
Genetic Algorithm Based Optimization of Convolutional Neural
Network for Respiratory Disease Detection
Vishwachetan D
a
, Nandini S B
b
Pranjal Shrivastava and Nihal Jahagirdar
c
Department of Computer Science and Engineering, Ramaiah Institute of Technology, Bangalore, India
Keywords: X-Ray Imaging, AI, Xception Neural Network, Pulmonary Disease, Genetic Algorithm.
Abstract:
The pandemic Covid 19 in the year 2019 highlighted the need for advanced diagnostic
methodologies to address a spectrum of pulmonary diseases. Although the major method of
COVID-19 detection is still conventional PCR testing, the combination of AI and X-ray imaging
presents a promising path toward a thorough diagnosis of pulmonary illness. Here, we provide a
new optimization framework based on the Xception neural network architecture and Genetic
Algorithm (GA) for precise pulmonary disease detection from X-ray pictures, including
coronavirus and pneumonis (viral, bacterial). By utilising deep learning and convolutional neural
networks, the main aim of this paper to improve the accuracy and efficiency of diagnosis. Using
GA, we explore the vast design space of deep CNN architectures, encompassing parameters such
as network depth, layer count, and type. Utilising an extensive dataset of X-ray pictures, the
suggested Xception-based neural network is rigorously assessed repeatedly through GA-driven
optimization. The result highlight how well the improved model distinguishes lung disorders
achieved with AI-driven approaches.
1 INTRODUCTION
The technology based on deep learning algorithms
has transformed traditional medical image diagnosis
and prognosis in recent years. X-ray imaging is of the
most readily available and used method for
diagnosing lung conditions like pneumonia, whether
bacterial or viral, as well as emerging threats like
COVID-19. The use of deep neural networks (DNNs)
for automated classification and detection tasks has
seen significant advancements, delivering remarkable
accuracy across various benchmarks. Among the
notable CNN architectures is Xception, introduced in
2017 by François Chollet, known for its exceptional
image recognition capabilities. Its innovative depth-
wise separable convolutions enhance effective
feature learning and extraction, making it a strong
candidate for medical image analysis where accuracy
and computational efficiency are paramount.
However, fine-tuning Xception's architecture and
parameters to meet specific medical imaging
a
https://orcid.org/0009-0009-9676-8279
b
https://orcid.org/0009-0001-5708-2954
c
requirements remains a challenge. This study
suggests a way to enhance the detection of pulmonary
diseases, including COVID-19, viral, and bacterial
pneumonia, in X-ray images. The approach involves
a hybrid methodology that combines the optimization
capabilities of genetic algorithms (GAs) with
Xception's properties. Genetic algorithms, inspired
by natural selection, serve as a powerful tool to
identify and build optimal DNN configurations. This
research seeks to determine whether using genetic
algorithms to refine the Xception model's architecture
and hyperparameters—specifically for lung disease
detection from X-ray images—is effective. The
objective is to improve the precision and robustness
of disease diagnosis by iteratively adjusting neural
network architectures using genetic algorithms,
facilitating early identification and timely diagnosis
or treatment. The optimized Xception-based DNN
model will be evaluated on benchmark datasets
through extensive experiments, including cases of
COVID-19, viral pneumonia, bacterial pneumonia,
804
D, V., S B, N., Shrivastava, P. and Jahagirdar, N.
Genetic Algorithm Based Optimization of Convolutional Neural Network for Respiratory Disease Detection.
DOI: 10.5220/0013733200004664
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 3rd International Conference on Futuristic Technology (INCOFT 2025) - Volume 3, pages 804-810
ISBN: 978-989-758-763-4
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

and healthy controls. The aim of this study is to
advance the creation of reliable and efficient
automated pulmonary disease diagnosis tools,
supporting clinical decisions by healthcare
professionals and ultimately improving patient
outcomes.
2 LITERATURE REVIEW
Over the years, several techniques have been
suggested for medical image analysis, shifting from
traditional feature-based approaches to advanced
machine learning techniques. Early investigations in
medical image analysis focused on basic image
processing techniques like thresholding,
morphological operations, and edge detection. While
these methods laid the groundwork for future
research, they often fell short in meeting the accuracy
and consistency required in clinical practice.
Apostolopoulos and Mpesiana (Apostolopoulos,
Mpesiana, et al. 2020) fine-tuned Convolutional
Neural Networks (CNNs) for the automatic detection
of COVID-19 from X-ray images, demonstrating that
pre-trained models can enhance diagnostic accuracy.
