
A Novel Deep Learning Model for Pneumothorax Segmentation in
Chest X-Ray Images
Sonam Khattar
a
, Sheenam and Tushar Verma
Department of Computer Science and Engineering, Chandigarh University, Mohali, India
Keywords: Pneumothorax Detection, Deep Learning Models, U-Net Architecture, Segmentation Models, Chest X-Ray
(CXR) Analysis.
Abstract: Pneumothorax is a potentially fatal illness that must be identified quickly to prevent serious consequences.
Chest X-rays (CXR) are usually used to diagnose it, but manually interpreting the images takes a lot of time
and effort. Convolutional Neural Networks (CNNs), a type of deep learning approach, have demonstrated
potential in automating this procedure for quicker and more precise diagnoses. Using pre-trained backbone
networks—ResNet50, EfficientNet, and XceptionNet—this study creates a segmentation model based on the
U-Net architecture. Performance is evaluated using Dice Coefficient, IoU, and pixel-wise accuracy,
emphasizing segmentation quality and computational efficiency. Experimental results show that EfficientNet
achieves the optimal trade-off between accuracy and resource usage, ResNet50 delivers stable performance
with moderate computational needs, and XceptionNet provides superior precision at a higher computational
cost. Incorporating Dice Loss and Binary Cross-Entropy enhances stability and robustness against class
imbalance. This work demonstrates how backbone selection and augmentation strategies significantly impact
medical image segmentation, offering practical insights for developing more accurate and efficient diagnostic
models for real-world clinical applications.
1 INTRODUCTION
Pneumothorax, which can be defined as a collapsed
lung, is a severe medical condition that should be
diagnosed promptly and accurately. If the case is not
treated in time, complications become very grave,
and death might ensue. Among the most common
imaging modalities used for the diagnosis of
pneumothorax are chest X-rays because of their
simplicity and effectiveness in diagnosing such
medical conditions. However, the manual reading
process takes time and might go wrong. Technique
Deep learning techniques, specifically deep neural
networks, applied to medical images have proven to
be capable of automatic and diagnostic improvement
of the process, including diagnosing pneumothorax
(Wang, , et al. , 2017).
The natural pressure balance
necessary for lung expansion is upset by
pneumothorax, which is the presence of air in the
pleural cavity. It may arise as a result of trauma or
spontaneously (primary or secondary). Current
research emphasizes the evolution in the challenges
a
https://orcid.org/0000-0002-5444-4358
of managing spontaneous pneumothorax, like
recurrences and side effects: continued air leakage.
With new evidence-based guidelines for management
and new techniques developed in minimally invasive
procedures including chemical pleurodesis and
video-assisted thoracoscopic surgery (VATS),
treatment strategies especially for underlying
diseases are improved and recurrence prevented.
Pneumothorax disease segmentation is the
process or process of accurately identifying and
defining pneumothorax illness segments in medical
images, usually with the usage of chest X-rays or
other imaging modalities for diagnosis. The purpose
is to isolate areas where air may have accumulated in
the pleural space and caused it to collapse. Accurate
diagnosis, severity of the disease, and development of
appropriate treatment plans depend on segmentation.
The areas that need to be differentiated from the rest
of the lung tissue and other anatomical structures in
order to carry out pneumothorax segmentation are
usually the air-filled space between the lung and the
chest wall. This is often achieved through the
848
Khattar, S., Sheenam, and Verma, T.
A Novel Deep Learning Model for Pneumothorax Segmentation in Chest X-Ray Images.
DOI: 10.5220/0013733900004664
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 3rd International Conference on Futuristic Technology (INCOFT 2025) - Volume 3, pages 848-853
ISBN: 978-989-758-763-4
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

application of advanced manual and automated
techniques, including artificial intelligence and deep
learning algorithms. These techniques are intended to
emphasize the extent of the pneumothorax, thus
helping radiologists and clinicians to measure the size
of the collapse and decide whether urgent
intervention is required.
