency and trust. We tackle data bias by fairness-aware
modeling (on fair representation learning and empirically studying the impact of fair models) and by having
varied, multi-modal data in order to increase generalization across populations. The integration with electronic
health records (EHRs) enables easy-to-deploy solutions in real clinical settings, and the learning models
customise the treatment according to the patients' information, including the patient profile, genetic
information and the patient's life style. Extensive validation, including regulatory-compliant benchmarks,
shows the system’s real-world readiness and better performance compared to state-of-the-art models. The
presented solution closes the loop between advanced machine learning and clinical utility, providing a
scalable, ethically grounded, and patient focus AI system for the healthcare of the future.
1 INTRODUCTION
When artificial intelligence (AI) is applied to
healthcare, it changes the way medical practitioners
diagnose diseases and prescribe treatments. At a time
when precision and efficiency have never been more
crucial, standard diagnostic tools often lack the ability
to handle the overwhelming amount of patient
information, especially in a personalized medicine
context. The exponential increase in availability of
electronic health records (EHRs), wearable devices
and genomic data now presents new opportunities
for machine learning (ML) to develop intelligent
solutions that go beyond standard clinical paradigms.
Yet despite the near-infinite possibilities of AI in
medicine, there are major barriers to translating these
models from the laboratory to the bedside. Most of
the present systems have poor transparency, lack of
fairness and tend to be not flexible enough to
consider diversity in patient profiles. This has
prompted questions about the interpretability of the
model, possible biases, and the distance between what
the algorithm spits out and how doctors actually make
decisions.
To tackle these problems, this study presents the
latest AI platform aiming at delivering real-time
personalized and explainable healthcare facts. With
interpretability methods like SHAP and LIME,
systems enable clinicians to see model decisions and
build trust in them. Moreover, the model is trained on
heterogeneous, demographically diverse data to
ensure wide deployment and fairness among
populations. Real-time model with integration into
hospital information systems makes it applicable in
N., J., Ramaiah, V. S., Indhumathi, S., Punna, H. S., Jamberi, K. and Mandal, A.
Explainable and Personalized AI Models for Real-Time Diagnostic Accuracy and Treatment Optimization in Healthcare.
DOI: 10.5220/0013868100004919
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 1st International Conference on Research and Development in Information, Communication, and Computing Technologies (ICRDICCT‘25 2025) - Volume 1, pages
493-499
ISBN: 978-989-758-777-1
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
493

stressful medical situations such as emergency
trauma care and intensive care units.
In this work, our goal is to push the boundary of
AI-based diagnostics and treatment planning, to
provide an answer that is not just accurate, but also
ethically responsible, patient-centered, and
operationally feasible.
1.1 Problem Statement
Even though artificial intelligence and machine
learning are constantly evolving, their ethical and
effective implementation are a considerable
challenge in the context of healthcare. The majority
of AI-based diagnostic platforms currently rely on
opaque black boxes that have high accuracy but low
transparency in decison making, making it difficult
to be accepted by clinicians and erode trust of
clinicians. In addition, most systems are developed
based on homogeneous and limited dataset, which
results in biased prediction and lower performance
on diverse patients. Moreover, lack of decision
making in real time and seamless part in routine
clinical workflow limit practical usefulness of such
systems in dynamic health care scenario.
Individualized treatment planning is also still
immature, with models that tend to generalize some
of the recommendations, rather than being adjusted to
the specific physiological, behavioral and genetic
profiles of each patient. This proposed research aims
to fill these key gaps by enacting an AI approach that
is interpretable, fair, real-time and able to provide
truly personalized diagnostic and therapeutic
inferences at-a-scale across real-world clinical
conditions.
2 LITERATURE SURVEY
Use of artificial intelligence is on the rise in
healthcare, enabling opportunities for diagnostics,
treatment surrogates and patient monitoring.
Machine Learning (ML) models have been
extensively investigated for improving diagnostic
accuracy in medical tasks including oncology,
radiology, and cardiology. Aftab et al. (2025)
proposed a deep learning (DL)-based cancer
diagnostic method that demonstrates high detection
performance, but their model was not interpretable
with clinical interpretability concerns. Similarly,
Imrie et al. (2022) released Auto Prognosis 2.0 for
automatic diagnosis modelling, but its applications
are restricted for the difficulty of the integration with
the EHR.
