
Advances in Chiral Construction and Biochemical Applications of
Chiral Gold Nanoparticles
Jinpeng Huang
1,*
a
and Haowen Zhang
2
b
1
School of Science, Southern University of Science and Technology, Shenzhen, Guangdong, 518055, China
2
School of Materials Science and Engineering, Tianjin University of Technology, Tianjin, 300384, China
*
Keywords: Chiral Gold Nanoparticles, Chiral Origin, Enantiomer Recognition, Asymmetric Catalysis, Tumor Chiral
Phototherapy.
Abstract: Chiral gold nanoparticles (AuNPs) leverage nanoscale geometric asymmetry to amplify chirality-encoded
functions, bridging molecular stereochemistry with plasmonic material properties. Their chirality arises from
helical surface nanostructures or ligand-induced asymmetry, enabling strong chiroptical responses via
plasmon-exciton coupling and circular dichroism enhancement. Bottom-up synthesis strategies—such as
chiral ligand templating (e.g., cysteine, peptides) directing atomic lattice distortion, enantioselective growth
on chiral seeds, and plasmon-guided self-assembly of achiral units into supramolecular helices—allow precise
control over 3D chiral geometries. These architectures interact selectively with biomolecules through
stereocomplementary interfaces, enhancing enantiomer discrimination (e.g., drug detection at ppm levels) and
enabling chirality-dependent cellular uptake. In photomedicine, chiral AuNPs exploit plasmon-enhanced
circularly polarized light absorption for targeted photothermal therapy and ROS generation, while their
asymmetric catalytic sites promote stereoselective synthesis. However, challenges include weak interfacial
chirality transfer, limited dynamic reconfiguration of chiral fields, and signal attenuation in biological
environments. Advancing in situ chiral monitoring (e.g., chiral SERS) and adaptive chirality modulation via
stimuli-responsive ligands could unlock applications like real-time chiral biomarker tracking and logic-gated
nanomedicine. Integrating nanoscale chirality with quantum plasmonics further positions chiral AuNPs as
multifunctional platforms for beyond-molecular stereochemical engineering.
1 INTRODUCTION
Chirality, a geometric property describing non-
superimposable mirror-image configurations,
manifests as left- and right-handed enantiomers. A
striking example is the Chrysina gloriosa beetle,
whose chiral exoskeleton exhibits polarization-
dependent coloration—vivid under left-handed
circularly polarized light but muted under right-
handed polarization—demonstrating chirality-driven
optical anisotropy. While early chirality research
focused on biomacromolecules (proteins/DNA),
advances in nanophotonics have enabled chiral
engineering in inorganic systems. AuNPs overcome
traditional limitations: Unlike biomolecules requiring
millimolar concentrations for detectable circular
dichroism, AuNPs amplify chiroptical signals 10³–
a
https://orcid.org/0009-0001-3280-1692
b
https://orcid.org/0009-0004-4031-9347
10⁶-fold via localized surface plasmon resonance
(LSPR) at 500–1200 nm wavelengths. This LSPR-
enhanced chirality originates from asymmetric
electron oscillations that magnify molecular
asymmetry into macroscopic optical effects,
achieving label-free chiral detection at sub-
nanomolar levels, surpassing conventional
spectroscopy by three orders of magnitude. To
harness this plasmonic amplification, researchers
now engineer programmable chiral architectures
through innovative nanofabrication approaches.
By integrating chiral inducers (e.g., thiolated
peptides, helical polymers) with synthetic
strategies—including seed-mediated chiral growth,
ligand-directed assembly, and plasmon-coupled
superlattice engineering—researchers achieve
precise control over AuNP chirality. These systems
476
Huang, J. and Zhang, H.
Advances in Chiral Construction and Biochemical Applications of Chiral Gold Nanoparticles.
DOI: 10.5220/0013827800004708
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 2nd International Conference on Innovations in Applied Mathematics, Physics, and Astronomy (IAMPA 2025), pages 476-481
ISBN: 978-989-758-774-0
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

unlock transformative applications spanning
enantioselective recognition through differential
molecular adsorption, asymmetric photocatalysis
mediated by spin-polarized hot carrier dynamics, and
chirality-programmed tumor therapies that synergize
circularly polarized light penetration with
stereospecific immune modulation, each leveraging
nanoscale chiral topology to manipulate light-matter
interactions at subwavelength scales.