Similarly, Duran-Lopez et al. (Duran-Lopez,
ominguez-Morales, et al. 2020) proposed COVID-
XNet, a deep learning model designed to diagnose
and localize COVID-19 in chest X-rays, aiming to
improve both detection accuracy and efficiency.
Sethy and Behera (Sethy, Behera, et al. 2020)
investigated deep learning potential in medical
imaging, using neural networks to extract features for
the identification of COVID-19 in X- rays images.
This study highlighted how deep learning techniques
can streamline the analysis of X-ray data, offering a
reliable solution for disease diagnosis and reducing
unnecessary examinations.Narin et al. (Narin, Kaya,
et al. 2021) applied various deep learning models for
classification of coronavirus and normal cases, with
their ResNet50 model achieving 98.0% accuracy in
the best-case scenario. Zhang et al. (Zhang, Xie, et al.
2020) introduced another ResNet-based model that
achieved an AUC of 0.952, effectively highlighting
areas affected by pneumoniavari using Grad-CAM.
Wang et al. (Wang, Lin, et al. 2020) proposed a deep
CNN for classifying viral and bacterial infections and
normal cases, achieving 83.5% accuracy.Image
segmentation has also played a critical role in
COVID-19 applications, including diagnostics
(Chen, et al. 2019), (Wang, et al. 2021), (Jin, et al.
2020), (Song, et al. 2021). For example, Li et al. (Li,
et al. 2020) used a U-Net architecture to segmentin
lung images to differentiate COVID-19 from
pneumonia acquired from the community using CT
scans of the chest region. Jin et al. (Jin, et al. 2020)
developed an AI system for rapid COVID-19
detection, where segmented CT slices serve as input
for the classification model.Segmentation techniques
also prove valuable in quantification tasks within
medical applications (Jin, et al. 2020), (Shan, et al.
2021). A new model, XcepCovidNet, was introduced
to identify features in X-rays of the chest region,
utilizing transfer learning combined with
hyperparameter tuning to address limitations in the
training dataset (Juneja, Kumar, et al. 2024). Beyond
X-rays, recent studies have turned to CT scans for the
same purpose. For instance, Khan et al. (Khan, Shah,
et al. 2020) developed CoroNet, a classification
system consisting of four classes for COVID-19,
achieving accuracies of 89.6% and 95% for chest X-
ray (CXR) and CT scanned images, respectively.
COVNet, designed by Li et al. (Li, et al. 2020), was
based on ResNet50 and trained on a dataset of 4,356
images of CT scans of the chest region. Lastly,
Joloudari et al. (Joloudariet, et al. 2023) proposed a
deep learning-based global feature extractor for
COVID-19 detection, further contributing to the
research on using deep learning in medical image
analysis.
3 PROPOSED METHOD
The suggested model in this paper makes use of three
fundamental algorithms. The deep convolutional
neural network Xception model is used for the
detection of respiratory disease in lung X-ray images.
The Genetic algorithm is then used to tune the
hyperparameters of the Xception model to achieve the
best possible architecture. This section explains the
Genetic Algorithm, CNN, Xception model and finally
the model suggested.
3.1 CNN
CNNs are a class of deep learning models for
processing structured grid data, notably images and
videos. Convolutional, pooling, and fully connected
layers are how CNNs work to extract hierarchical
characteristics from input data.Components of a
CNN: Convolutional Layers: These layers are made
up of filters, sometimes known as kernels, that
execute convolutions by sliding across the input data.
Each filter specializes in detecting specific features,
such as edges or textures, by capturing spatial
correlations. Activation functions such as ReLU
introduce non-linearity. Pooling Layers:
Genetic Algorithm Based Optimization of Convolutional Neural Network for Respiratory Disease Detection
805

Convolutional layer feature maps are down sampled
by pooling layers, which reduces spatial dimensions
without losing important information. For example,
max pooling selects the greatest value within local
regions, effectively shrinking feature map sizes.
Feature maps are converted into vectors and run
through one or more fully connected layers following
a number of convolutional and pooling layers.The
layers here handle classification or regression tasks
by learning intricate relationships between extracted
features and target labels. CNN architectures may
incorporate additional elements such as dropout
layers for regularization, batch normalization layers
for accelerated convergence, and skip connections for
improved gradient flow during training. Training
CNNs involves optimizing parameters (weights and
biases) using gradient-based optimization algorithms
like SGD or its variants. During training, the network
minimizes a loss function, quantifying the disparity
between predicted outputs and ground truth labels.