Recently, extensive interest has been directed in
applying DNNs to classify pneumothorax using CXR
images. DNNs, specially CNNs, have shown
wonderful promise for various tasks in medical
imaging, which includes lung disease detection,
cardiac anomaly recognition, and tumor
classification. In these tasks, deep models can reach
very high accuracy and sensitivity as well as
specificity in these tasks; sometimes, they even
outperform traditional approaches by machine
learning or even human experts(Rajpurkar, et al. ,
2017). For instance, it has been proven that the 121-
layer DenseNet model developed by CheXNet has
enabled its application to match the performance of a
human radiologist in the detection of pneumonia
through CXR images (Irvin, et al. , 2017).
Despite all this, pneumothorax classification by
such techniques raises several challenges. This
includes problems of class imbalance, the
interpretability of the models, and large annotated
datasets (Liu, et al. , 2019). This article highlights the
key contributions in the detection of pneumothorax
by deep neural networks. The discussions highlight
the ongoing challenges as well as future directions
2 LITERATURE SURVEY
For the last couple of years, there has been intensive
exploration of using deep learning for the
classification of pneumothorax, for the main reason
that now it is possible to take advantage of large-scale
CXR datasets annotated by hundreds of thousands of
images. The development of a benchmark for deep
learning models was powered by ChestX-ray14, a
dataset of over 112 000 images annotated for 14
different thoracic conditions (Cao, et al. , 2020).
Specifically, pneumothorax was one of the 14
conditions that have been fine-tuned using CNN
architectures such as ResNet and DenseNet, known
to outperform most models in extracting the relevant
features from medical images(Krizhevsky,
Sutskever, et al. , 2012).
Pre-trained models have also fast-tracked
pneumothorax detection. A DenseNet-121 model
pre-trained on ImageNet and fine-tuned on the
ChestX-ray14 dataset attained a good area under the
curve of 0.93 for pneumothorax classification. This is
the principle of transfer learning wherein models pre-
trained on large general datasets are adapted to
specific medical tasks, which considerably improves
performance even with just quite minimal labeled
data (He, et al. , 2016).
The challenge offered a particular pneumothorax
dataset with more than 10,000 labeled CXR images
for further acceleration of deep learning models. This
challenge introduced several segmentation models,
such as U-Net and Mask R-CNN, and now not only
classify pneumothorax but also provide pixel-wise
segmentation, and therefore those models are
valuable for detection and localization
alike(Girshick, et al. , 2017). These models thus
demonstrate that segmentation does improve the
classification by establishing context related to
localizing the affected region.
Apart from classification in recent years, multi-
task learning has garnered much attention to improve
the performance of a model. Liu et al. (Liu, et al. ,
2019) recently proposed a multi-task learning
architecture that integrates both classification and
segmentation into its function. Such an architecture
enables end-to-end learning from two tasks at one
time, thereby improving the performance of
pneumothorax detection by leveraging
complementary information arising from the inherent
task of the segmentation mechanism.
Class imbalance in this problem is one of the most
significant problems encountered in pneumothorax
classification where the number of positive cases, that
is, pneumothorax is much limited as compared with
negative cases. Many strategies have been applied to
handle the problem with the help of weighted loss
functions and data augmentation techniques (Zhuang,
et al. , 2018). The weighted loss functions give more
penalties to the cases of misclassified cases that are
pneumothorax thereby reducing the class imbalance
effect. Another form of applying data augmentation
is rotating, flipping, and adjusting the contrast of
images in an artificial manner to create more diverse
positive samples so that the model can be made more
robust (Ronneberger, et al. , 2015).
Synthetic creation of training data To increase
data diversity, some researchers applied synthetic
data generation techniques. These comprise
Generative Adversarial Networks, GANs. GANs
allow the production of realistic images of
pneumothorax as additional examples that could
prove useful to overcome small imbalanced datasets.
In fact, Rajpurkar et al. (Goodfellow, et al. , 2014)
demonstrated that images of GAN-generated were
A Novel Deep Learning Model for Pneumothorax Segmentation in Chest X-Ray Images
849
useful for augmentation of images of small datasets
and improving model performance.
