
Development of a Control System for an Innovative Parallel Robot
Used in Laparoscopic Pancreatic Surgery
Doina Pisla
1,3 a
, Andra Ciocan
1,2 b
, Bogdan Gherman
1,* c
, Diana Schlanger
2d
,
Alexandru Pusca
1,* e
, Nadim Al Hajjar
2f
, Emil Mois
2g
, Andrei Cailean
1h
, Nicoleta Pop
1i
,
Paul Tucan
1j
, Ionut Zima
1k
and Calin Vaida
1l
1
CESTER, Technical University of Cluj-Napoca, 400114 Cluj-Napoca, Romania
2
Department of Surgery, “Iuliu Hatieganu” University of Medicine and Pharmacy, 400347 Cluj-Napoca, Romania
3
Technical Sciences Academy of Romania, B-dul Dacia, 26, 030167 Bucharest, Romania
Keywords: Parallel Robot, Master-Slave, Control System, Minimally Invasive Pancreatic Surgery, Robotic Assisted.
Abstract: This paper presents the development of the control architecture for an innovative parallel robot, designed to
assist surgeons during the minimally invasive pancreatic cancer surgery. Based on the defined medical
protocol and surgeon requirements. The robot was designed to serve as a surgical assistant and to manipulate
a third active instrument. The system features a 3-DOF parallel active module coupled to a passive spherical
module guiding the instrument through a Remote Center of Motion (RCM). The master-slave control
architecture enables surgeons to operate the robot using a 3D Space Mouse or haptic device (Omega.7). The
system automatically calculates RCM position using IMU sensors, validated through optical tracking.
1 INTRODUCTION
Pancreatic cancer ranks 7
th
globally in terms of
mortality rate and 14
th
in incidence. In Europe, it
ranks 2
nd
in terms of the number of new cases, with
countries such as Hungary, Slovakia, the Czech
Republic, and Serbia reporting the highest rates
(McGuigan, 2018) with a higher incidence in
developed countries (Wong MCS, 2017). Risk factors
include modifiable factors (obesity, diet, alcohol,
smoking) and non-modifiable factors (age, gender,
genetics) (McGuigan, 2018). The prognosis remains
challenging with a 5-year survival rate of only 5%,
increasing to 30% with early detection. However,
early detections increase the 5-year survival rate to
30%. Treatment involves surgery combined with
oncological therapy (Nortunen, 2023; Nießen, 2022).
Three main surgical procedures are used for
a
https://orcid.org/0000-0001-7014-9431
b
https://orcid.org/0000-0003-0126-6428
c
https://orcid.org/0000-0002-4427-6231
d
https://orcid.org/0000-0002-4427-6231
e
https://orcid.org/0000-0002-5804-575X
f
https://orcid.org/0000-0001-5986-1233
g
https://orcid.org/0000-0002-2972-3777
pancreatic cancer: Pancreaticoduodenectomy
(Whipple Procedure) (Cawich, 2023), Distal
Pancreatectomy (De Pastena, 2023), and Total
Pancreatectomy (Balzano, 2023).
The first robotic-assisted distal pancreatectomy
was performed using the da Vinci system in 2003
(Melvin, 2003), improving safety and feasibility
compared to manual laparoscopic surgery leading to
wider robotic surgery adoption (Damoli, 2015).
Robotic systems (da Vinci, Senhance, Versius) offer
advantages including the elimination of the
triangulation effect, enhanced precision, improved
dexterity, and increased patient safety, driving
adoption in surgical interventions. These surgical
robots reduce hand tremors, enable motion scaling
and multi-instrument manipulation, improve
ergonomics through master-slave control (Pisla,
2021), and support remote telesurgery interventions
h
https://orcid.org/0009-0004-4758-0468
i
https://orcid.org/0000-0001-5660-8259
j
https://orcid.org/0000-0001-5660-8259
k
https://orcid.org/0009-0007-0483-7691
l
https://orcid.org/0000-0003-2822-9790
*
Corresponding author
Pisla, D., Ciocan, A., Gherman, B., Schlanger, D., Pusca, A., Al Hajjar, N., Mois, E., Cailean, A., Pop, N., Vaida, C., Tucan, P. and Zima, I.