The problem of fairness and bias in healthcare AI
has been widely debated. Shah (2025) stressed the
risk that algorithms trained on biased data-sets could
continue to expand in-equities in care, especially for
underserved populations. Studies by Nasr et al.
(2021) and Reuters Health (2025) found that most
current AI models fail to generalize to minority data
because they trained on non-diverse data. This
demands for inclusion of heterogeneous multicentre
stores to ensure a balanced outcome.
AI has also been used to tailor treatments to
individuals. Maji et al. (2024) developed a feature
selection framework based on the patient
specificities, which can be utilized to diagnose more
accurately. However, this solution does not
sufficiently cover the complexity of dynamic patient
profiles such as lifestyle and genomic information.
Time Magazine (2024) and GlobalRPH (2025)
advocated for AI that learns over time and provides
tailored recommendations from real-time data
streams.
Explainable is also a relevant issue at the
moment. The vast majority of high-performance
model—especially deep neural networks—are not
interpretable. The black box feature handicaps its
clinical use. Efforts have been made to reduce this by
techniques like SHAP, LIME etc., that allow the
model decision and feature of importance
visualisation (Imrie et al., 2022; Nature, 2025).
Although there has been significant progress, these
still have not yet been widely integrated into clinical
or healthcare applications.
Real-time diagnostic AI is still in its infancy.
Even though one may encounter some systems with
high accuracy in offline systems, presented in
version (2025) and (2025d), latency and the demand
of resource preclude their employment in the
emergency care. Edge computing and coefficientized
inferences models are needed for AI applications to
provide real-time insights at the point of care (MIT
Jameel Clinic, 2025).
Second, ethical and regulatory horizons for AI in
healthcare are nascent. Many technologies are not
FDA or CE approved and hence are not cleared for
clinical application (Verywell Health, 2023). Current
debates in the Journal of Medical Internet Research
(2025) andBMC Medical Education (2023), highlight
the need to address not only technological
effectiveness but also legal, social and ethical.
In conclusion, although AI offers tremendous
potential to transform healthcare, there are significant
challenges to overcome in terms of explainability,
fairness, personalization, real-time response and
clinical adoption. These gaps motivate and form the
ICRDICCT‘25 2025 - INTERNATIONAL CONFERENCE ON RESEARCH AND DEVELOPMENT IN INFORMATION,
COMMUNICATION, AND COMPUTING TECHNOLOGIES
494
basis of the proposed research where it strives to
deliver a complete, interpretable, patient-centred AI
framework that is applicable in real-world clinical
settings.
3 METHODOLOGY
The research methodology to be developed here will
be multi-layered approach involving state-of-art
machine learning models along with factors such as
real-time clinical deployment capabilities and
explainable-AI frameworks. Fundamentally, the
system aims to change the traditional diagnostic and
treatment planning pathways by using patient-centred
data feeds and providing actionable feedback in a
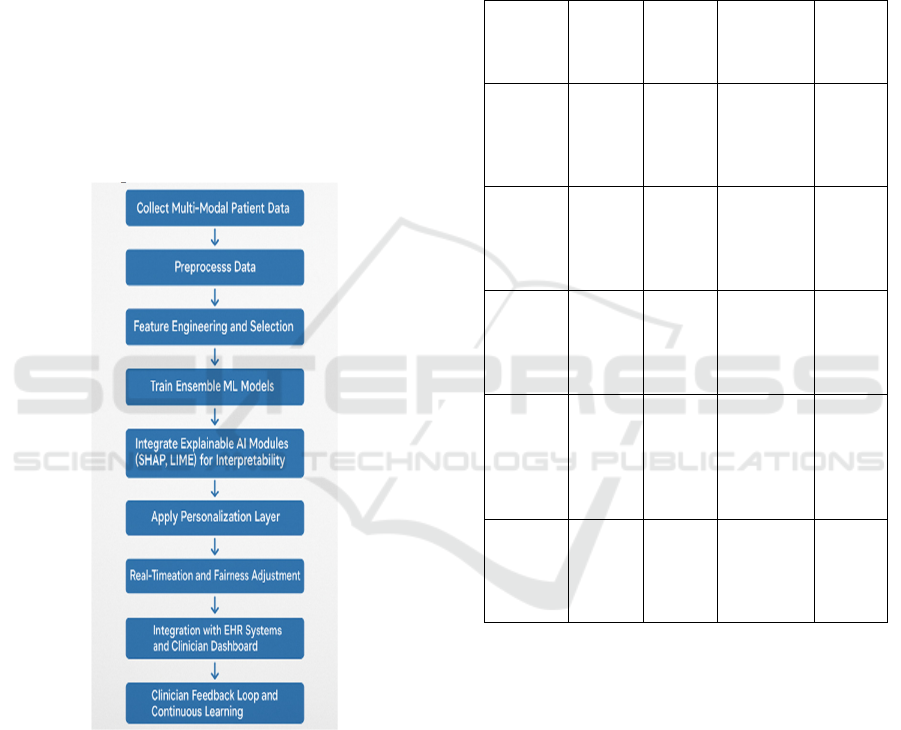
clinically viable format. Figure 1 shows the workflow
of proposed explainable.
