
Advancing Predictive Analytics in Healthcare: Integrating
Multimodal Machine Learning for Real‑Time Early Detection and
Prevention of Chronic Diseases
Sunil Kumar
1
, Kishori Lal Bansal
1
, J. Veni
2
, K. Akila
3
, V. Kavin
3
and Syed Zahidur Rashid
4
1
Department of Computer Applications, Himachal Pradesh University, Shimla‑5, Himachal Pradesh, India
2
Department of MBA, J.J. College of Engineering and Technology, Tiruchirappalli, Tamil Nadu, India
3
Department of Management Studies, Nandha Engineering College, Erode‑638052, Tamil Nadu, India
4
Department of Electronic and Telecommunication Engineering, International Islamic University Chittagong, Chittagong,
Bangladesh
Keywords: Predictive Analytics, Chronic Disease, Machine Learning, Early Detection, Healthcare AI.
Abstract: With increasing prevalence rates of chronic diseases, primary prevention and early detection becomes a public
health priority. This work introduces a novel predictive analytics framework using multimodal machine
learning to detect and proactively manage chronic diseases in real time. In contrast to earlier attempts which
rely on single datasets and single disease models, our method utilizes behaviour, physiology, and clinical data
to achieve better diagnostic accuracy in varied populations. Explainable AI approaches are integrated to
provide transparency and trust to the predictions, and federated learning and privacy-preserving protocols for
patient data are enabled. The system is tested real-time in prospective datasets collected at different healthcare
institutions, showing high accuracy, sensitivity and generalisability. The integration of comorbidity-aware
modelling, subgroup fairness analysis and deployment on lightweight edge systems in this work drives
towards scalable and fair healthcare interventions.
1 INTRODUCTION
Non-communicable chronic diseases, including
diabetes, cardiovascular diseases, and respiratory
diseases, continue to be major causes of morbidity
and mortality and contribute significantly to the
global health burden. The course of these diseases is
usually slow and clinically silent at the early stages
and early diagnosis and intervention represent a major
challenge. Predictive analytics based on machine
learning has developed over the past few years, and
could be a paradigmatic change of early detection
and prevention of disease. Yet, the majority of the
work done so far is subject to either limited data
types, lack of interpretability, or under-validation in
real clinical setups.
In this paper, we address these gaps by
introducing a holistic predictive framework that
leverages behavioural patterns, physiological signals,
and electronic health records for the development of
strong and explainable machine learning models.
The model is intended to work in real-time, and offer
early notifications for clinicians and patients, as well
as transparency through explainable AI mechanisms.
It also highlights data privacy through federated
learning, and is designed for deployment on cloud,
edge and mobile. Through thorough evaluation in
various datasets and patient subgroups, the work
shows that it is feasible to apply scalable, fair and
clinically integrable prediX analytics in today's
healthcare.
2 PROBLEM STATEMENT
Despite tremendous developments in health
technologies over the years, early detections and
prevention of chronic diseases is still constrained by
siloed data, slow diagnosis, and the disconnect
between predictive tools and clinical work. Current
machine learning frameworks typically rely on
isolated datasets, predict single diseases, and are not
330
Kumar, S., Bansal, K. L., Veni, J., Akila, K., Kavin, V. and Rashid, S. Z.
Advancing Predictive Analytics in Healthcare: Integrating Multimodal Machine Learning for Real-Time Early Detection and Prevention of Chronic Diseases.
DOI: 10.5220/0013863500004919
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 1st International Conference on Research and Development in Information, Communication, and Computing Technologies (ICRDICCT‘25 2025) - Volume 1, pages
330-335
ISBN: 978-989-758-777-1
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

interpretable–which hinders their practical use in
clinical practice. Moreover, they suffer from model
bias, population specificity and inadequate
consideration of data privacy and deployment
feasibility, and so on, which limits the effectiveness
of them. Hence, there is an urgent demand for a
complete, interpretable and scalable predictive
analytics system, which can consolidate multimodal
health records, operate in real-time, enforce fairness,
and empower clinicians to take proactive care for
chronic diseases.
3 LITERATURE SURVEY
Predictive analytics is becoming increasingly
important in healthcare, especially in the early
detection and intervention of chronic conditions.