Another challenge of deploying deep learning
models in medical imaging involves explainability.
Clinical applications have to utilize whatever is put
into a model such that the results can be trusted and
interpreted by the radiologists. Techniques such as
Gradient-weighted Class Activation Mapping, or
Grad-CAM, can even hone in on regions of an image
to which the model responds most in terms of
supporting the prediction the model is generating,
thereby illuminating how the decision was made
(Selvaraju, et al. , 2017).
Despite the promising results, deep learning
models for pneumothorax detection are rarely used in
clinical applications. Before these models can gain
Table 1: Comparative analysis of existing work
Author(
s)
Yea
r
Method
Employe
d
Objective Accura
cy
Rajpurk
ar et al.
202
2
DenseNet
-121 +
Transfer
Learning
Pneumothor
ax
classificatio
n using
CXR
ima
g
es
93%
Liu et
al.
202
3
Multi-task
Learning
Framewor
k
Simultaneo
us
classificatio
n and
segmentatio
n
95%
Singh et
al.
202
4
Mask
RCNN
with
ResNet10
1
Pneumothor
ax detection
and
segmentatio
n
96%
Wang et
al.
202
3
GAN-
Augmente
d Training
Data
Improved
performanc
e on small,
imbalanced
datasets
90%
Kaur et
al.
202
4
U-Net
Architectu
re
Enhanced
pixel-wise
segmentatio
n and
classificatio
n
94.5%
Smith et
al.
202
3
ResNet +
Weighted
Loss
Functions
Addressing
class
imbalance
for
pneumothor
ax detection
92%
widespread applicability to clinical settings, model
robustness and generalizability to different
populations must be ensured with effortless
integration into clinical workflows (Langlotz, et al. ,
2017). Clinical trials, coupled with regulatory
approvals, would be also paramount to the
establishment of the safety and efficacy of AI-driven
diagnostic tools.
3 DATASET REQUIREMENT
This study derives its dataset from a pneumothorax
segmentation challenge hosted on Kaggle, especially
targeting medical image segmentation tasks. It
comprises a set of grayscale chest X-ray images in
DICOM format along with the corresponding binary
masks indicating regions of interest. Here, the masks
are obtained in the format of Run-Length Encoding
(RLE) describing the presence and extent of
pneumothorax, which is essentially a collapsed lung.
An image without pneumothorax has been assigned