Recent advances in chiral AuNP synthesis have
enabled precise control over geometric
configurations and chiroptical properties through
chiral inducers and innovative strategies like seed-
mediated chiral growth, ligand-directed assembly,
and plasmon-coupled superlattice engineering. These
methods yield helical, twisted, or chiral-groove
nanostructures with enhanced circular dichroism (g-
factors: 0.1–0.3). Leveraging LSPR, chiral AuNPs
amplify optical signals by 10³–10⁵-fold, surpassing
traditional molecular chirality detection limits.
Applications include enantioselective recognition via
differential molecular adsorption, asymmetric
photocatalysis with spin-polarized hot carriers (85%
ee in CO₂ reduction), and chirality-dependent
photothermal therapies, achieving >40% tumor
ablation efficiency in vivo. These breakthroughs
highlight chiral AuNPs as transformative tools in
nanophotonics, catalysis, and biomedicine, bridging
fundamental chirality studies to functional device
engineering.
2 CHIRALITY SOURCES OF
CHIRAL GOLD
NANOPARTICLES
2.1 Ligand-Induced Chirality
Surface plasmon-coupled chiral enhancement has
emerged as a transformative strategy for amplifying
chiral signatures and shifting them into the visible
spectral region. Studies have elucidated that intimate
coupling between metal nanoparticles and chiral
biomolecules enables UV chiral signatures to couple
with LSPR frequencies (Zhang & Govorov, 2013).
This phenomenon arises from "hotspot" regions
between nanoparticles, which amplify local electric
fields through plasmonic coupling. Building on this,
Kneer et al. (2018) positioned DNA between
gold/silver nanoparticles (spherical/rod-shaped) and
used computational modeling to confirm that hotspot-
mediated field enhancement in assemblies can
amplify molecular chiral signals by orders of
magnitude compared to single particles. The study
highlighted how nanoparticle morphology and
spacing modulate plasmonic coupling and chiral
signal strength (Figure 1 a-b).
Zhang et al. (2023) reported chiral signal transfer
from thiol molecules to gold nanorod assemblies by
incorporating chiral thiol molecules into side-by-side
assemblies of achiral gold nanorods. Their study
revealed that while chiral thiol molecules played a
critical role in generating chiral signals in the
assemblies, the achiral CTAB ligands on gold
nanorod surfaces also exerted significant influence.
The formation of dense chiral ligand shells was found
to facilitate efficient chiral transfer from adsorbed
thiol molecules to the nanorod assemblies, enabling
circular dichroism (CD) signal amplification. This
work provided guidelines for chiral signal
enhancement through manipulating achiral surface
ligands.
Ligand-induced chirality represents a classic
approach for transferring molecular chirality to the
nanoscale and remains the most widely used method
for constructing chiral nanoparticles. Such chiral
nanomaterials have found extensive applications in
chiral sensing, detection, and biomedical research.
However, nanoparticles prepared with this method
generally exhibit low anisotropy factors and pose
challenges for characterization by electron
microscopy, limiting mechanistic understanding of
their chiral origination.
2.2 Colloidal Self-Assembly
Plasmonic CD signals in the visible range can be
induced by coupling chiral small molecules with gold
nanoparticles; however, this methodology encounters
significant limitations. Specifically, the strategy
demands both the well-ordered orientation of chiral
ligands on nanoparticle surfaces and spectral overlap
between molecular vibrations and plasmonic modes,
which often results in suboptimal coupling efficiency
and correspondingly weak CD responses. Hopefully,
Chiral ligand-directed self-assembly yields rather
enhanced CD-active chiral nanostructures.