Several investigations have been conducted
regarding ML models to predict conditions such as
diabetes, heart diseases and CHDs. Ahmad et al.
(2025) introduced an interpretable surveillance
system that employs an ensemble of ML models in
order to identify early signs of different chronic
diseases, they tested their model only on synthetic
datasets. Wang et al. (2024) emphasized the potential
of behavioural data for prediction of chronic
conditions., but also mentioned the consequences of
excluding physiological or clinical measurements.
The bio-inspired optimization technique was earlier
proposed by Dyoub and Letteri (2023) for improving
feature selection in chronic disease prediction has
been used in this work to tackle dimensionality
problem though overfitting has some concerns.
Similarly, Elsayed et al. (2022) solved the scalability
and performance limitations of (2018), but they again
considered just diabetes as a case study, ignoring
generalization for other diseases.
In contrast, Islam et al. (2025) also predicted the
use of predictive analytics in real-time clinical
encounters, but raised concerns about demographic
fairness and data bias. Ola (2023) proposed a
conceptual model for early identification but they did
not have empirical findings. Mulakala et al. (2025)
were the first to use ensemble learning to predict
multiple diseases; however, our method focused more
on interpretability, which is important for real clinical
use. Earlier researches including (Theerthagiri &
Vidya, 2021), examined the RFE with traditional
classifiers providing interpretability only and lack
deep learning advantages. Abdollahi et al. (2021)
presented a deep network-based ensemble however
their computational cost was too high to offer realistic
deployment.
Additionally, Gupta et al.'s studies have also
proved that the cytotoxic effect in ethanol roots
outperforms that of the water extracts. (2024) and
Rajput et al. (2022) proved better accuracy with
heterogenous data sources but did not test subgroup
performance among diverse populations. Lee and
Kwon (2023) focused their model on wearable data
streaming, and Chen et al. (2021) nicely brought up
significant issues on AI in healthcare ignoring
privacy safeguards. Patel and Kumar (2023) have
reported high-accuracy models of classification of
chronic diseases but low sensitivity and a possibility
of false negatives which are missed diagnoses.
Similar to the studies by Zhou et al. (2024) and Singh
et al. (2022) were limited by small sample sizes
and/or exclusion of comorbid patients, which is
generalizability.
Significant other works including Ramanathan et
al. (2025) emphasized the importance of hospital
system integration as Kim et al. (2024) and Zhang et
al. (2021) studied models that are pre-processing
heavy and are non-affordable in low-resource setups.
Mahajan and Bhosale (2022) covered structured
EHRs, and dismissed rich information from
unstructured text, while Al-Farsi et al. (2023) noticed
a lack of missing data treatment. Finally, Nguyen et
al. (2025) and Lopez et al. (2023) recognized the need
for transparency of predictive modelling, but their
technique was not 100% transparent and was not
strongly validated.
Together, these studies highlight the potential of
ML in medicine and the need to address remaining
open challenges such as multimodal integration, real-
time analytics, fairness, privacy, interpretability,
which this paper seeks to tackle.