Development of a Control System for an Innovative Parallel Robot Used in Laparoscopic Pancreatic Surgery.
DOI: 10.5220/0013708700003982
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 22nd International Conference on Informatics in Control, Automation and Robotics (ICINCO 2025) - Volume 2, pages 243-250
ISBN: 978-989-758-770-2; ISSN: 2184-2809
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
243
(Li, 2023). Robotic-assisted surgery presents several
disadvantages, including the high cost of surgical
intervention, steep learning curves, potential arm
collisions, significant space, the lack of haptic
feedback, and the limited intraoperative space
(Haidegger, 2022).
Most robots for laparoscopic surgery use master-
slave architecture (Rus, 2023; Pisla, 2024 a). These
robots operate without autonomy in minimally
invasive surgery (Khachfe, 2022). These robotic
systems lack haptic feedback for surgeons
(Minamimura, 2024). Studies demonstrate haptic
device integration in the Senhance robotic system
(Kastelan, 2021). Force feedback sensors have been
placed near the robot flange, but the research in this
direction is in the early stages of development
(Bergholz, 2023). Alternative control approaches
using contactless interfaces have been explored.
Korayem demonstrated Leap Motion controller
integration for surgical robot control with Kalman
filtering (Korayem, 2021), hand tremor detection and
compensation techniques (Korayem, 2022 a), and
complete laparoscopic robot system development
(Korayem, 2022 b; Najafinejad, 2023).
Minimally invasive pancreatic procedures are
complex, requiring multiple instruments, extended
operative time, and involving high risks (Asbun,
2023), indicating significant research gaps in the
field. To address these challenges, a new parallel
robot was developed for minimally invasive
pancreatic surgery (Vaida, 2025). The proposed robot
acts as a surgical assistant during pancreatic
laparoscopy, performing tissue manipulation tasks
using an active surgical instrument.
The paper is structured as follows: Section 1
presents the introduction, section 2 outlines medical
protocol, robot design and integration, and
mathematical model implementation, section 3
illustrates the control architecture of the robot; section
4 illustrates the experimental testing and validation,
while section 5 presents conclusions and future
developments.
2 DESIGN OF THE INNOVATIVE
SURGICAL PARALLEL ROBOT
AND ACTIVE INSTRUMENT
The Athena parallel robot was developed based on a
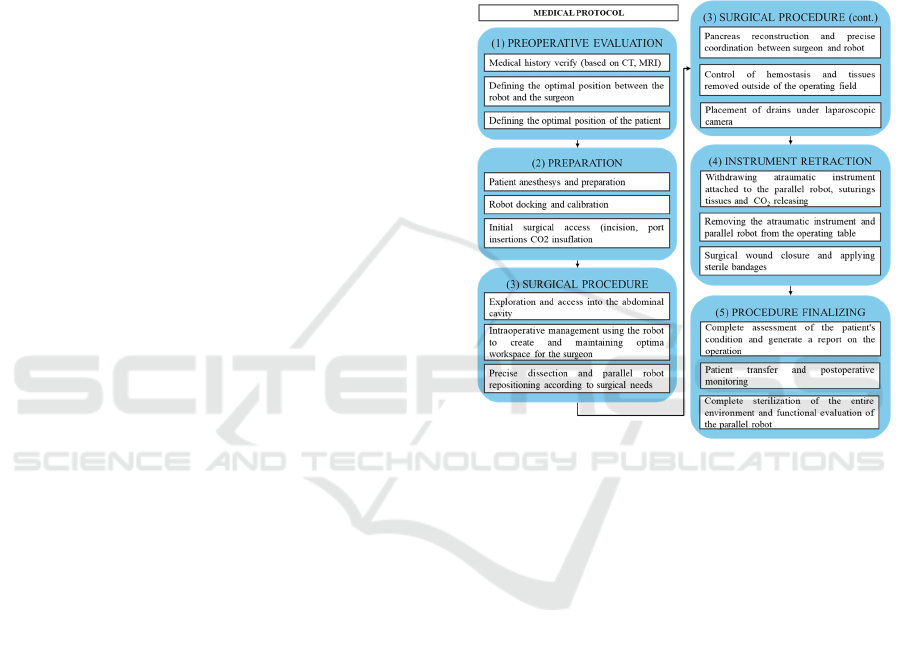
medical protocol (Figure 1) established in
collaboration with surgeons. This protocol outlines
the steps for laparoscopic pancreatic surgery targeting
the head of the pancreas (Whipple Procedure).