3.2 Xception
Xception is a CNN architecture innovated by
François Chollet, renowned for his contribution to the
Keras deep learning library. Termed as "Extreme
Inception," Xception builds upon the foundational
concepts of the Inception architecture while
introducing notable advancements. Central to
Xception's design is the utilization of depth wise
separable convolutions, a variant of conventional
convolutional operations. This methodology
effectively segregates spatial and channel-wise
convolutions into distinct processes, resulting in a
significant reduction in both parameters and
computational complexity compared to conventional
convolutions. Consequently, Xception achieves
enhanced efficiency and model lightweightness. The
architecture of Xception heavily draws from the
Inception modules featured in the Inception v3
model. However, Xception distinguishes itself by
replacing conventional convolutions within these
modules with depth wise separable convolutions.
This architectural refinement facilitates an improved
utilization of computational resources, ensuring the
modeling of intricate patterns and relationships across
various scales. One notable advantage of Xception
lies in its capability to capture both local and global
dependencies within input data. The decomposition
of the convolution operation into spatial and channel-
wise components allows Xception to effectively
model complex structures and correlations present in
the data. Furthermore, Xception's architecture boasts
expedited training and inference times, surpassing
preceding CNN architectures. This attribute renders
Xception particularly suitable for applications
characterized by resource-constrained environments,
where computational efficiency is paramount.
3.3 Genetic Algorithm
Genetic Algorithms (GAs) are commonly applied
to optimization problems, such as tuning
hyperparameters in machine learning. A population
of potential solutions, known as people or
chromosomes, is used by a genetic algorithm to solve
problems. Each chromosome is a potential solution.
In the context of hyperparameter tuning, these
solutions usually correspond to different sets of
hyperparameters for a machine learning model. As
the algorithm runs through a sequence of stages called
generations, selection, crossover, and mutation are
applied to create a fresh set of potential solutions.
3.3.1 Initialization
The algorithm begins by generating an initial
population of chromosomes, typically done
throughrandomness or certain heuristics.
3.3.2 Evaluation
Each chromosome is assessed according to its fitness,
which measures how effectively the solution
performs the given optimization task. In
hyperparameter tuning, the fitness is determined by
how well the machine learning model performs when
trained with the hyperparameters encoded in the
chromosome.
3.3.3 Selection
Greater fitness values in chromosomes are preferable
for reproduction, similar to the concept of "survival
of the fittest."
3.3.4 Crossover
Selected chromosomes are paired to produce
offspring through crossover or recombination. This
process involves mixing genetic information from
parent chromosomes to create new solutions. In
hyperparameter tuning, crossover allows for the
exploration of different hyperparameter
combinations.
In hyperparameter tuning, where finding the ideal
set of hyperparametersfor a machine learning model is
the goal, GAs provide an effective way to navigate the
large search space. Initially, a population of potential
INCOFT 2025 - International Conference on Futuristic Technology
806
solutions (chromosomes) is created either randomly or
using heuristics. Each chromosome encodes some
hyperparameters for the machine learning model,
which might include factors like learning rates,
regularization strengths, or network
architectures.Next, the fitness of each chromosome is
assessed by training and testing the machine learning
model using the hyperparameters it contains.
Performance metrics, such as accuracy or loss, are
used to assess fitness. Selection methods like
tournament or roulette wheel selection are then
applied to choose chromosomes to be reproduced
based on their fitness, favoring those with greater
values.Crossover and mutation are triedon these
selected chromosomes to generate new offspring.
Crossover mixes genetic information from parent
chromosomes, enabling the exploration of new
hyperparameter combinations. Mutation introduces
random changes, maintaining diversity in the
population and preventing early convergence to
suboptimal solutions. The offspring replace the
previous generation, with fitter individuals more
likely to survive.Until a termination requirement is
satisfied, for example, by reaching a certain number of
generations or attaining adequate performance, this
iterative process keeps going. Through this cycle of
selection, crossover, and mutation, genetic algorithms
efficiently search the hyperparameter space, slowly
converging toward optimal or near-optimal
configurations that enhance the ML model's
performance.