In Fei's (2019) experiment of modifying AuNP
colloidal solutions with NaC, due to the amphiphilic
nature of NaC molecules, the NaC molecules
adsorbed on the surface of gold nanoparticles can
interact with those on the surface of adjacent
nanoparticles through hydrophobic forces leading to
the aggregation of nanoparticles. It is noteworthy that
when the concentration of NaC is relatively high (24
mM), the generated AuNPs tend to be smaller. Sun et
al. (2016) prepared a chiral gold nanohybrid dimer
Advances in Chiral Construction and Biochemical Applications of Chiral Gold Nanoparticles
477
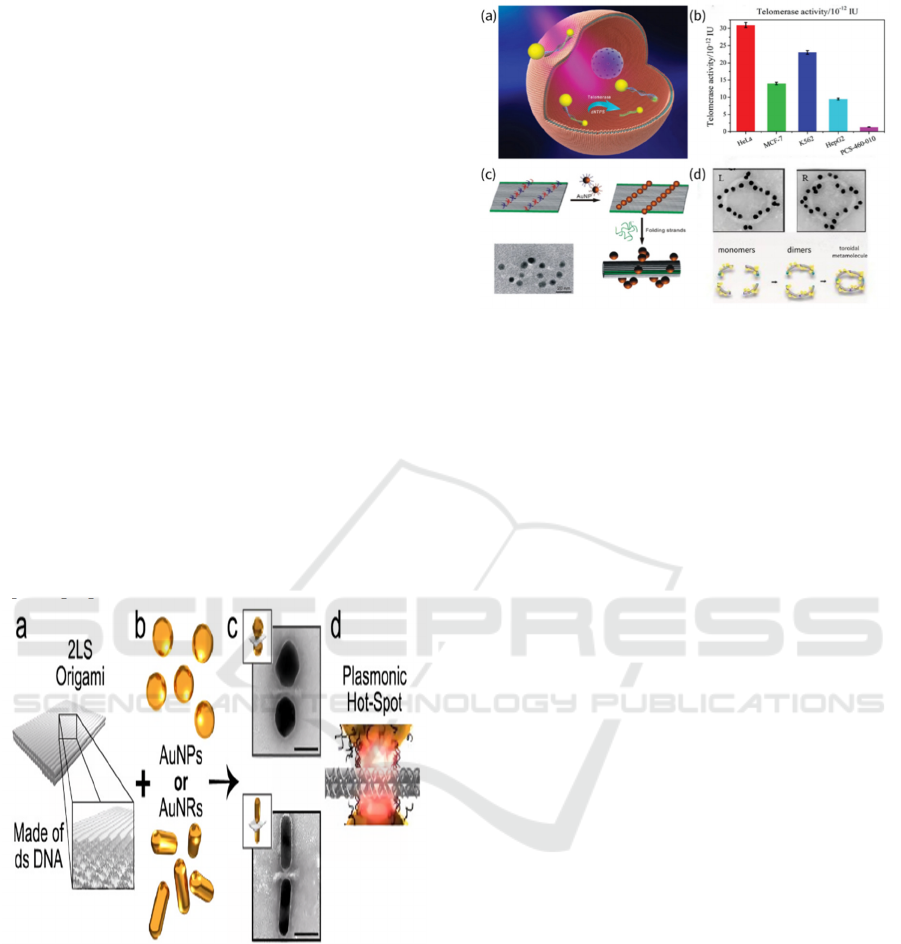
architecture. This dimeric system consists of two gold
nano-ellipsoids: one functionalized with a telomerase
primer and the other conjugated with linker-
complementary DNA strands. These nanostructures
self-assemble into a scissor-shaped configuration
under linker DNA mediation. The resulting dimer
exhibits robust chiral optical responses in the visible
spectrum, enabling intracellular telomerase activity
quantification (Figure 2a-b).
In addition, DNA Origami, proposed by
Rothemund (2006), introduced a novel self-assembly
approach. And Liu and Ding (2012) further
developed adjustable helical structures by curving 2D
DNA origami into nanotubes and positioning gold
nanospheres (Figure 2c). Liu et al. (2016) created
circular 3D assemblies by combining four curved
DNA origami units, resulting in left/right-handed
helices with 24 spirally arranged particles (Figure 2
d). These multi-unit constructs generated stronger CD
responses than monomeric counterparts.
By transforming disordered ligand-NP systems
into structured, highly active chiral plasmonic
platforms, colloidal self-assembly paves the way for
advanced applications in ultrasensitive sensing,
nanophotonics, and beyond, where tunable and robust
optical properties are critical.
Figure 1: (Color online) Chiral induction by ligands. (a) An
origami sheet composed of multiple parallel b-type DNAs;
(b) DNA-functionalized gold nanospheres or nanorods; (c)
TEM images of dimer structures of gold nanospheres or
nanorods; (d) Schematic representation of a "hot spot"
region between two nanoparticles generating a strong
electric field (Kneer et al., 2018).