Based on the medical protocol and surgeon’s
requirements, the kinematic scheme of the robot
(Figure 2) was developed, together with an active
instrument, both subject to two patents (Pisla, 2025 b;
Vaida, 2025 a). The robot assists the main surgeon by
manipulating a third active atraumatic instrument to
create an intraoperative workspace and support
organs around the pancreas. A detail of the tasks that
the robot performs is extensively presented in (Vaida,
2025 b) and in (Tucan, 2025).
Figure 1: Medical protocol for laparoscopic pancreatic
surgery.
The ATHENA parallel robot mechanical
architecture (Figure 2) consists of two modules: an
active 3 degrees-of-freedom (DOF) parallel robot and
a passive parallel spherical mechanism. The spherical
mechanism fixes and holds the Remote Center of
Motion (Zhang, 2024), while the parallel robot
manipulates the active surgical instrument. The robot
consists of 9 passive revolute joints, three passive
prismatic joints, three active prismatic joints (qi,
i=1....3) and two passive universal joints. The global
coordinate system is placed on the robot base. The
spherical mechanism features five passive revolute
joints and one passive cylindrical joint that provide
rotation and insertion motion of the active instrument.
The spherical mechanism connects to the robot base
via a link and two spherical joints for RCM placement
and adjustment.
Detailed description of parameters is presented in
(Vaida, 2025), and integration of the robot into the
medical environment is illustrated in Figure 3.
ICINCO 2025 - 22nd International Conference on Informatics in Control, Automation and Robotics