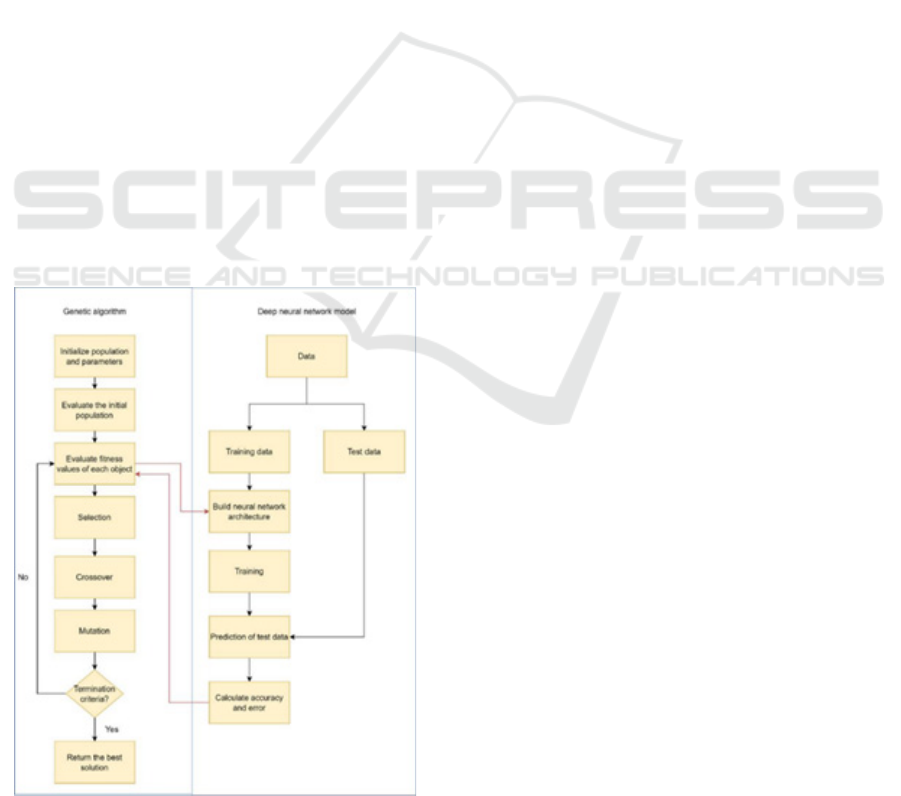
Figure 1: Flowchart of proposed model
This research employs Genetic Algorithm (GA) in
conjunction with the Xception architecture, a
sophisticated Convolutional Neural Network (CNN).
First, a deep Xception network is constructed
utilizing parameters computed from the Genetic
Algorithm. Subsequently, these deep neural
networks undergo training and evaluation using a
dataset aimed at discerning COVID-19 presence in
individuals. Each network's performance is assessed
based on its error rate, with lower error rates
indicative of more desirable solutions. These
evaluated solutions undergo further refinement via
iterations of the GA algorithm. With each iteration,
novel networks are generated, leading to
progressively improved outcomes. The rationale
behind selecting the Xception model stems from its
proven efficacy in prior research endeavours,
consistently yielding commendable results. Its
selection is particularly apt given its tailored focus on
COVID-19 detection, aligning closely with the
objectives of this study. Figure 1 explains the flowof
the proposed model.
4 IMPLEMENTATION
4.1 Dataset for Respiratory Disease
Detection Training
The dataset for respiratory disease detection training
encompasses four primary classes: COVID, normal
lung conditions, bacterial pneumonia, and viral
pneumonia. Initially split into training, testing, and
validation sets, the dataset underwent meticulous
cleaning due to the presence of noise, including
random letters and unnecessary watermarks, ensuring
data integrity and reliability. With close to 1400
images solely for training purposes, augmentation
techniques were employed to expand the dataset size,
enhancing the model's ability to generalize and learn
diverse patterns. This comprehensive dataset,
meticulously curated and augmented, serves as a
robust foundation for training and evaluating deep
learning models aimed at accurate and effective
respiratory disease detection and classification.