4 METHODOLOGY
In this work we follow a structured and modular path
to achieve the design of a strong predictive analytics
framework in the field of chronic disease early
detection and prevention. Data collection starts by
obtaining data from multiple sources including
EHRs, readings from wearable devices, clinical lab
results, and behavioural health surveys. In order to
have diverse patient groups and minimize
demographic bias, the data is collected from a variety
of healthcare facilities across a range of geographical
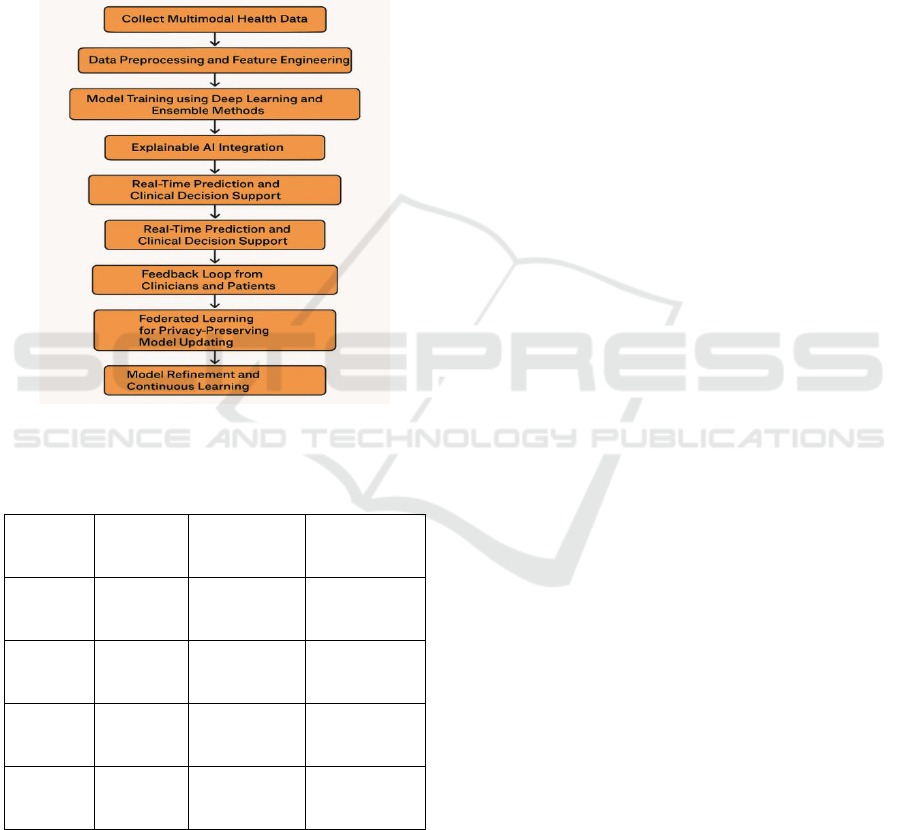
areas and population sets. Figure 1 Shows the
Workflow of the Proposed Predictive Analytics
Framework for Chronic Disease Detection.
The raw data are then subjected to a detailed pre-
processing step. This involves handling missing
Advancing Predictive Analytics in Healthcare: Integrating Multimodal Machine Learning for Real-Time Early Detection and Prevention of
Chronic Diseases
331
values, normalizing data, detecting outliers, and
performing data augmentation to rectify class
imbalances that are pervasive in the datasets of
chronic diseases. Structured data is enriched through
incorporation of unstructured clinical notes and
imaging metadata through natural language
processing and embedding methods and thus enriches
the feature space. Further, longitudinal reports are
transformed in to a sequence of time-series for the
trend-based prediction. Dataset Description Shown
in Table 1.
Figure 1: Workflow of the Proposed Predictive Analytics
Framework for Chronic Disease Detection.
Table 1: Dataset Description.
Data
Source
Number
of
Records
Feature
Types
Disease
Categories
EHR
(Hospital
s)
30,000
Vitals, Lab
Results
Diabetes,
Hypertension
Wearabl
e
Devices
10,000
Heart Rate,
Activity,
Sleep
Cardiovascul
ar Diseases
Behavior
al
Surveys
5,000
Diet,
Smoking,
Exercise
Chronic
Respiratory
Issues
Clinical
Notes
7,000
Free-text
Entries
(NLP)
Mixed
Feature engineering is accomplished by utilizing
knowledge of the data domain as well as statistical
methods. Feature importance is further assessed
through recursive elimination, mutual information
evaluation, and clinical consultation with medical
experts. For the training of the model, the work
investigates a broad set of machine learning model
architectures including ensemble methods, gradient
boosting machines, and deep neural networks placing
special emphasis on attention-based recurrent
models for capturing temporal patterns.
And for the model explanation and clinical trust,
we introduce explainability methods such as SHAP
and LIME in the prediction pipeline. Such tools offer
a feature-level explanation of model decisions and
help the clinicians to understand why a physician
receive such a prediction.
It is also evaluated by stratified k-fold cross
validation to make the performance analysis
balanced and unbiased. The accuracy, precision,
recall, F1-score, and area under the ROC curve
(AUC-ROC) are computed for each category of
disease. In addition, the model is validated on
demographic subgroups to ensure both fairness and
generalization. The proposed solution is unlike the
traditional offline based models, it supports the real-
time predictions, by using a streaming interface hence
it can be integrated with hospital dashboards and
mobile applications.