244
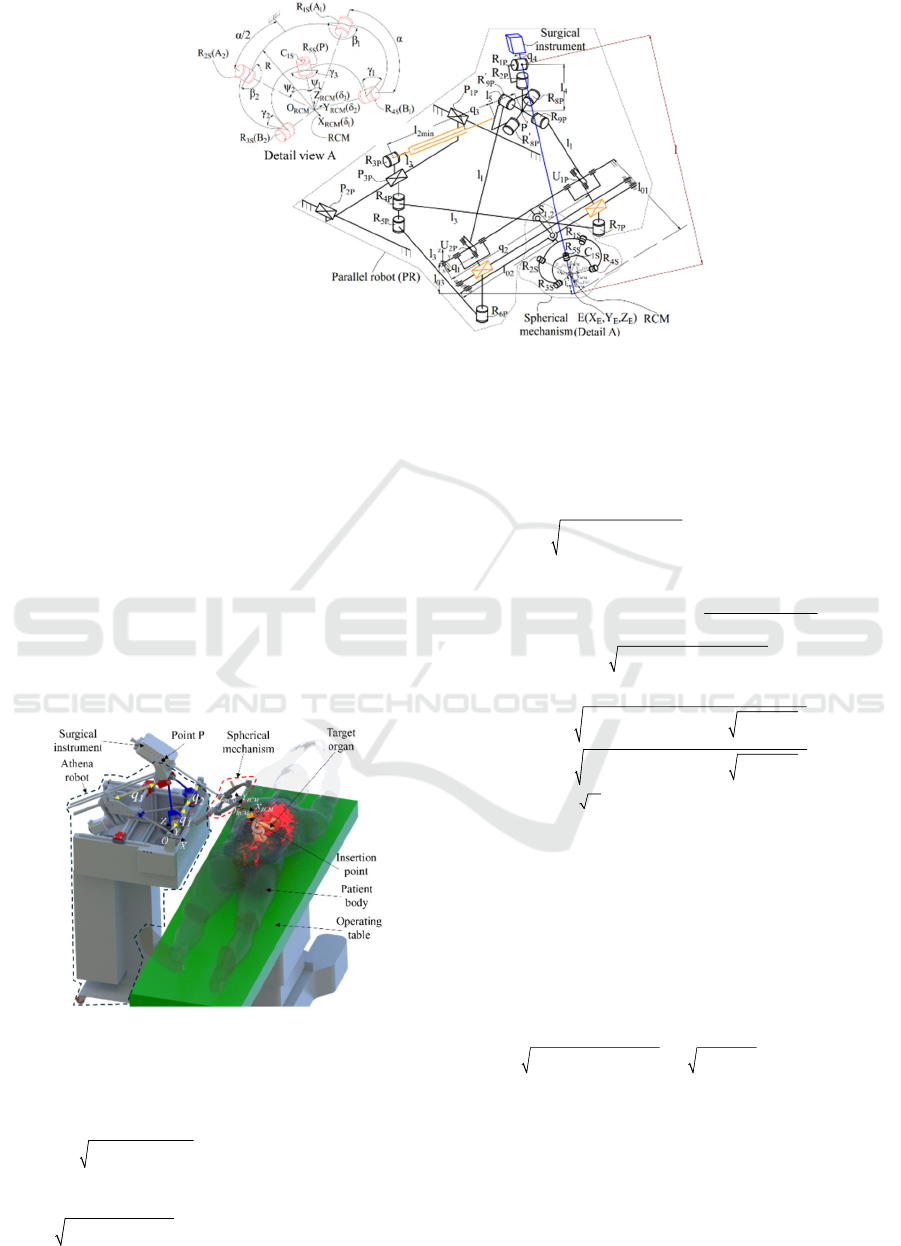
Figure 2: Kinematic scheme of the Athena robot.
Based on the kinematic scheme and parameters
presented in (Vaida, 2025), kinematic models
(forward and inverse) of the Athena robot were
developed and integrated into the control system
through input/output equations. Four mathematical
models are used to control the robot: forward (Eqs. 2-
5) and inverse (Eqs. 7-9) kinematic models without
RCM, used for robot positioning near the patient and
RCM before instrument attachment. Once the RCM
is defined, the instrument is positioned at the
abdominal insertion position and attached to the
robot. In this configuration, inverse (Eqs. 5-6) and
forward (Eqs. 7-9) kinematic models with RCM
control both the robot and the surgical instrument.
Figure 3: Athena robot integrated into the medical
environment.
The equations for these models are:
()()( )
02 1 2
2
222
2
41 2 03 0
1
21 1
:0
:/2) 0
((/2))
(
++ −
+−− −− − −
=
=
P
PP
fl q q Y
llqq Z Xlfl
(1)
() ()
(
()
)
()
()
2
32min5 014
2
2
2
2
321 4 03
3
:
/2) 0
cos
(sin
P
P
fql l Xll
lqq Zl l
λ
λ
++− −− +
−− − − =−
where:
()
ins ins
ins 03 01
() ()( ); () ()( )
()( ); , .
P
P
PP
P
cos sin l l sin sin l lXY
ataZcosll ZlXln
ψθ ψθ
θλ
−−
=− −−
==
=
(2)
() ()
44
;= ;
PPc PPcP Pc
XX lcos YYZZ lsin
ββ
+==+
(3)
and:
()
()
()
()
()
()
() ()
2
321 532min
21 32min
2
;
;
= / 2
/2; =
pc
pc pc
Xlqq lqlcos
Yqq Zqlsin lsin
α
αβ
+
−
−− −++
=− +
4
(4)
()
()
()
()
()
()
2222
1
32min
22 2
32min 3 1 21 2 5
21
2, ;
2
; 4 2 /2;
/2.
;
pc pc
ABlC
arctan Z X arccos
ql B
Aq l B l q qq q l
Cqq
βα
+−+
==
+
=+ = −+
=−
⋅
−+
()()
()()
()
()
22 22 22
114 4
22 22 22
214 4
3
.