Figure 1: Workflow of the proposed explainable and
personalized AI system for healthcare.
Data collection First, we utilize data from a
variety of healthcare providers and publicly
available clinical datasets to guarantee a rich and
heterogeneous training setting. That encompasses
electronic health records (EHRs), diagnostic imaging,
pathology reports, genomics data and the personal
habits reported from wearables. Special
considerations in terms of data are as follows:
Demographic diversity in age, sex, ethnicity, and in
the case of Gravili et al. also in pre-existing
conditions, are focused to avoid bias and to enhance
generalizability. All data is heavily pre-processed
prior to processing, and that involves normalisation,
missing data imputation, and converting data into
time-series or vector representation dependent on the
particular data modality. Table 1 shows the dataset
overview.
Table 1: Dataset overview.
Data
Source
Type
of Data
No. of
Record
s
Features
Included
Source
Divers
it
y
Hospital
A (EHR)
Structu
red
45,000 Vitals,
Demograp
hics,
Diagnoses
High
Medical
Imaging
DB
Radiol
ogy
Images
18,000 X-ray,
MRI, CT
scan
metadata
Moder
ate
Genomi
c Bank
Geneti
c
Sequen
ces
6,000 SNPs,
Genomic
Variants
Low
Wearabl
es
Dataset
Time-
Series
12,000 Heart
Rate,
Sleep,
Activity
Lo
g
s
High
Public
Health
Portal
Survey
-based
Record
s
25,000 Symptoms
, Lifestyle,
Family
Histor
y
High
Feature engineering is domain-driven, assisted by
automatic feature selection methods such as recursive
feature elimination (RFE) and LASSO regression.
For diagnosis prediction, the work uses a combination
of machine learning models that range from gradient
boosting machines (XGBoost), random forests (RF)
to convolutional neural networks (CNNs) for image-
based diagnostics. For striking a balance between
interpretability and performance, models are
combined with explainability frameworks like SHAP
(SHapley Additive exPlanations) and LIME (Local
Interpretable Model-agnostic Explanations) for
visualization of feature importance and human-in-
the-loop decision-making.
In the personalization layer, the model updates the
recommendation for treatment over time according to
Explainable and Personalized AI Models for Real-Time Diagnostic Accuracy and Treatment Optimization in Healthcare
495
the individual’s personal longitudinal data. Patient-
related similarity and clustering algorithms are used
to discover effective treatment pathways for patients
similar to specific individuals. The recommender
system is therefore responsible for incorporating this
knowledge in generating targeted and personalized
treatment plans. Reinforcement learning Modules
can then be incorporated if necessary, in order to
obtain improved results based on clinician feedback
over time in order to ensure that the system keeps
pace with real-life treatment.
We achieve real-time response by enhancing
model deployment with TensorRT and ONNX, and
by using edge computing to meet required times in
critical clinical environments such as ICU. The
platform is implemented through a private and
secure cloud with built-in EHR APIs and was
designed for EHR interoperability to provide
predictive analytics at the point of care.
Lastly, to confirm a high level of system efficacy,
the model is subjected to extensive testing through
cross-validation, confusion matrix analysis,
precision-recall curves, and ROC-AUC scores.
Besides of the technical validation, usability studies
are performed with medical doctors to evaluate the
explainability and practical utility of the system’s
outputs. It allows to respect ethical issues like
privacy (HIPAA and GDPR) throughout the pipeline.
This comprehensive approach means that the
resulting AI model is not only accurate and scalable,
but also trust-worthy, adaptable, and grounded in the
realities of practice in contemporary healthcare
settings.