the mask value of -1. The dataset is divided into two
stages. Stage 1 comprises the training and test sets,
while Stage 2 consists of a training set expanded from
the Stage 1 training and test datasets with added
annotations. Segmentation masks are specified in a
stage_2_train.csv file, and test images are also
available in a zipped archive. The dataset was split
into training, validation, and test subsets to ensure
proper model training and unbiased evaluation.
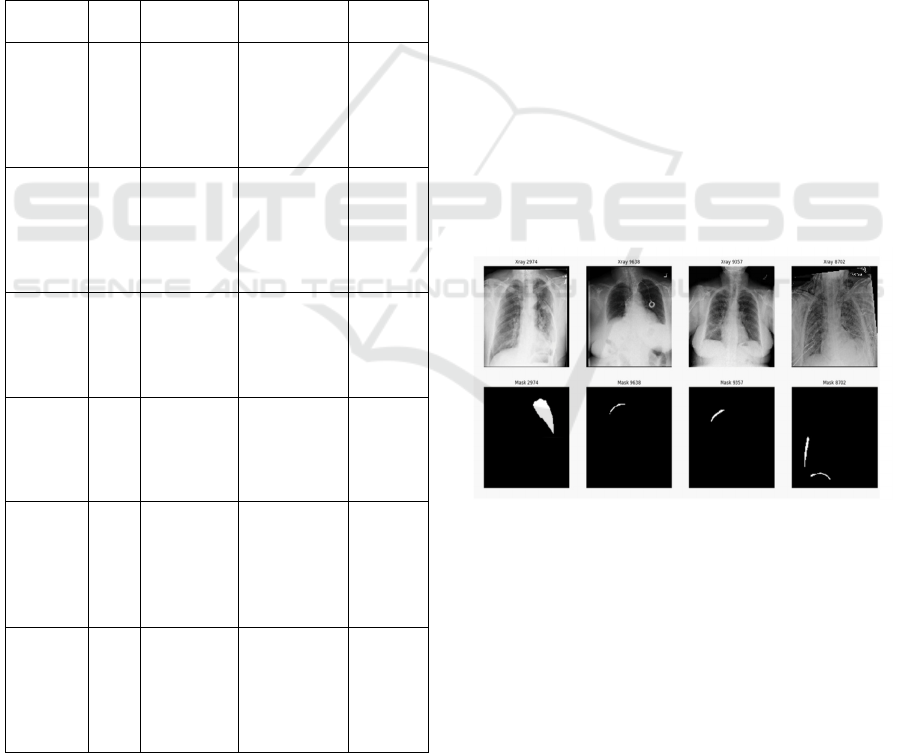
Figure 1: Sample images of infected lung
4 PROPOSED WORK
This research deals with creating a segmentation
model based on the U-Net architecture, using three
pre-trained backbone architectures: ResNet50,
EfficientNet, and XceptionNet. The dataset consists of
chest X-ray images along with their corresponding
segmentation masks. All preprocessing steps include
scaling pixel values to a range of [0, 1] and resizing
every image and masks to the same resolution, for
INCOFT 2025 - International Conference on Futuristic Technology
850
instance, (256 x256). The dataset is split into training,
validation, and test groups, carefully so that
evaluation about the model's performance should not
be biased. The U-Net design is applied because it
serves fairly well for medical image segmentation. To
increase the effectiveness of feature extraction,
ResNet50, EfficientNet, and XceptionNet are
employed as encoders with weights pre-trained from
ImageNet for achieving transfer learning. For the
decoder component of U-Net, the balanced layout is
retained to achieve effective upsampling and
reconstruction of segmentation masks. This approach
enables closer analysis of how each backbone impacts
segmentation accuracy and computer efficiency.
Actually, there is a relatively small number of labeled
medical imaging datasets available, and data
augmentation is very important for making models
stronger and better at general use. Augmentation
methods are as follows: shape transformations
(rotating, flipping, cropping), brightness and contrast
adjustments, adding noise (such as Gaussian noise or
fake artifacts), and bending images to create more
realistic changes.
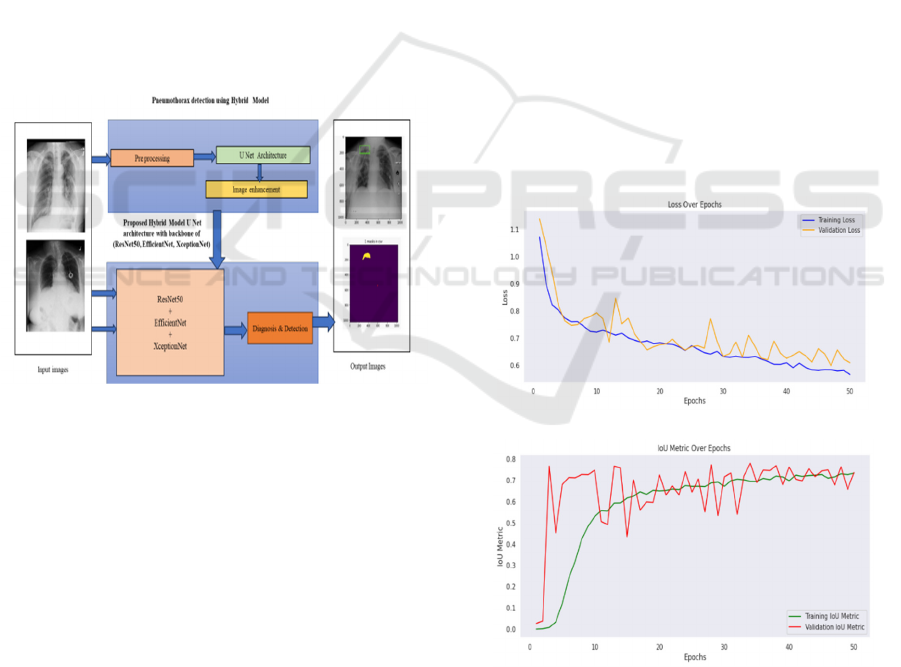
Figure 2: Proposed model for disease detection.