2
+
;
2
/
;
2²
PPPPP
PPPPP
qY l l X Z l X Z
qY l l X Z l X Z
qND r
=− −− + + +
=−−+++
=
+
(5)
where:
( )() ()
66 24 5 32 4
1P P PP2 P5 5 5
22 2222
3345
5
222
2min 123456
2222 2 222
4
2
12 12 12
22
1
4
5
P
2
P
2;²
2
;;
²42
4;
4
T = 4Z + 4X + 1 X 4Z ; 8X 16 8 ;
4 ( )( );
XX
P
PP
PP
P
PP
P
PP
P
P
P
N
l
T
rl r X Z D T T T T T T
rX Z q q rqq XZ
l
lXZlXZl
TrXZll
T
qq
XZq
A
T
q
=− = + = + + + + +
−+ ++
+
−+=+
+
=+ +
=++
=
()
()
()
P
32 22 4 4
555
32
4
22 4 3 2
455
22 2 2 2
31
3
22 P
6
2
;
.
X
2
82
4
;
2;X
PP PP PP P
PPP
PP P P PP
P
XZ XZl XZ Z
Br X X Z l
Brl X Z Z X l X Z l
AlqqqqB Z
T
−
++ ++
+
−++++
=−
=
−=+
+
1
(6)
()
()
atan 2 sin , cos
atan 2 sin , cos
θθ
ψψ
θ
ψ
=
=
(7)
where:
Development of a Control System for an Innovative Parallel Robot Used in Laparoscopic Pancreatic Surgery
245

()()()
22 2
2
cos
sin 1 cos
RCM P RCM P RCM P
RCM P
XXYYZZ
ZZ
θ
θθ
−+ −+ −
=
−
=−
(8)
()()()
()
()()()
()
22 2
22 2
sin
1
sin
cos
1
sin
RCM P
RCM P RCM P RCM P
RCM P
RCM P RCM P RCM P
XX
XX YYZZ
YY
XX YYZZ
ψ
ψ
θ
θ
−
=⋅
−+ −+ −
−
=⋅
−+ −+ −
Finally, the end-effector coordinates are:
() ()
() ()
()
cos sin
sin sin
cos
EP
EP
EP
XL X
YL Y
ZL Z
ψθ
ψθ
θ
=⋅ +
=⋅ +
=⋅ +
(9)
An innovative active instrument (Pisla, 2024 b)
was developed and integrated with the robot.
The active instrument features a 10 mm diameter
shaft with an articulated distal head and four degrees
of freedom (DOF) to improve the workspace
compared to standard rigid laparoscopic instruments,
q
insi
, i=1…4.
The 3D design of the instrument and the
movements are illustrated in Figure 4.
Four actuators are used to control the instrument
movements (Figure 4): opening/closing of the distal
head (q
1
), rotation of the distal head (q
2
), flexible
element bending (q
3
) and the rotation of the entire rod
(q
4
).
The flexible segment (Figure 4) provides distal
head bending capability. The instrument is
manufactured using 3D printing (Stratasys J5 Prime
Med).
Figure 4: The 3D design of the active instrument and its
movements.
3 THE CONTROL SYSTEM
The control system converts surgeon hand motions
into robot commands using either a 3D Space Mouse
(3DConnexion, Munich, Germany, 2001) or
Omega.7 haptic device (Force Dimension,
Switzerland, 2001) as master console. The robot
reproduces the surgeon's movements following
established safety measures (Vaida, 2016).
Figure 5: Hardware architecture of the Athena parallel robot.
ICINCO 2025 - 22nd International Conference on Informatics in Control, Automation and Robotics
246

Figure 5 illustrates the hardware architecture of
the Athena parallel robot. The entire system can be
divided into three main modules, the surgical
instrument, the Athena robot and the spherical
module.
To control the surgical instrument, five Pololu
micro metal gear-box motors are used and to obtain
position feedback five Pololu Romi encoders are
used. To control the motors, two Motoron M2H18v18
controllers are used, with two different addresses. The
controllers are receiving data that is converted to
PWM signals from Raspberry Pi 5-2 via I²C BUS
communication protocol that allows the Raspberry Pi
to control multiple devices. The 12V supply powers
the Motoron controllers and Pololu motors, while the
5V supply operates the Raspberry Pi 5-2.