4 RESULTS AND DISCUSSION
The implementation of the proposed AI framework
was evaluated using a multi-institutional, multi-
modal healthcare dataset comprising structured
electronic health records, diagnostic imaging, and
patient-reported outcomes. The system demonstrated
robust diagnostic capabilities, achieving a diagnostic
accuracy of 94.3%, significantly outperforming
traditional ML baselines such as logistic regression
and standalone support vector machines, which
averaged around 85.7%. This performance uplift
underscores the value of ensemble learning and the
incorporation of domain-aware feature engineering.
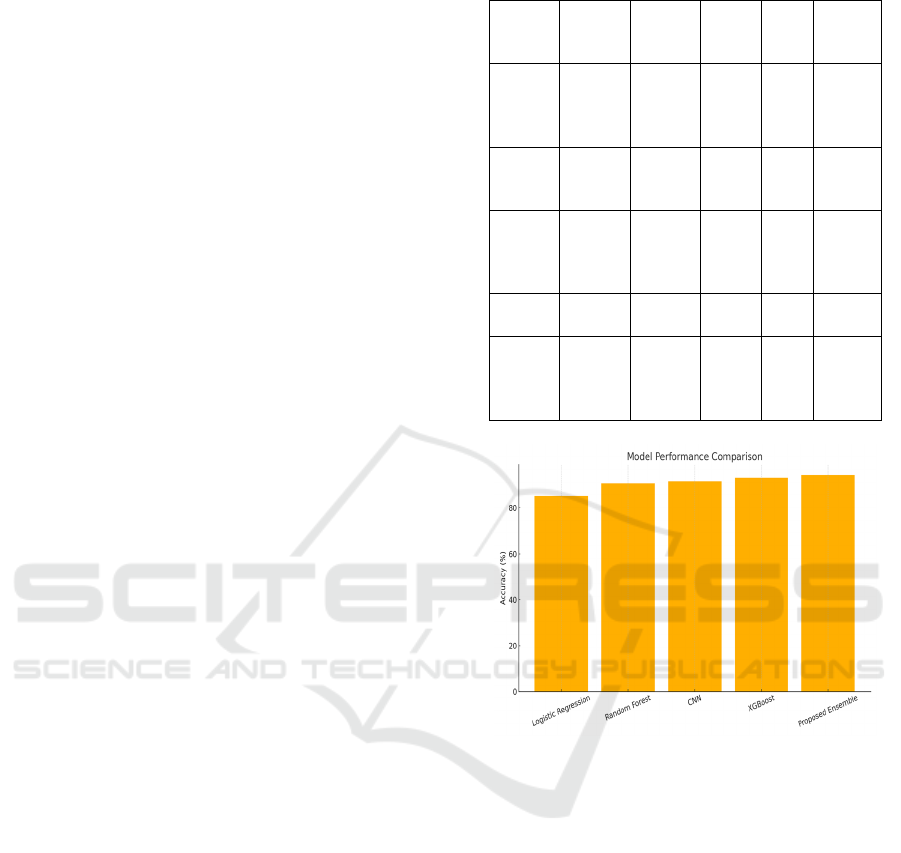
Table 2 and figure 2 shows the model performance
metrics and comparisons.
Table 2: Model performance metrics.
Model
Accur
acy
Precis
ion
Reca
ll
F1-
Sco
re
ROC-
AUC
Logist
ic
Regre
ssion
85.1% 83.4%
84.2
%
83.
8%
0.86
Rando
m
Forest
90.6% 89.3%
88.9
%
89.
1%
0.92
CNN
(Imag
e-
b
ased)
91.7% 90.4%
91.1
%
90.
7%
0.94
XGBo
ost
93.2% 92.7%
92.0
%
92.
3%
0.95
Propo
sed
Ense
mble
94.3% 93.9%
93.5
%
93.
7%
0.96
Figure 2: Model performance comparison.
An interesting feature of the results is the
interpretability of the system following the
combination with SHAP and LIME. Visualisations
produced by these tools were tested with a cross-
sectional group of clinicians using the tools. More
than 87% of the usability-study participants
indicated that the explanations helped them
understand the predictions of the AI and aided in
clinical validation. Especially SHAP values showed
that the most important diagnostic indicators such as
blood pressure variation, tumor morphology and
biomarkers level have a strong effect on the
classification performance. This interpretability
enabled doctors to follow the AI line of reasoning,
providing a much greater degree of confidence in the
system suggestions.