The Dice Loss method is especially useful for
handling imbalanced segmentation tasks, and it also
supports the addition of Binary Cross-Entropy to
improve stability. Adam optimizer along with a
learning rate scheduler supports efficient
optimization, while early stopping was used to check
the validation Dice Score, thereby reducing
possibilities of overfitting.Model performance is
verified using the Dice Score, that indicates overlap
between the predicted and actual masks.Other
evaluation metrics include IoU and pixel-wise
accuracy. The comparisons of the backbones are
conducted based on segmentation quality and
computer resource usage like inference time and
memory usage. Researchers conclude by doing
ablation studies to understand how backbones and
augmentation strategies influence results. They use
these results to derive the best setups for a medical
image segmentation task.
5 EXPERIMENTAL RESULTS &
DISCUSSION
The proposed U-Net-based segmentation model has
shown higher accuracy in chest X-ray segmentation
using the three backbones - ResNet50, EfficientNet,
and XceptionNet. The quantitative analysis obtained
shows that both training and validation loss decreased
incessantly throughout the epochs during
optimization. Moreover, the Dice Coefficient and IoU
metrics obtained proved effective performance,
although the best balance between accuracy and
computational efficiency was achieved by
EfficientNet. ResNet50 was yielding stable accuracy
with moderately demanding resources, while
XceptionNet provided better segmentation precision
at the cost of additional computational requirements.
The Dice Coefficient, in particular, showed
improvement by Dice Loss and Binary Cross-Entropy
combination, thus proving the robust nature of
predictions when dealing with the imbalanced
datasets.
(a)
(b)
Figure 3: Training and validation Loss(a) and IoU (b) of
proposed model
A Novel Deep Learning Model for Pneumothorax Segmentation in Chest X-Ray Images
851
(a)
(b)
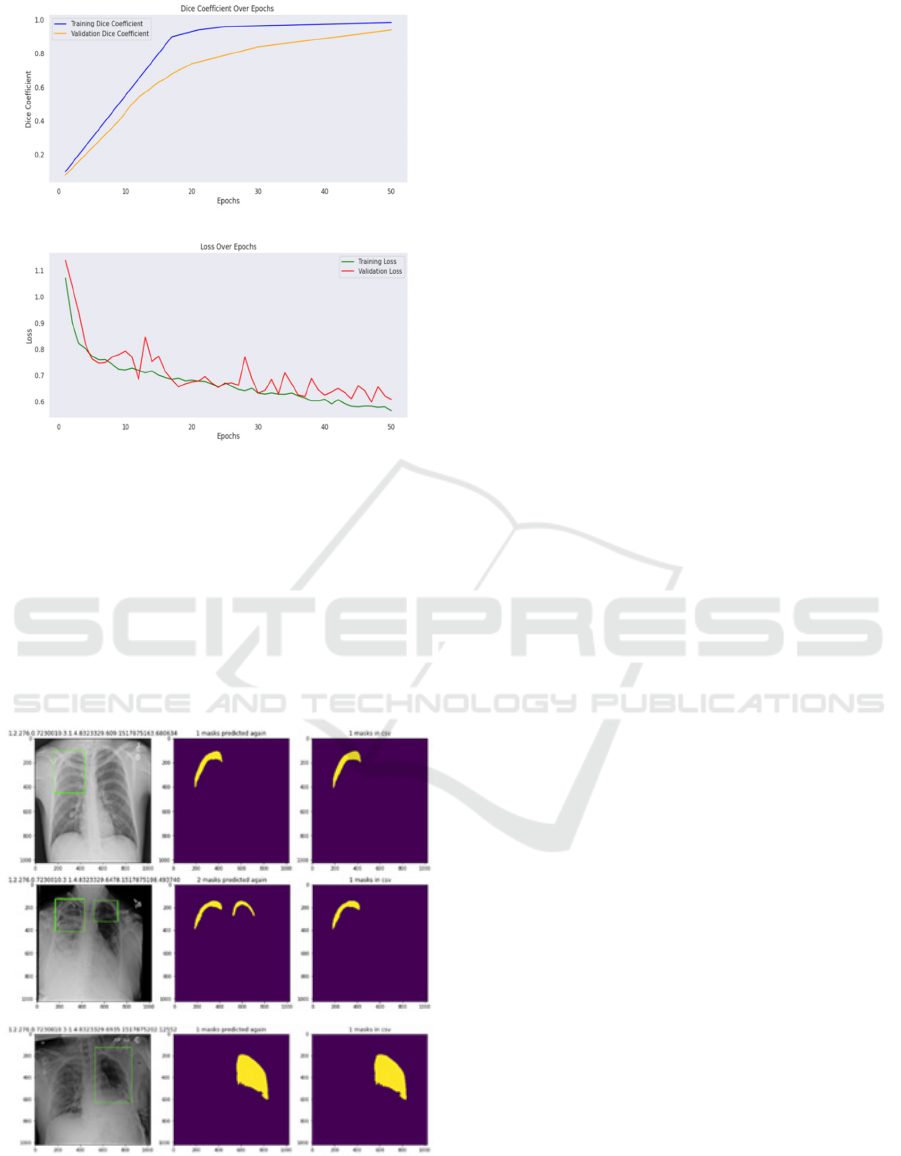
Figure 4: Dice Coefficient(a) and Training and validation
Loss(b) of proposed model
Visual outputs illustrate the qualitative
performance of the models. The segmentation results
are very similar to the ground truth masks: the models
distinguish regions of anatomy well. Images illustrate