4.2 Model Implementation
For implementing the proposed model, after
initializing the population, the evaluate_population
function is called to train and evaluate each candidate
solution (CNN) on the training and validation
datasets. This step involves training the CNN model
with the training data, then evaluating its execution
Genetic Algorithm Based Optimization of Convolutional Neural Network for Respiratory Disease Detection
807
using the validation data. The fitness scores are
computed based on the performance metrics obtained
during evaluation. Within the loop that iterates
through generations, the training of CNN models is
implicitly done during the evaluation step, as part of
the evaluate_population function. The CNN models
are trained with the training data before their
performance is evaluated on the validation data. The
training process typically involves using the training
data on the CNN model, computing the loss, and then
modifying the model's parameters. Algorithms for
optimization such as SGD is used. The performance
of each CNN model on the validation dataset is then
evaluated using metrics like accuracy, loss, or other
relevant measures, and these evaluations are used to
compute the fitness scores. The process continues
until a termination condition is reached, such as
reaching a set number of generations.
5 RESULTS
Accuracy and categorical cross entropy loss were
theperformance metrics used to find thebest
architecture discovered by Genetic algorithm. One
frequent metric used to assess a classification model's
performance is accuracy. Out of all the anticipated
classifications, it calculates the percentage of accurate
classifications. Accuracy is mathematically
calculated as shown in Figure 2.
The categorical cross-entropy loss function given
in Equation 1serves as a pivotal evaluation metric in
various machine learning tasks, particularly in
classification problems where the output is
represented in a categorical format. This metric
quantifies the disparity between the true distribution
of class labels and the predicted probabilities assigned
by the model. By computing the logarithmic
difference between the predicted probabilities and the
actual class labels across all categories, the
categorical cross-entropy loss penalises deviations
from the true distribution, effectively guiding the
model towards better classification performance. Its
formulation makes it particularly suited for multi-
class classification tasks, providing a continuous,
differentiable measure of the model's performance
that can be optimised through gradient descent
methods.
Loss =
∑
𝑦
⋅𝑙𝑜𝑔𝑦
(1)
where, loss is the categorical cross-entropy loss, n
is the output size, y is the correct probability
distribution of class labels (one-hot encoded) and y^
is the estimated probability distribution of class
labels.
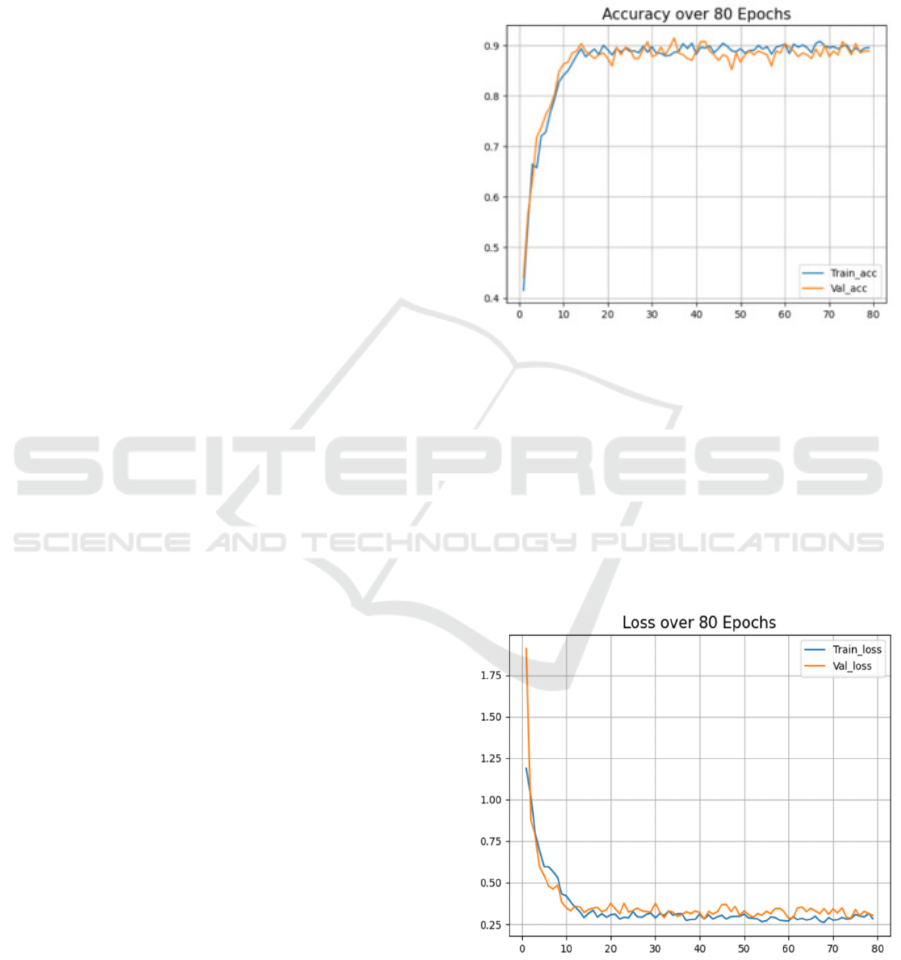
The accuracy metric demonstrates a notable
enhancement, with a consistent increase of 2-3%,
indicating improved model performance in correctly
classifying data points.