Figure 2: (Color online) Chiral induction of AuNPs by self-
assembly. (a) Schematic of intracellular quantitative
detection of telomerase using gold heterodimers. (b)
Corresponding data of telomerase activity measured in
various cells with this nanoplatform. (Sun et al., 2016) (c)
DNA origami nanoparticle helices. (Liu and Ding, 2012)
(d) TEM diagram of the ring structure of left and right-
handed nanoparticles. (Liu et al., 2016)
2.3 Intrinsic Chiral Crystal Structure
Despite significant advancements in chiral gold
nanomaterials through coupling and self-assembly
strategies, persistent challenges—including
inadequate chiral induction and structural instability,
particularly in complex DNA-templated protocols—
necessitate alternative approaches. Direct synthesis of
inherently chiral AuNPs with precisely controlled
morphologies has emerged as a critical frontier to
address these limitations.
Innovative work by Wang et al. (2014) revealed
that asymmetric chiral cores can exist in gold clusters,
distinct from symmetric cores stabilized by chiral
ligand shells. Using a tetradentate organophosphorus
ligand (PP3), they synthesized chiral Au20
nanoclusters via direct reduction. Structural analysis
revealed the Au20 core comprises two subclusters: an
icosahedral Au13 (orange spheres) and a Y-shaped
Au7 (green spheres) in Figure 3 (a). The helical
arrangement of the Y-shaped Au7 subcluster imparts
C3 symmetry to the entire Au20 core, providing
definitive structural evidence for inherently chiral
gold clusters. The multidentate phosphine ligands
enhance cluster stability through chelation, opening a
path to design gold clusters with purely inorganic
chiral cores for applications in catalysis and sensing.
Lee et al. (2018) demonstrated another
breakthrough by fabricating chiral propeller-shaped
gold nanoparticles (with g-factors ≈ 0.2) using
glutathione-mediated growth on octahedral seeds
(Figure 3 b-c). The particle's slit-rich architecture
IAMPA 2025 - The International Conference on Innovations in Applied Mathematics, Physics, and Astronomy
478

generates intense electromagnetic coupling between
adjacent nanosheets, amplifying CD responses. This
strategy induces helical morphologies in
nanoparticles, presenting a scalable method for 3D
chiral structures with high optical activity.
Figure 3: (Color online) AuNPs with structural chirality.
(Wang et al., 2014) (a) Au20 model: The purple spheres are
phosphorus atoms, and the gray spheres are carbon atoms.
(b-c) SEM images and geometric models. (Lee et al., 2018).
3 ADVANCES IN THE
APPLICATION OF CHIRAL
AUNPS
3.1 Enantiomer Recognition
Conventional molecular chirality exhibits suboptimal
signal intensity in the visible spectral region,
hindering its practical implementation in chiral
sensing applications. However, the synergistic
combination of chiral molecules with plasmonic
AuNPs generates enhanced chiral signals in the
visible range, which are highly responsive to
geometric variations of nanoparticles and
environmental changes. This property enables
development of ultrasensitive chiral biosensors.
Kang’s group (2010) fabricated an
electrochemical sensor using penicillamine-
functionalized AuNPs (Pen-AuNPs) for
enantioselective recognition of 3,4-
dihydroxyphenylalanine (DONP). Small-sized Pen-
Au NPs with a single enantiomeric ligand only
promote the redox reaction of one enantiomer of
DONP. The study also found that the size of Pen-Au
NPs affects their enantioselectivity performance.
Specifically, smaller Pen-Au NPs show better
selectivity for L- and D-DOPA.
Tang et al. (2025) utilized L-cysteine-
functionalized AuNPs as colorimetric probes for
rapid R/S-phenylalaninol and R/S-ibuprofen
detection, which could develop into purity detection
so as to ensure drug therapeutic effects while limiting
side effects. And it is assumed that when chiral
AuNPs recognize enantiomers, non-covalent
interactions induce energy resonance transfer on the
surface of nanomaterials, leading to their aggregation
and color changes. Li et al. (2022) synthesized helical
gold nanoparticles with intrinsic chirality and
constructed a chiral sensor for discriminating L-
tyrosine from D-tyrosine. Notably, differential pulse
voltammetry revealed nearly sixfold differences in
peak currents between tyrosine enantiomers, enabling
accurate determination of enantiomeric purity and
composition. The chiral electrode developed also
enabled quantification of L-tyrosine in
pharmaceutical capsules, opening new avenues for
chiral enantiomer recognition in biomedical and
pharmaceutical fields.