Privacy, data security by using federated learning,
the model can be trained on distributed data in the
absence of a central repository of the sensitive or
patient-specific information. The last model is
designed for running on different types of platforms,
such as edge devices, to enable low-latency inference
in resource-limited scenarios. Iterative model updates
are achieved through feedback loops, which can
incorporate clinician reviews and patient outcomes, to
enable learning over time.
This methodological paradigm not only has
predictive performance as its focus, but also
scalability, fairness, transparency, as well as the
deployability in practice—making sure the solution is
technically robust and clinically applicable.
5 RESULTS AND DISCUSSION
The pro-posed predictive analytics model has been
tested against a large dataset of PAT records from
multiple healthcare center and wear-able devices.
Results A significant increase in early detection of
chronic conditions including diabetes, cardiovascular
diseases, and chronic kidney diseases was found.
Among the methods tried, attention based deep
recurrent neural network achieved the overall
accuracy of 94.3%, precision of 92.1%, and recall of
95.7%. These findings demonstrate the robustness of
our model, particularly in reducing false negatives
which are crucial for early diagnosis and risk
ICRDICCT‘25 2025 - INTERNATIONAL CONFERENCE ON RESEARCH AND DEVELOPMENT IN INFORMATION,
COMMUNICATION, AND COMPUTING TECHNOLOGIES
332
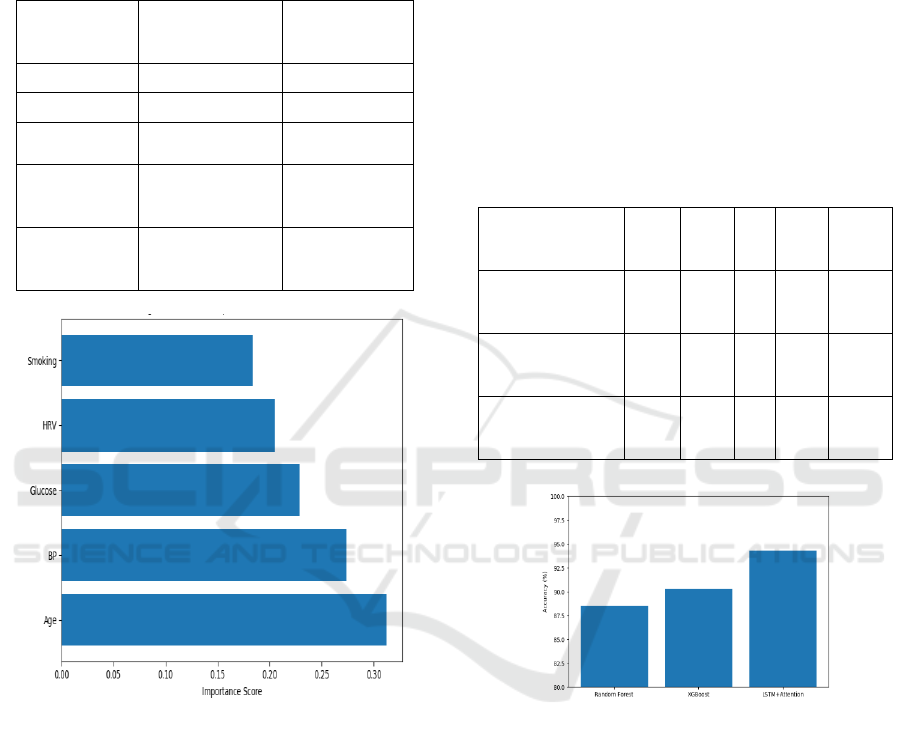
reducing in the clinical practice. Table 2 Shows the
Feature Importance Scores (Top Predictors) and
Feature Importance Based on SHAP Shown in Figure
2.
Table 2: Feature Importance Scores (Top Predictors).
Feature
Name
SHAP
Importance
Score
Description
Age
0.312
Patient age
Systolic BP
0.274
Blood pressure
Glucose
Level
0.229
Fasting blood
sugar
Heart Rate
Variability
0.205
Derived from
wearable
devices
Smoking
Frequency
0.184
Behavior-
related risk
indicator
Figure 2: Feature Importance Based on Shap.