Figure 2: Accuracy obtained over 80 Epochs
The cross-entropy loss in Figure 3 exhibits a
significant improvement, with a remarkable decrease
of 30%, reflecting the model's enhanced ability to
minimize discrepancies between predicted and true
class probabilities. These advancements, coupled
with the absence of significant spikes in loss, suggest
the efficacy of hyperparameter tuning and the
refinement of the dataset, contributing to a more
stable and robust model performance with reduced
noise interference.
Figure 3: Categorical Cross Entropy Loss over 80 Epochs
The model trained was then tested across various
images of lungs from the dataset to evaluate its
performance. The accuracy on the test dataset was
INCOFT 2025 - International Conference on Futuristic Technology
808
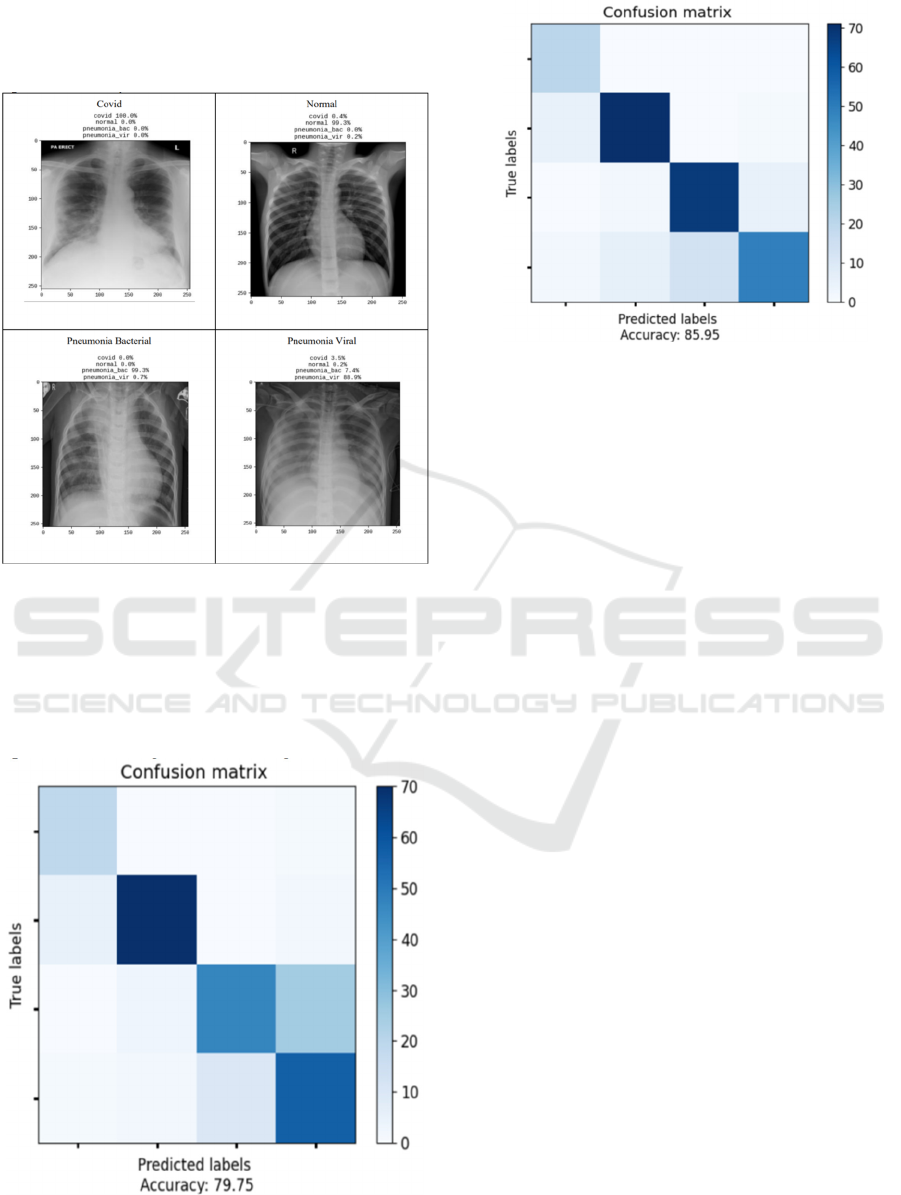
found out to be 0.79 and after optimization using GA,
an accuracy of 0.85 was obtained. Categorical cross
entropy loss of 0.79 was also reduced to 0.44 upon
optimization by GA.