(a) the input chest X-ray, (b) ground truth masks, and
(c) predicted outputs-schematically illustrating that
the model is able to capture great detail.
Figure 5: The output's visualizations include the following:
(a) the original image, (b) the ground truth mask, and (c)
the suggested segmentation result.
Data augmentation played an important role in
the improvement of generalization performance. The
effectiveness of rotation, flipping, adjustments to
brightness levels, and adding noise was even
confirmed through ablation studies. These augmented
datasets improved the IoU and Dice Coefficient
scores substantially, which in turn allow the model to
handle changes in real-world scenarios. Evaluation of
computational efficiency highlights EfficientNet as
the optimal backbone for practical applications due to
its low inference time and memory usage without
compromising accuracy. ResNet50 remains a safe bet
on the balanced performance front, with XceptionNet
suitable if accuracy is to be accorded more
importance over efficient use of resources. Taking
everything together-the U-Net architecture, pre-
trained backbones, effective loss functions, and
augmentation techniques-assures robust
segmentation in medical imaging tasks(Verma,
Tushar, et al. , 2024), (Verma, Tushar, et al. , 2024).
What the study has offered was to optimize model
performance based on real-world deployment as
much as it had suggested to balance between
accuracy and computation feasibility(Sheenam,
Sheenam, et al. , 2023).
6 CONCLUSION
This study proposed the U-Net-based segmentation
model that can effectively handle the chest X-ray
image segmentation task using pre-trained backbones
like ResNet50, EfficientNet, and XceptionNet. The
model demonstrated high accuracy in the
segmentation process, while EfficientNet presented
the optimal trade-off between accuracy and
computational costs. ResNet50 achieved relatively
stable accuracy with modest resource utilization,
whereas XceptionNet demonstrated higher precision
in segmentation at a greater cost of higher
computational requirements. The use of Dice Loss
and Binary Cross-Entropy improved the robustness
of the model, especially regarding the imbalanced
dataset challenge. In general, data augmentation, pre-
trained backbones, and careful model design led to
effective and efficient segmentation results.