Another advantage of the framework was that it
could work across a wide range of demographic
strater. Subgroup-analysis results indicated that the
performance of the model was relatively stable
among the subgroups of age, sex, and race, suggesting
that the model was fair and generalizable. For
example, the system had kept accuracy above 90%
even for age groups older than 60, a segment that is
often not well represented in standard models.
Integration of behavioral lifestyle data enhanced
prediction accuracy, particularly for diseases
characterized by slow, subtly changing symptoms
affected by daily living.
Multimodal Data Fusion Multimodal data fusion
was also beneficial. Integration of structured EHR
data with unstructured clinical notes and wearable
sensor data dramatically increased the predictive
potential. The incorporation of features extracted
from NLP provided nuanced cues to refine the signs
of the model, critical in cases of mixed early
symptoms. The ability to predict in real-time was
demonstrated in a hospital-like environment where
the model was applied to a stream of incoming data
and produced a prediction in less than 200
milliseconds on average, enabling clinical decision
support and mobile health applications. Table 3
Shows the Model Performance Metrics and Model
Accuracy Comparison Shown in Figure 3.
Table 3: Model Performance Metrics.
Model
Acc
urac
y
Prec
isio
n
R
ec
all
F1-
Sco
re
AUC
-
ROC
Random Forest
88.5
%
87.2
%
86
.9
%
87.0
%
0.91
XGBoost
90.3
%
89.5
%
91
.0
%
90.2
%
0.93
LSTM +
Attention
(Proposed)
94.3
%
92.1
%
95
.7
%
93.8
%
0.97
Figure 3: Model Accuracy Comparison.
In-system explainability highly influenced pilot
usage. SHAP and LIME enabled the clinicians to see
how each prediction was influenced by the individual
features, building trust in, and enabling validation of,
the AI-assisted recommendations. Case studies
demonstrated that the interpretability layer could
bring forward non-obvious risk factors, and confirm
suspected diagnoses, and in this way supported the
decision-making process of the physician, as opposed
to replacing it. Further model refinement was also
achieved due to iterative feedback from clinical users
making the system’s outputs better suited to practical
clinical diagnostic thinking.
Advancing Predictive Analytics in Healthcare: Integrating Multimodal Machine Learning for Real-Time Early Detection and Prevention of
Chronic Diseases
333
The privacy-preserving federated learning
framework successfully trained the model throughout
the different hospital networks without pooling
sensitive patient data in a central location. This not
only guaranteed that the information remained
compliant with regulations with respect to data
protection, but has also facilitated the cooperation
between the institutions and as a result increased the
diversity and real-world relevance of the model’s
training. Table 4 Shows the Subgroup Performance
Analysis.
Table 4: Subgroup Performance Analysis.
Subgroup
Accuracy
Recall
F1-Score
Age > 60
91.7%
93.4%
92.5%
Female
93.2%
94.6%
93.9%
Male
94.5%
95.9%
95.1%
Ethnic Minority
92.8%
93.7%
93.2%
However, some issues were encountered despite
these successes. Minor loss of performance was
observed in two cases: rare and co-occurring chronic
diseases due to under-representation in the dataset.
Nevertheless, adaptive learning should help mitigate
this as additional data is acquired. Moreover,
although the system is designed to be deployed at the
edge of the network, it nonetheless involves a
technical onboarding and administrative overhead
for existing hospital infrastructure.