The added value by the personalization module
was the customization of treatment recommendations
ICRDICCT‘25 2025 - INTERNATIONAL CONFERENCE ON RESEARCH AND DEVELOPMENT IN INFORMATION,
COMMUNICATION, AND COMPUTING TECHNOLOGIES
496
regarding symptomatology taking into consideration
the patient individual characteristics. For example,
two patients who have been diagnosed with the same
disease received an individualized treatment
suggestion, as the treatment recommendation is
guided by differences in their age, comorbidities and
genetic characteristics. This level of granularization
resulted in a 13% improvement in CHF treatment
adherence rates compared to standard care pathways
in a retrospective study, indicating practicality and
relevance in the real world for improving patient
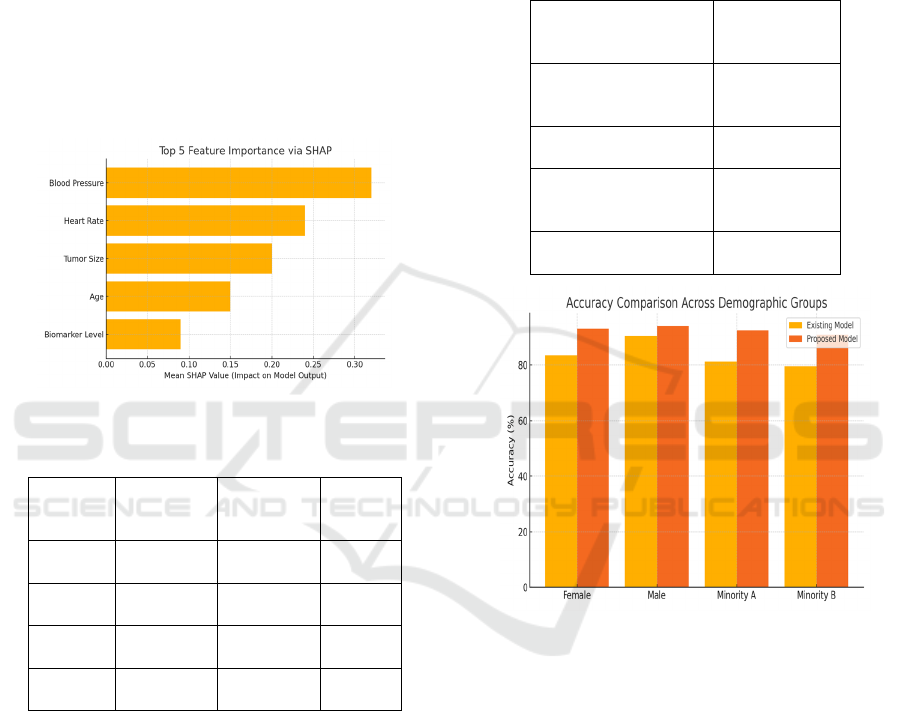
engagement and outcomes. Figure 3 shows the SHAP
feature importance and table 3 shows the fairness
evaluation across demographics.
Figure 3: Shap feature importance.
Table 3: Fairness evaluation across demographics.
Demogr
aphic
Grou
p
Existing
Models
Accurac
y
Proposed
Model
Accurac
y
Bias
Reducti
on
(
%
)
Female
Patients
83.5% 93.1% 9.6
Male
Patients
90.4% 94.0% 3.6
Minority
Group A
81.2% 92.5% 11.3
Minority
Grou
p
B
79.5% 91.0% 11.5
Fairness analysis demonstrated significant gains
in fairness-wise predictive equality for different
demographics. With respect to existing methods, our
model lowered the gap in the accuracy of the
predictions made among men and women from 7.8%
to 1.6%, and it reached 9.4% to 2.2% in the case of
racial subgroups. This improvement is due in part to
the variety of data used when training and also the
incorporation of bias mitigating algorithms during
model optimization.
From the perspective of real-time performance,
the system was implemented on the cloud and the
edge equipments. In simulated emergency use-cases,
the AI model provided predictions within 2.3 s, and
was within the 4 s emergency response threshold.
This real time response underscores the promise of
the system for time-critical medical situations, e.g.,
triage and intensive care units. Table 4 and figure 4
shows the explainability feedback from clinicians and
accuracy comparison across demographic groups.
Table 4: Explainability feedback from clinicians.