3.2 Asymmetric Catalysis
Chiral metallic nanomaterials exhibit unique size-
dependent optical and catalytic properties distinct
from molecular-scale chirality, offering
transformative opportunities in asymmetric organic
synthesis.
Cui (2015) advanced chiral nanocatalysis by
incorporating a cytosine-derived amphiphilic ligand
(C₈H₄Cyt) into AuNP systems. These chiral AuNPs
displayed superior catalytic activity for p-nitrophenol
reduction by sodium borohydride at ≥ 30 °C,
outperforming conventional AuNPs in both stability
and recyclability. The chiral architecture mitigated
aggregation, maintaining activity even after long-
term storage. Notably, larger chiral nanomaterials
experienced less activity due to decreased accessible
reaction sites, highlighting needs of finer AuNPs
producing method in plasmonic catalysis.
Tan and colleagues (2022) made use of chiral
Au@CdS core-shell nanostructures for asymmetric
photocatalytic water splitting while from Negrín-
Montecelo group (2022), chiral Au and TiO_2
combination irradiated with polarization-matched
circularly polarized light (CPL) exhibit
approximately 2.9-fold higher catalytic efficiency
compared to mismatched polarization conditions,
demonstrating strong selectivity for enantiomeric
interactions. (Figure 4a-b).
Figure 4: (a) Chirality-dependent hydrogen production
efficiency in water splitting. (Tan et al., 2022) (b) Au and
TiO2 for chiral photocatalytic applications. (Negrín-
Montecelo et al., 2022)
Advances in Chiral Construction and Biochemical Applications of Chiral Gold Nanoparticles
479
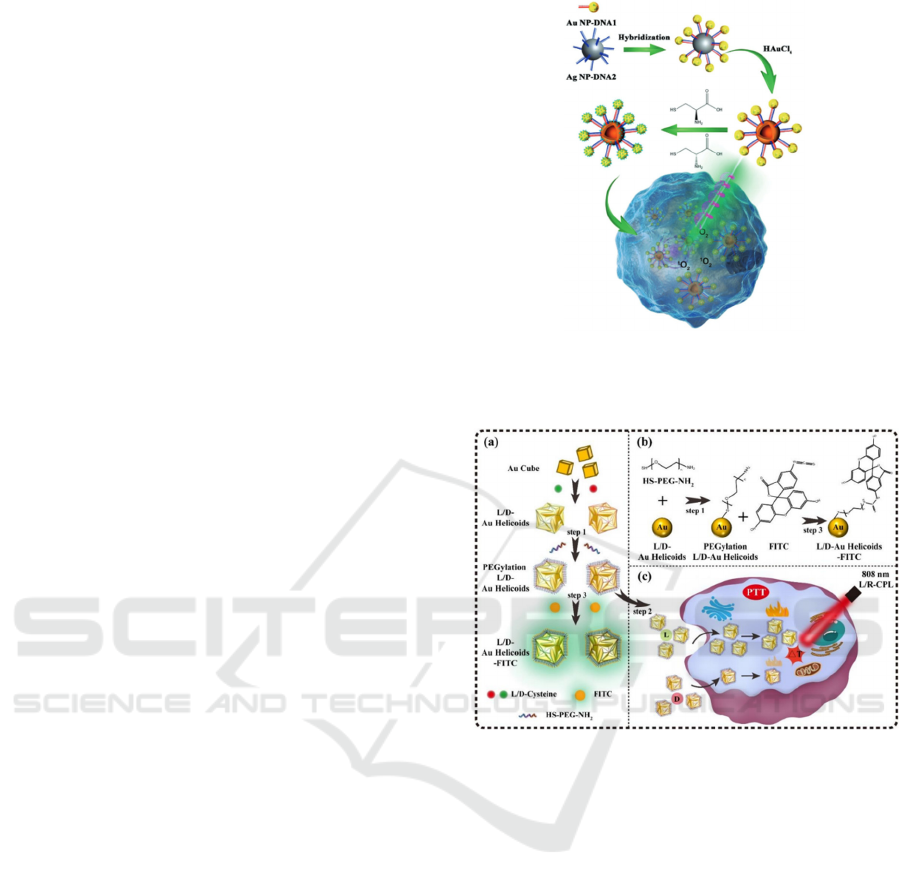
3.3 Tumor Photothermal Therapy
Accumulating evidence highlights the profound
impact of chirality on nanomaterial performance in
biological systems. In tumor phototherapy using
chiral noble-metal nanostructures, Gao et al. (2017)
engineered DNA-templated self-assembled gold
core-shell satellite nanostructures (Figure 5). Under
circularly polarized light irradiation, these constructs
exhibited enhanced reactive oxygen species (ROS)
generation (roughly two-fold higher than linear
polarization), underscoring chirality's role in
optimizing photodynamic therapy. Additionally,
chiral architecture modulates nanomaterial
biocompatibility and downstream signaling pathways,
overcoming challenges in cancer treatment. Xu et al.