The Athena robot is controlled using a B&R PLC
system employing two B&R hybrid stepper motors
and one Nanotec stepper motor with integrated
encoders. Motor control is achieved using two B&R
stepper controllers. Three LANBAO inductive
sensors are used for the homing procedure.
To control both robot and the instrument, the
Raspberry pi 5 - 1 is receiving the data from a 3D
space mouse or from the haptic device via USB. The
PLC is receiving data from the rasp Raspberry pi 5 –
1 via Modbus, being connected to the LAN network
of the system though the Network Switch. The same
protocol and LAN network is used to send data from
the PLC to the second Raspberry Pi 5.
The spherical module represents the last element
of the system. To know the position and the
orientation of the module, three BNO055 Absolute
orientation modules and four AS5600 magnetic
encoders are used.
Three absolute orientation modules with 9-axis
IMUs determine the spherical module's position and
orientation.
Four magnetic encoders provide precise angle
feedback for each revolute joint of the spherical
mechanism. IMU sensors and encoders communicate
with Raspberry Pi 5 via I²C protocol through a
multiplexer powered by a 3.3 V supply. The
multiplexer manages multiple I²C devices and
prevents address conflicts, enabling simultaneous
data acquisition from all sensors and encoders.
Two lasers powered from a 3.3 V supply are used
to aid in the positioning of the RCM, with the RCM
located at the intersection of both laser beams.
Figure 6 presents the state machines of the PLC
and two raspberry PI 5 boards. On the PLC side, the
initial state that is set on system power up is INIT,
which waits for every component of the system to be
powered up and ready for communication. If
successful, the system proceeds to the IDLE state in
which it awaits user inputs via the GUI such as:
Homing, Power, Reset etc. Once the user selects the
structure to control (Robot or active instrument) and
a method of controlling said structure (Haptic or
Space Mouse) the state machine switches to
MOVE_STATE. A secondary state machine allows
the smooth change between controlling the robot and
the active instrument and switching between control
peripherals. The Auxiliary Raspberry PI 5 that is used
for gathering data from the control peripherals and
secondary sensors (BNO-055 and AS5600) is
connected via MODBUS to the system’s LAN
network and presents a state machine of its own. A
simple one that connects, reads and sends the data
back to the PLC via Modbus. The instrument’s
Raspberry PI 5 also has a state machine that connects,
reads commands and data from the PLC and moves
the instrument’s tip according to the user’s input to
grasp, bend, twist and rotate.
4 EXPERIMENTAL TESTING
AND VALIDATION
Figure 7 illustrates the experimental setup that was
developed. Robot calibration is required before
testing (Pisla, 2009). The calibration consists of a
homing procedure for the active linear joints of the
robot (q
i
, i=1…3).
One IMU positioned on the robot frame represents
the reference, the second is positioned on the link
between the two spherical joints connecting the robot
frame with the spherical mechanism and one on the
spherical mechanism. The fixed one, positioned on
the robot frame, is aligned with the robot reference
system (OXYZ).
These sensors are only used once in the
determination of the RCM, specifically at system
power up, ensuring that angle drift has a minimal or
no impact on the Euler angles.