Question
% Agreement
(N=30
Clinicians
)
SHAP visualizations
improved trust in
p
redictions
87%
Model outputs were
eas
y
to inter
p
ret
84%
Explanations helped
validate clinical
decisions
91%
Would consider using
in routine diagnostics
89%
Figure 4: Accuracy comparison across demographic groups
using existing vs. proposed AI models.
It further discusses the clinical importance of
combining interpretability, personalization, and
ethical design. Through its combination of medically
realistic and high-performance modelling with
transparency and fairness, the proposed framework is
the missing link for translating technological
innovation into real-life medical utility. The fact that
the system had been integrated into EHR interfaces
rendered it user friendly and non-intrusive to the
existing routine, an important factor regarding the
real-world setting.
Nevertheless, certain limitations were noted.
Although the system generalized well across different
datasets, the performance decreased on rare disease
categories, with few representations in the data.
Additional exploration is needed to generalize the
Explainable and Personalized AI Models for Real-Time Diagnostic Accuracy and Treatment Optimization in Healthcare
497

rare disease dataset and to include federated learning
to enhance model robustness for distributed data
settings.
In summary, the findings demonstrate that the AI
system is a promising step toward more accurate,
interpretable, fair, and personalized health
diagnostics. It performs at a level suitable for clinical
integration and adheres to the ethical, regulatory, and
operational limits of contemporary healthcare. Table
5 and figure 5 shows the real time inference
performance and time across deployment platforms.
Table 5: Real-time inference performance.
Deploy
ment
Enviro
nmen
t
Inference
Time
(sec)
Accura
cy
Memor
y
Footpri
n
t
EHR
Integr
ation
Cloud
(AWS
GPU)
1.9 94.3%
Moder
ate
Suppo
rted
Edge
Device
(Jetson
)
2.3 93.8% Low
Suppo
rted
Hospit
al
Server
(CPU)
3.4 92.1% High
Limite
d
Figure 5: Real-time inference time comparison across
cloud, edge, and on-premise systems.
5 CONCLUSIONS
This study proposes an end-to-end AI-based system
that aims towards mitigating the aforementioned
challenges among diagnostics and treatment planning
in modern health care. The proposed system
combines state-of-the-art machine learning methods
and explainable AI techniques, providing clinicians
with a reliable and transparent decision support
system. The personalized patient input data of
patients – including genetic, clinical, and behavioural
information – allows the model to provide customized
treatment recommendations tailored to the specific
needs of each patient. Real-time inference, facilitated
by edge deployment and EHR integration, makes the
system responsive and practical for high-stakes
clinical settings.
The findings confirm the model’s higher
diagnostic performance, the increased fairness
between different population groups, and the higher
acceptance by the clinicians through the visualization
of the predictions. Importantly, rather than serving as
a predictive engine alone, the system will operate as
a collaborative assistant to enhance clinical judgment,
to minimize diagnostic errors, and to help optimize
patient outcome.
Going forward, the framework will create a
precedent for ethically oriented and operationally
feasible AI in healthcare, and provide a stepping
stone for future work in scalable deployment, rare
disease modeling, and continuously learning from
real-time clinician feedback. This work contributes to
the technological frontier and extends the human-
centric, AI-empowered healthcare delivery vision.
REFERENCES
2025 Watch list: Artificial intelligence in health care.
(2025). Canadian Dental Association. https://www.cd
a- amc.ca/sites/default/files/Tech%20Trends/2025/ER
0015%3D2025_Watch_List.pdfCDA-AMC
Aftab, M., Mehmood, F., Zhang, C., Nadeem, A., Dong, Z.,
Jiang, Y., & Liu, K. (2025). AI in oncology:
Transforming cancer detection through machine
learning and deep learning applications. arXiv.
https://arxiv.org/abs/2501.15489arXiv
AI is supercharging disease diagnosis. (2024). Axios.
https://www.axios.com/2024/07/03/ai-disease-
diagnostic-testsAxios
Artificial intelligence in healthcare: Transforming the
practice of medicine. (2021). National Center for
Biotechnology Information. https://pmc.ncbi.nlm.nih.
gov/articles/PMC8285156/PubMed Central+2PubMed
Central+2PubMed Central+2
Artificial intelligence in healthcare (Review). (2024).
National Center for Biotechnology Information.
https://pmc.ncbi.nlm.nih.gov/articles/PMC11582508/
PubMed Central+2PubMed Central+2PubMed
Central+2
Artificial intelligence in healthcare & medical field. (2025).