Figure 4: Predictions obtained for various classes
Figure 4 shows the results of classification along
with their predicted conditions obtained from the
trained model. The confusion matrices shown in
Figure 5 and Figure 6 depict the accuracy of
predictions of the model before and after
optimizations using the Genetic Algorithm.
Figure 5: Confusion Matrix on test dataset before GA
optimization
Figure 6 Confusion Matrix on test dataset after GA
optimization
6 CONCLUSIONS
In conclusion, the enhancements made to the model
have yielded significantly improved stability and
robustness, as evidenced by the obtained results.
Notably, there is a consistent enhancement in
accuracy ranging between 2-3%, indicating the
effectiveness of the implemented changes. Equally
significant is the remarkable 30% decrease in
Validation Loss, underscoring the model's improved
generalisation capability. It's important to note that
while training loss measures the performance of the
model during the training phase, validation loss
provides insight into how well the model generalises
to unseen data, making it a crucial metric in assessing
real-world performance. The fact that both training
and validation accuracy and losses closely match
underscores the absence of overfitting, signifying that
the model has learned to generalise well to unseen
data. This alignment between training and validation
metrics further validates the reliability and efficacy of
the model's performance. Overall, these results affirm
the success of the enhancements implemented, paving
the way for more reliable and accurate predictions in
practical applications.
7 FUTURE WORK
For future work, a larger dataset should be assembled
to encompass a more diverse set of lung diseases,
potentially enhancing the model's ability to detect and
classify a broader range of conditions. Additionally,
fine-tuning the existing architecture with progressive
Genetic Algorithm Based Optimization of Convolutional Neural Network for Respiratory Disease Detection
809

techniques such as transfer learning or ensemble
methods could potentially elevate the model's
performance to even greater heights.
REFERENCES
D. Apostolopoulos and T. A. Mpesiana, “Covid-19:
automatic detection from X-ray images utilizing
transfer learning with convolutional neural networks,”
Physical and Engineering Sciences in Medicine, vol.
43, no. 2, pp. 635–640, Apr. 2020, doi:
10.1007/s13246-020-00865-4. Available:
https://doi.org/10.1007/s13246-020-00865-4
L. Duran-Lopez, J. P. Dominguez-Morales, J. Corral-
Jaime, S. Vicente-Diaz, and A. Linares-Barranco,
“COVID-XNet: A Custom Deep Learning System to
Diagnose and Locate COVID-19 in Chest X-ray
Images,” Applied Sciences, vol. 10, no. 16, p. 5683,
Aug. 2020, doi: 10.3390/app10165683. Available:
https://doi.org/10.3390/app10165683
P. K. Sethy, S. K. Behera, P. K. Ratha, and P. Biswas,
“Detection of coronavirus Disease (COVID-19) based
on Deep Features and Support Vector Machine,”
International Journal of Mathematical Engineering and
Management Sciences, vol. 5, no. 4, pp. 643–651, Aug.
2020, doi: 10.33889/ijmems.2020.5.4.052. Available:
https://doi.org/10.33889/ijmems.2020.5.4.052
A. Narin, C. Kaya, and Z. Pamuk, “Automatic detection of
coronavirus disease (COVID-19) using X-ray images
and deep convolutional neural networks,” Pattern
Analysis and Applications, vol. 24, no. 3, pp. 1207–
1220, May 2021, doi: 10.1007/s10044-021-00984-y.
Available: https://doi.org/10.1007/s10044-021-00984-
y
Zhang J, Xie Y, Li Y, Shen C, Xia Y. COVID-19 Screening
on Chest X-ray Images Using Deep Learning based
Anomaly Detection. arXiv; 2020.