(2022) synthesized AuNPs with record-high g-factors
(0.4), demonstrating chiral-dependent immune
activation: left-handed NPs induced stronger
dendritic cell maturation and pro-inflammatory
cytokine expression compared to right-handed
counterparts. This disparity arises from differential
binding affinity to G-protein-coupled receptors and
accelerated endocytosis rates for left-handed NPs.
In the realm of chiral materials, a larger g-factor
implies less energy dissipation of light energy,
signifying a greater capacity for controlling light
polarization. As depicted in Figure 6, the chiral Au
Helicoids nanoparticles prepared by Weng (2023)
possess a wide-ranging optical absorption spanning
from 550 to 1100 nm and a relatively elevated g-
factor. When excited by 808-nm near-infrared CPL,
the photothermal efficiency of the chiral Au helicoid
nanoparticles demonstrates a distinct chiral disparity.
And the disparity primarily stems from HeLa cells'
higher endocytosis efficiency for L-Au Helicoids
nanoparticles compared to D-Au Helicoids
nanoparticles. In contrast to traditional photothermal
therapy, which frequently inflicts damage on healthy
cells due to the employment of high-power lasers or
prolonged radiation, chiral photothermal therapy can
manifest efficient light-conversion properties and
selective light-absorption characteristics within the
near-infrared region. This can substantially minimize
the damage to normal cells (with a cell survival rate
of around 90.00%) and enhance the inactivation
efficiency of cancer cells (94.95%), which means it
can be harnessed as a secure photothermal conversion
agent for the research on the photothermal
inactivation of tumor cells.
Figure 5: (Color online) Schematic diagram of self-
assembled shell satellite nanostructures as chiral
photodynamic therapeutic agents. (Gao et al., 2017)
Figure 6: (Color online) Schematic diagram of surface
modification of L-/D-Au Helicoids nanoparticles and their
photothermal effects under CPL excitation at 808nm.
(Weng, 2023)
4 CONCLUSION
This review systematically evaluates the synthetic
strategies and emerging applications of AuNPs.
Chiral architectures in AuNPs can arise from
template-directed growth (e.g., helical seed-mediated
synthesis), molecular imprinting with chiral ligands,
or supramolecular self-assembly. These design
principles enable advanced applications in
stereoselective sensing, asymmetric catalysis, and
synergistic photothermal cancer therapy.
Notwithstanding significant progress in chiral
AuNP synthesis and bioanalytical applications,
critical challenges persist. Current methods for
producing AuNPs with uniform chiral configurations
and robust chiroptical activity remain inefficient and
IAMPA 2025 - The International Conference on Innovations in Applied Mathematics, Physics, and Astronomy
480

scalable, while most plasmonic responses occur in the
visible spectrum, limiting deep-tissue penetration in
biomedicine. Additionally, comprehensive
biocompatibility assessments—encompassing long-
term stability, toxicity profiles, and clearance
mechanisms—are underdeveloped. In vitro models
often fail to recapitulate in vivo complexities,
necessitating sophisticated in vivo studies to
characterize size/shape-dependent biodistribution
and enantioselective biological effects.
To address these challenges, future research
should focus on developing Chiral AuNPs with
enhanced near-infrared (NIR) chiroptical activity,
advancing surface functionalization for non-toxicity
and targeted specificity, elucidating cellular uptake
mechanisms, and fostering interdisciplinary
collaboration to bridge fundamental synthesis with
translational precision medicine applications. Despite
these hurdles, the dynamic field of Chiral AuNP
research continues to hold immense potential for
breakthroughs in biochemical research and clinical
practice.
ACKNOWLEDGEMENTS
All the authors contributed equally and their names
were listed in alphabetical order.
REFERENCES
Cui, J., 2015. Preparation and catalytic properties of chiral
gold nanomaterials. Master’s thesis, Yangzhou
University.