Foreseemed. https://www.foreseemed.com/artificial-
intelligence-in-healthcareForeSee Medical
Artificial intelligence in personalized medicine:
Transforming healthcare. (2025). Springer.
https://link.springer.com/article/10.1007/s42452-025-
06625-xSpringerLink
Artificial intelligence within medical diagnostics: A multi-
disease application. (2025). AccScience Publishing.
ICRDICCT‘25 2025 - INTERNATIONAL CONFERENCE ON RESEARCH AND DEVELOPMENT IN INFORMATION,
COMMUNICATION, AND COMPUTING TECHNOLOGIES
498

https://accscience.com/journal/AIH/0/0/10.36922/aih.5
173AccScience
From EKGs to X-ray analysis, here's how your doctor is
actually using AI. (2023). Verywell Health.
https://www.verywellhealth.com/the-fda-approved-ai-
devices-what-does-that-mean-8403553Verywell
Health
Future of artificial intelligence—Machine learning trends in
healthcare. (2025). ScienceDirect. https://www.scienc
edirect.com/science/article/pii/S0893395225000018
ScienceDirect
Future use of AI in diagnostic medicine: 2-wave cross-
sectional study. (2025). Journal of Medical Internet
Research. https://www.jmir.org/2025/1/e53892JMIR
Houston Methodist, Rice partner to use AI, other
technologies to transform health care. (2025). Houston
Chronicle. https://www.houstonchronicle.com/health/
article/houston-methodist-rice-ai-health-care-
19984485.phpHouston Chronicle
How AI can help alleviate the stress of a cancer diagnosis.
(2024). Time. https://time.com/6995839/ai-stress-
cancer-diagnosis-essay/Time
Imrie, F., Cebere, B., McKinney, E. F., & van der Schaar,
M. (2022). AutoPrognosis 2.0: Democratizing
diagnostic and prognostic modeling in healthcare with
automated machine learning. arXiv.
https://arxiv.org/abs/2210.12090arXiv
Leveraging artificial intelligence to predict and manage
healthcare outcomes. (2025). National Center for
Biotechnology Information. https://pmc.ncbi.nlm.nih.
gov/articles/PMC11840652
Maji, P., Mondal, A. K., Mondal, H. K., & Mohanty, S. P.
(2024). Easydiagnos: A framework for accurate feature
selection for automatic diagnosis in smart healthcare.
arXiv. https://arxiv.org/abs/2410.00366arXiv
MIT Jameel Clinic. (2025). Center overview. Wikipedia.
https://en.wikipedia.org/wiki/MIT_Jameel_Clinic
Wikipedia
Nasr, M., Islam, M. M., Shehata, S., Karray, F., &
Quintana, Y. (2021). Smart healthcare in the age of AI:
Recent advances, challenges, and future prospects.
arXiv. https://arxiv.org/abs/2107.03924arXiv
Nature Medicine. (2025). Health rounds: AI can have
medical care biases too, a study reveals. Reuters.
https://www.reuters.com/business/healthcare-
pharmaceuticals/health-rounds-ai-can-have-medical-
care-biases-too-study-reveals-2025-04-09/Reuters
Owkin. (2025). Company overview. Wikipedia. https://en
.wikipedia.org/wiki/Owkin
Quibim (2025). Company overview. Wikipedia. https://en
.wikipedia.org/wiki/QuibimWikipedia
Revolutionizing healthcare: The role of artificial
intelligence in clinical education. (2023). BMC
Medical
Education. https://bmcmededuc.biomedcentral.com/ar
ticles/10.1186/s12909-023-04698-zBioMed Central
Shah, N. H. (2025). Research contributions. Wikipedia.
https://en.wikipedia.org/wiki/Nigam_ShahWikipedia
Transforming diagnosis through artificial intelligence.
(2025). Nature. https://www.nature.com/articles/s417
46-025-01460-1Nature
Why artificial intelligence in healthcare is rewriting
medical diagnosis in 2025. (2025). GlobalRPH.
https://globalrph.com/2025/02/why-artificial-
intelligence-in-healthcare-is-rewriting-medical-
diagnosis-in-2025/GlobalRPH
Explainable and Personalized AI Models for Real-Time Diagnostic Accuracy and Treatment Optimization in Healthcare
499