Development of a Control System for an Innovative Parallel Robot Used in Laparoscopic Pancreatic Surgery
247
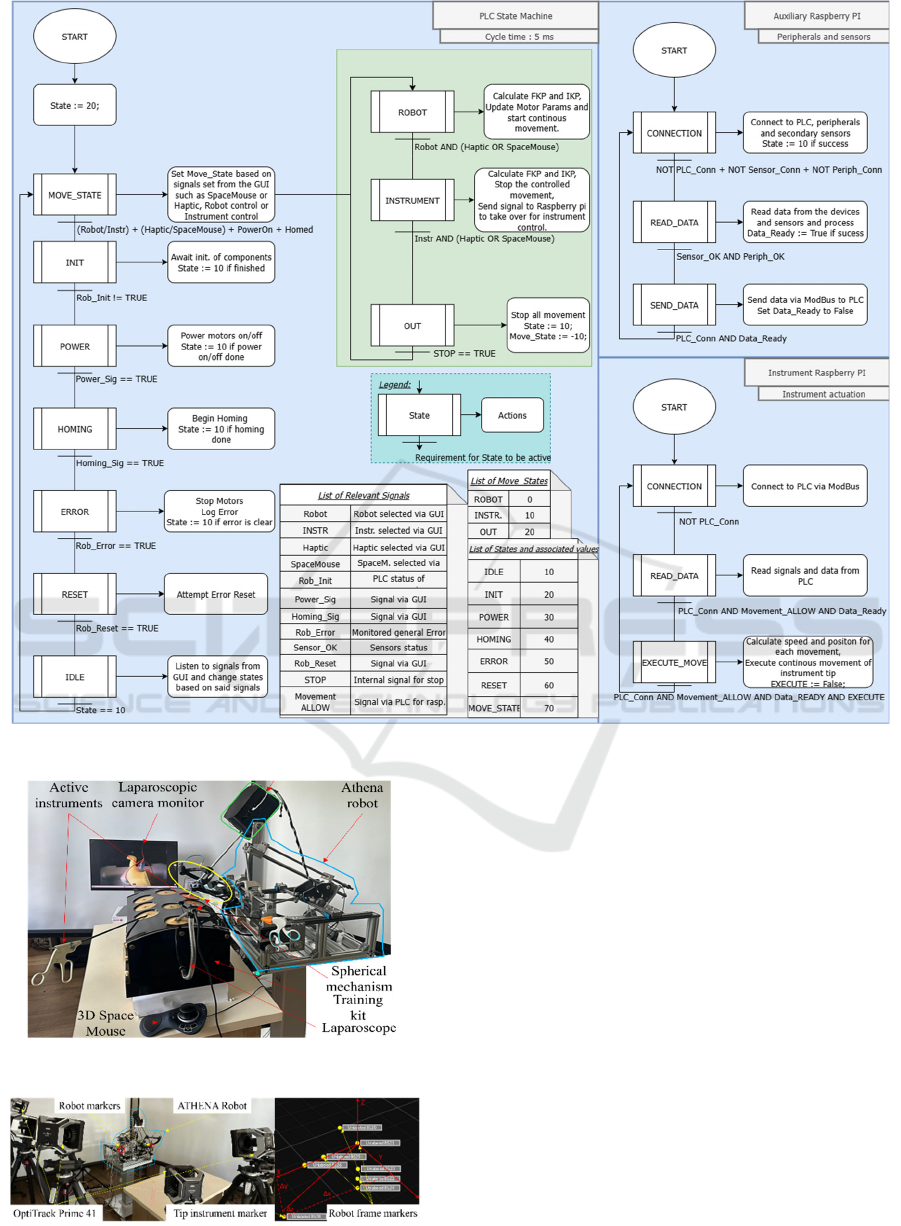
Figure 6: Athena parallel robot state machines.
Figure 7: Experimental setup of the Athena robot.
Figure 8: Setup for RCM validation using Optitrack.
The RCM position within the robot coordinate
frame is automatically calculated using the IMU
sensors output, namely the yaw-pitch-roll angles
(following the z-y-x rotation convention). Thus, the
RCM position in the robot coordinates frame is:
12
01 2
1 001
T
Sph Sph SphMec T
RCM RCM RCM
Sph Sph
XYZ T T T R=⋅⋅ ⋅
(10)
where
1
0
Sph
T
is the transformation matrix from the
robot coordinate system to the first spherical joint,
2
1
Sph
Sph
T
the transformation matrix from the first to
second spherical joint,
2
SphMec
Sph
T
the transformation
matrix from the second spherical joint to the passive
spherical mechanism (the attaching point) and R is its
radius (Vaida, 2025). Knowing the coordinates of
point P (Eq. 3), the orientation of the instrument can
be determined using Eqs. 7-9.