L. Wang, Z. Q. Lin, and A. Wong, “COVID-Net: a tailored
deep convolutional neural network design for detection
of COVID-19 cases from chest X-ray images,”
Scientific Reports, vol. 10, no. 1, Nov. 2020, doi:
10.1038/s41598-020-76550-z. Available:
https://doi.org/10.1038/s41598-020-76550-z
O. Gozes et al., “Rapid AI Development Cycle for the
Coronavirus (COVID-19) Pandemic: Initial Results for
Automated Detection & Patient Monitoring using Deep
Learning CT Image Analysis,” arXiv (Cornell
University), Jan. 2020, doi:
10.48550/arxiv.2003.05037. Available:
https://arxiv.org/abs/2003.05037
L. Li et al., “Using Artificial Intelligence to Detect COVID-
19 and Community-acquired Pneumonia Based on
Pulmonary CT: Evaluation of the Diagnostic
Accuracy,” Radiology, vol. 296, no. 2, pp. E65–E71,
Aug. 2020, doi: 10.1148/radiol.2020200905.
Available: https://doi.org/10.1148/radiol.2020200905
J. Chen et al., “Deep learning-based model for detecting
2019 novel coronavirus pneumonia on high-resolution
computed tomography,” Scientific Reports, vol. 10, no.
1, Nov. 2020, doi: 10.1038/s41598-020-76282-0.
Available: https://www.nature.com/articles/s41598-
020-76282-0
B. Wang et al., “AI-assisted CT imaging analysis for
COVID-19 screening: Building and deploying a
medical AI system,” Applied Soft Computing, vol. 98,
p. 106897, Jan. 2021, doi: 10.1016/j.asoc.2020.106897.
Available: https://doi.org/10.1016/j.asoc.2020.106897
C. Jin et al., “Development and Evaluation of an AI System
for COVID-19 Diagnosis,” medRxiv (Cold Spring
Harbor Laboratory), Mar. 2020, doi:
10.1101/2020.03.20.20039834. Available:
https://doi.org/10.1101/2020.03.20.20039834
Y. Song et al., “Deep Learning Enables Accurate Diagnosis
of Novel Coronavirus (COVID-19) With CT Images,”
IEEE/ACM Transactions on Computational Biology
and Bioinformatics, vol. 18, no. 6, pp. 2775–2780, Nov.
2021, doi: 10.1109/tcbb.2021.3065361. Available:
https://doi.org/10.1109/tcbb.2021.3065361
Y. Cao et al., “Longitudinal Assessment of COVID-19
Using a Deep Learning–based Quantitative CT
Pipeline: Illustration of Two Cases,” Radiology
Cardiothoracic Imaging, vol. 2, no. 2, p. e200082, Apr.
2020, doi: 10.1148/ryct.2020200082. Available:
https://doi.org/10.1148/ryct.2020200082
F. Shan et al., “Abnormal lung quantification in chest CT
images of COVID‐19 patients with deep learning and
its application to severity prediction,” Medical Physics,
vol. 48, no. 4, pp. 1633–1645, Mar. 2021, doi:
10.1002/mp.14609. Available:
https://doi.org/10.1002/mp.14609
A. Juneja, V. Kumar, M. Kaur, D. Singh, and H.-N. Lee,
“XcepCovidNet: deep neural networks-based COVID-
19 diagnosis,” Multimedia Tools and Applications, Jun.
2024, doi: 10.1007/s11042-024-19046-6. Available:
https://doi.org/10.1007/s11042-024-19046-6
A. I. Khan, J. L. Shah, and M. M. Bhat, “CoroNet: A deep
neural network for detection and diagnosis of COVID-
19 from chest x-ray images,” Computer Methods and
Programs in Biomedicine, vol. 196, p. 105581, Nov.
2020, doi: 10.1016/j.cmpb.2020.105581. Available:
https://doi.org/10.1016/j.cmpb.2020.105581
L. Li et al., “Using Artificial Intelligence to Detect COVID-
19 and Community-acquired Pneumonia Based on
Pulmonary CT: Evaluation of the Diagnostic
Accuracy,” Radiology, vol. 296, no. 2, pp. E65–E71,
Aug. 2020, doi: 10.1148/radiol.2020200905.
Available: https://doi.org/10.1148/radiol.2020200905
J. H. Joloudariet al., “Developing a Deep Neural Network
model for COVID-19 diagnosis based on CT scan
images,” Mathematical Biosciences & Engineering,
vol. 20, no. 9, pp. 16236–16258, Jan. 2023, doi:
10.3934/mbe.2023725. Available:
https://doi.org/10.3934/mbe.2023725
INCOFT 2025 - International Conference on Futuristic Technology
810