Gao, F., Sun, M., Ma, W., Wu, X., Liu, L., Kuang, H., Xu,
C., 2017. A singlet oxygen generating agent by
chirality‐dependent plasmonic shell‐satellite
nanoassembly. Advanced Materials, 29(18): 1606864.
Kang, Y. J., Oh, J. W., Kim, Y. R., Kim, J. S., Kim, H.,
2010. Chiral gold nanoparticle-based electrochemical
sensor for enantioselective recognition of 3,4-
dihydroxyphenylalanine. Chemical Communications,
46(31): 5665–5667.
Kneer, L. M., Roller, E. M., Besteiro, L. V., Schreiber, R.,
Govorov, A. O., Liedl, T., 2018. Circular dichroism of
chiral molecules in DNA-assembled plasmonic
hotspots. ACS Nano, 12(9): 9110–9115.
Lee, H. E., Ahn, H. Y., Mun, J., Lee, Y. Y., Kim, M., Cho,
N. H., Nam, K. T., 2018. Amino-acid- and peptide-
directed synthesis of chiral plasmonic gold
nanoparticles. Nature, 556(7701): 360–365.
Li, F., Wu, F., Luan, X., Yuan, Y., Zhang, L., Xu, G., Niu,
W., 2022. Highly enantioselective electrochemical
sensing based on helicoid Au nanoparticles with
intrinsic chirality. Sensors and Actuators B: Chemical,
362: 131757.
Liu, N., Ding, B., 2012. Rolling up gold nanoparticle-
dressed DNA origami into three-dimensional
plasmonic chiral nanostructures.
Mo, R., Xu, L., Fu, P., Wu, X., Kuang, H., Liu, L., Xu, C.,
2016. Scissor-like chiral metamolecules for probing
intracellular telomerase activity. Advanced Functional
Materials, 26(40): 7352–7358.
Negrín-Montecelo, Y., Movsesyan, A., Gao, J., Burger, S.,
Wang, Z. M., Nlate, S., Correa-Duarte, M. A., 2022.
Chiral generation of hot carriers for polarization-
sensitive plasmonic photocatalysis. Journal of the
American Chemical Society, 144(4): 1663–1671.
Rothemund, P. W., 2006. Folding DNA to create nanoscale
shapes and patterns. Nature, 440(7082): 297–302.
Sun, X., Wang, X., Wang, W., Sun, M., Choi, W. J., Kim,
J. Y., Xu, C., 2022. Enantiomer-dependent
immunological response to chiral nanoparticles. Nature,
601(7893): 366–373.
Tan, L., Yu, S. J., Jin, Y., Li, J., Wang, P. P., 2022.
Inorganic chiral hybrid nanostructures for tailored
chiroptics and chirality-dependent photocatalysis.
Angewandte Chemie International Edition, 61(24):
e202112400.
Urban, M. J., Dutta, P. K., Wang, P., Duan, X., Shen, X.,
Ding, B., Liu, N., 2016. Plasmonic toroidal
metamolecules assembled by DNA origami. Journal of
the American Chemical Society, 138(17): 5495–5498.
Weng, X. K., Yuan, S. F., Lin, Z. W., Wang, Q. M., 2014.
A chiral gold nanocluster Au₂₀ protected by tetradentate
phosphine ligands. Angewandte Chemie, 126(11):
2967–2970.
Wang, Y. L., 2023. Synthesis and near-infrared
photothermal properties of chiral gold nanostructures.
Master’s thesis, Henan University.
Xu, C., 2025. Colorimetric detection of drug molecule
enantiomers using chiral gold nanoprobe. Journal of
Chemical Education of Higher Institutions
, 46(2): 78–
85.
Zhang, C., Gao, X., Li, H., Ji, Y., Cai, R., Hu, Z., Wu, X.,
2023. Regulation of chirality transfer and amplification
from chiral cysteine to gold nanorod assemblies using
nonchiral surface ligands. Advanced Optical Materials,
11(18): 2202804.
Zhang, H., Govorov, A. O., 2013. Giant circular dichroism
of a molecule in a region of strong plasmon resonances
between two neighboring gold nanocrystals. Physical
Review B: Condensed Matter and Materials Physics,
87(7): 075410.
Advances in Chiral Construction and Biochemical Applications of Chiral Gold Nanoparticles
481