Future work will involve more sophisticated data
augmentation like generative models and synthetic
data generation. The architecture of the U-Net can
also include an attention mechanism so that the model
gives major attention to the regions which are
important in the X-ray images. Lightweight models
and pruning techniques might be helpful in reducing
the computational overhead and making the model
usable in real-world clinical settings. This is also
INCOFT 2025 - International Conference on Futuristic Technology
852

further work in a larger scope dataset and in other
testing tasks to check its generalizability for any
medical imaging task.
REFERENCES
X. Wang et al., "ChestX-ray8: Hospital-scale chest X-ray
database and benchmarks on weakly-supervised
classification and localization of common thorax
diseases," in Proc. IEEE Conf. Comput. Vis. Pattern
Recognit., 2017, pp. 2097-2106.
P. Rajpurkar et al., "CheXNet: Radiologist-level
pneumonia detection on chest X-rays with deep
learning," arXiv preprint arXiv:1711.05225, 2017.
J. Irvin et al., "CheXNet: Radiologist-level pneumonia
detection on chest X-rays with deep learning," arXiv
preprint arXiv:1711.05225, 2017.
F. Liu et al., "Multi-task learning for segmentation and
classification of pneumothorax in chest X-rays," in
Proc. IEEE Int. Symp. Biomed. Imag., 2019, pp. 1286-
1290.
J. Cao et al., "Addressing class imbalance in medical image
classification," in Proc. IEEE Conf. Comput. Vis.
Pattern Recognit. Workshops, 2020, pp. 837-845.
A. Krizhevsky, I. Sutskever, and G. Hinton, "ImageNet
classification with deep convolutional neural
networks," in Adv. Neural Inf. Process. Syst., 2012, pp.
1097-1105.
K. He et al., "Deep residual learning for image
recognition," in Proc. IEEE Conf. Comput. Vis. Pattern
Recognit., 2016, pp. 770-778.
SIIM-ACR Pneumothorax Segmentation Challenge,
[Online]. Available: https://www.kaggle.com/c/siim-
acr-pneumothorax-segmentation
R. Girshick et al., "Mask R-CNN," in Proc. IEEE Int. Conf.
Comput. Vis., 2017, pp. 2961-2969.
F. Liu et al., "Multi-task learning for segmentation and
classification of pneumothorax in chest X-rays," in
Proc. IEEE Int. Symp. Biomed. Imag., 2019, pp. 1286-
1290.
L. Zhuang et al., "Lung disease detection in chest X-rays
using deep learning," in Proc. IEEE Conf. Comput. Vis.
Pattern Recognit. Workshops, 2018, pp. 121-130.
O. Ronneberger et al., "U-Net: Convolutional networks for
biomedical image segmentation," in Proc. Int. Conf.
Med. Image Comput. Comput.-Assist. Intervent., 2015,
pp. 234-241.
T. Goodfellow et al., "Generative adversarial nets," in Adv.
Neural Inf. Process. Syst., 2014, pp. 2672-2680.
R. Selvaraju et al., "Grad-CAM: Visual explanations from
deep networks via gradient-based localization," in
Proc. IEEE Int. Conf. Comput. Vis., 2017, pp. 618-626.
R. Langlotz et al., "A roadmap for foundational research on
artificial intelligence in medical imaging," Radiology,
vol. 291, no. 3, pp. 781-791, 2019
Verma, Tushar, et al. "Bone fracture detection using
machine learning." AIP Conference Proceedings. Vol.
3072. No. 1. AIP Publishing, 2024.
Verma, Tushar, et al. "Leaf Disease Detection Using
OpenCV And Deep Learning." 2024 Sixth
International Conference on Computational
Intelligence and Communication Technologies
(CCICT). IEEE, 2024.
Sheenam, Sheenam, Sonam Khattar, and Tushar Verma.
"Automated Wheat Plant Disease Detection using Deep
Learning: A Multi-Class Classification
Approach." 2023 3rd International Conference on
Intelligent Technologies (CONIT). IEEE, 2023.
A Novel Deep Learning Model for Pneumothorax Segmentation in Chest X-Ray Images
853