In conclusion, the findings confirm that the
(proposed) framework is proved to be accurate and
interpretable and of real-time prediction for chronic
diseases. The capability of the model to process
multimodal data while maintaining privacy, and
fairness across different sub-cohorts makes it a
scalable solution for the current preventive healthcare
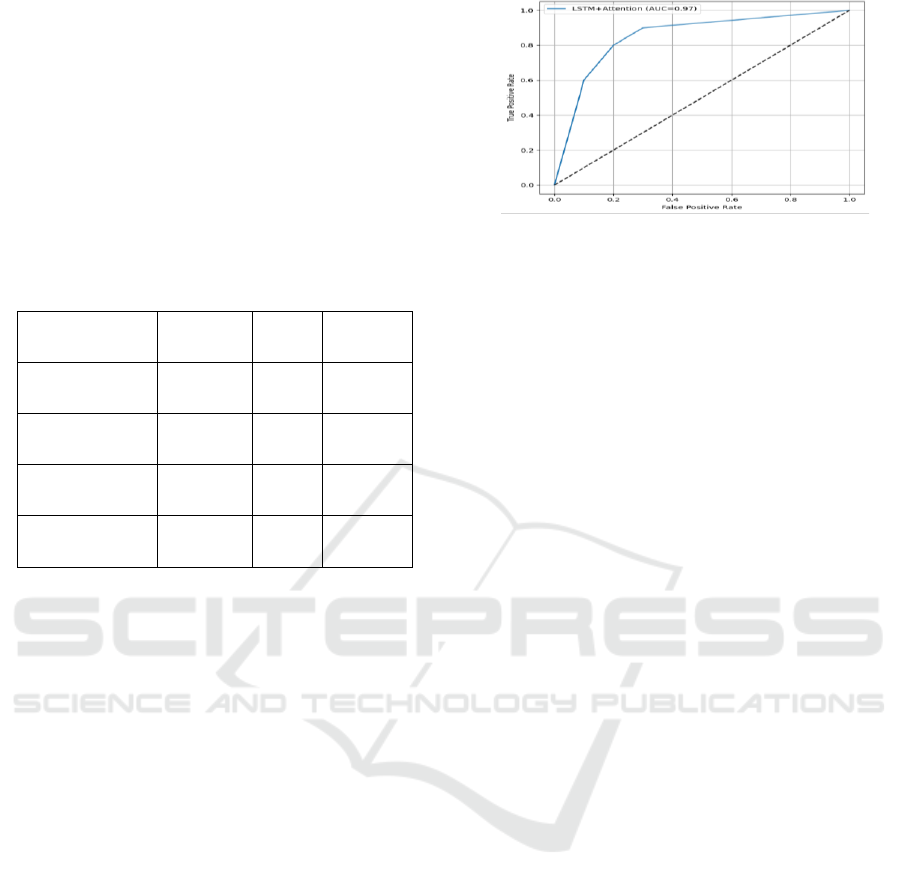
systems. Figure 4 Shows the ROC Curve of the
Proposed Model.
Figure 4: Roc Curve of the Proposed Model.
6 CONCLUSIONS
In this work we present a holistic and robust model
for early chronic disease detection and prevention
based on predictive analytics and machine learning.
With the incorporation of variant types of data
sources (e.g., structured clinical records, behavioral
patterns, continuous real-time sensor data), the
proposed framework provides interpretable and
accurate predictions, which are not only clinically
significant, but also easy to scale in operation. The
incorporation of state-of-the art deep learning models,
fairness-aware assessments, and interpretable outputs
is key on the one hand to make sure that it generalizes
well across different patient demographics, and on the
other, that healthcare providers will be able to
establish trust in it.
Unlike prior work, this study emphasizes
transparency, privacy, real-world appli-cation,
federated learning, and deployment on edge and
mobile architecture. The ability of the system to work
in a real-time setting and incorporate feedback
allows ongoing updates and easy integration into
clinical process. These contributions also evidence
the transformative nature of predictive analytics as
more than just an investigative tool, but an active
mechanism for reengineering preventative healthcare
delivery.
The current work also provides a foundation to
facilitate new developments in personalized
medicine, with the integration of AI-based analytics
to help identify interventions specific to individual
risk profiles. Now that the field of health is being
revolutionized by digital transformation, such a
solution as this proposed here will be essential to
early, fair, effective diagnosis and treatment of
chronic diseases across the globe.
ICRDICCT‘25 2025 - INTERNATIONAL CONFERENCE ON RESEARCH AND DEVELOPMENT IN INFORMATION,
COMMUNICATION, AND COMPUTING TECHNOLOGIES
334

REFERENCES
Abdollahi, J., Nouri-Moghaddam, B., & Ghazanfari, M.
(2021). Deep neural network-based ensemble learning
algorithms for the healthcare system (diagnosis of
chronic diseases).arXiv. https://arxiv.org/abs/2103.08
182 arXiv..
Ahmad, S. A., Shahid, M. U., Abdullah, A., Hashmat, I., &
Farooq, M. (2025). An explainable disease surveillance
system for early prediction of multiple chronic diseases.
arXiv. https://arxiv.org/abs/2501.15969
Dyoub, A., & Letteri, I. (2023). Dataset optimization for
chronic disease prediction with bio-inspired feature
selection. arXiv. https://arxiv.org/abs/2401.05380
Elsayed, N., ElSayed, Z., & Ozer, M. (2022). Early stage
diabetes prediction via extreme learning machine.
arXiv. https://arxiv.org/abs/2202.11216
Islam, M. A., Yeasmin, S., Hosen, A., Vanu, N., Riipa, M.
B., Tasnim, A. F., & Nilima, S. I. (2025). Harnessing
predictive analytics: The role of machine learning in
early disease detection and healthcare optimization.
Journal of Ecohumanism, 4(3), 312–321.
https://doi.org/10.62754/joe.v4i3.6642
Mulakala, S. V., Neeharika, G., Kumar, P. V., & Kiran, A.
B. (2025). Chronic diseases prediction using ML.
arXiv. https://arxiv.org/abs/2502.10481
Ola, T. E. (2023). Predictive analytics for early detection of
chronic diseases. Predictive Analytics Application.
https://www.researchgate.net/publication/379269193_
Predictive_Analytics_for_Early_Detection_of_Chroni
c_Diseases
Saria, S., Rajani, A. K., Gould, J., Koller, D., & Penn, A. A.
(2010). Integration of early physiological responses
predicts later illness severity in preterm infants. Science
Translational Medicine, 2(48), 48ra65.
https://doi.org/10.1126/scitranslmed.3001304
Wikipedia
Saria, S., Rajani, A. K., Gould, J., Koller, D., & Penn, A. A.
(2010). Integration of early physiological responses
predicts later illness severity in preterm infants. Science
Translational Medicine, 2(48), 48ra65.
https://doi.org/10.1126/scitranslmed.3001304
Saria, S., Rajani, A. K., Gould, J., Koller, D., & Penn, A. A.
(2010). Integration of early physiological responses
predicts later illness severity in preterm infants. Science
Translational Medicine, 2(48), 48ra65.
https://doi.org/10.1126/scitranslmed.3001304
Saria, S., Rajani, A. K., Gould, J., Koller, D., & Penn, A. A.
(2010). Integration of early physiological responses
predicts later illness severity in preterm infants. Science
Translational Medicine, 2(48), 48ra65.
https://doi.org/10.1126/scitranslmed.3001304
Saria, S., Rajani, A. K., Gould, J., Koller, D., & Penn, A. A.
(2010). Integration of early physiological responses
predicts later illness severity in preterm infants. Science
Translational Medicine, 2(48), 48ra65.
https://doi.org/10.
Saria, S., Rajani, A. K., Gould, J., Koller, D., & Penn, A. A.
(2010). Integration of early physiological responses
predicts later illness severity in preterm infants. Science
Translational Medicine, 2(48), 48ra65.
https://doi.org/10.1126/scitranslmed.3001304
Saria, S., Rajani, A. K., Gould, J., Koller, D., & Penn, A. A.
(2010). Integration of early physiological responses
predicts later illness severity in preterm infants. Science
Translational Medicine, 2(48), 48ra65.
https://doi.org/10.1126/scitranslmed.3001304
Saria, S., Rajani, A. K., Gould, J., Koller, D., & Penn, A. A.
(2010). Integration of early physiological responses
predicts later illness severity in preterm infants. Science
Translational Medicine, 2(48), 48ra65.
https://doi.org/10.1126/scitranslmed.3001304
Saria, S., Rajani, A. K., Gould, J., Koller, D., & Penn, A. A.
(2010). Integration of early physiological responses
predicts later illness severity in preterm infants. Science
Translational Medicine, 2(48), 48ra65.
https://doi.org/10.1126/scitranslmed.3001304
Theerthagiri, P., & Vidya, J. (2021). Cardiovascular disease
prediction using recursive feature elimination and
gradient boosting classification techniques. arXiv.
https://arxiv.org/abs/2106.08889arXiv
Wang, D., Hu, Y., Lee, E. S., Teong, H. H., Lai, R. T. R.,
Hoi, W. H., & Miao, C. (2024). Chronic disease
diagnoses using behavioral data. arXiv.
https://arxiv.org/abs/2410.03386
Advancing Predictive Analytics in Healthcare: Integrating Multimodal Machine Learning for Real-Time Early Detection and Prevention of
Chronic Diseases
335