The OptiTrack measurement system was used to
validate the detected RCM position obtained using
ICINCO 2025 - 22nd International Conference on Informatics in Control, Automation and Robotics
248

the IMU sensors and Eq 10. The setup for these
measurements is illustrated in Figure 8, which shows
the placement of the markers on the experimental
model to determine the coordinate system axes and
the actual position of the RCM (measured), as well as
their display in the virtual environment using the
Motive software program. The measurements were
performed after the IMU sensors calibration, which
reduces the drift) and the determined RMSE has been
of 0.58 mm, with a maximum positioning error of
1.56 mm within the experimental tests.
Experimental tests were performed using a
minimally invasive surgery kit (Figure 9) with 3D
printed pancreas and stomach. The soft-material
organs (Vaida, 2025) enable testing scenarios where
the instrument grasps and withdraws the stomach
from the pancreatic field, holding it outside the
intraoperative workspace required for pancreatic
procedures.
Figure 9: Stomach grasping and manipulation to generate
intraoperative field.
5 CONCLUSIONS
This paper presents the control architecture for the
Athena robot, a surgical assistant for laparoscopic
pancreatic surgery that manipulates an active
instrument. The master-slave control system uses
either a 3D Space Mouse or haptic device (Omega.7)
as the master console. The architecture integrates
hardware and software components to enable precise
surgical manipulation. Surgeons can define the RCM
position before insertion and modify it during surgery
as required. OptiTrack Motion Capture system
validation confirmed the approach's accuracy.
Experimental tests used a minimally invasive surgery
kit with 3D printed pancreas and stomach to
demonstrate the flexibility and dexterity for
pancreatic surgery. Future research will focus on
experimental tests to simulate resection and
reconstruction of the pancreas during the Whipple
procedure, using a pancreas realized at a scale of 1 to
1 based on 3D reconstruction using real CT scan data,
improvement of the GUI and integration of the robot
into a surgical simulator.
ACKNOWLEDGEMENTS
This research was funded by the project New smart
and adaptive robotics solutions for personalized
minimally invasive surgery in cancer treatment -
ATHENA, funded by European Union – Next
Generation EU and Romanian Government, under
National Recovery and Resilience Plan for Romania,
contract no. 760072/23.05.2023, code CF
116/15.11.2022, through the Romanian Ministry of
Research, Innovation and Digitalization, within
Component 9, investment I8.
REFERENCES
McGuigan, A., Kelly, P., Turkington, R. C., Jones, C.,
Coleman, H. G.,McCain, R. S. Pancreatic cancer: A
review of clinical diagnosis, epidemiology, treatment
and outcomes. W. J. of Gastroenterology, 24(43),4846–
4861, 2018. doi:10.3748/wjg.v24.i43.4846.
Asbun, D., Lluis, N., Jimenez, R.E., Asbun, H. J. A
narrative review of minimally invasive pancreatic
surgery: from pipedreams to pancreatoduodenectomies,
DMR 7(0), DMR9393, 2023.
Balzano, G., Zerbi, A., Aleotti, F., Capretti, G., Melzi, R.,
Pecorelli, N. et al. Total Pancreatectomy With Islet
Autotransplan-tation as an Alternative to High-risk
Pancreatojejunostomy After Pancreaticoduodenecto-
my: A Prospective Randomized Trial. Annals of
Surgery 277(6): p894-903, 2023. doi:
10.1097/SLA.0000000000005713.
Bergholz, M., Ferle, M. & Weber, B.M. The benefits of
haptic feedback in robot assisted surgery and their
moderators: a meta-analysis. Sci Rep 13, 19215, 2023.
doi: 10.1038/s41598-023-46641-8.
Cawich, S. O., Cabral, R., Douglas, J., Thomas, D. A.,
Mohammed, F. Z., Naraynsingh, V., & Pearce, N. W.
Whipple's procedure for pancreatic cancer: training and
the hospital environment are more important than
volume alone. Surgery in Practice and Science, 14,
100211, 2023. doi: 10.1016/j.sipas.2023.100211.
Damoli, I., Butturini, G., Ramera, M., Paiella, S.,
Marchegiani, G., Bassi, C. Minimally invasive
pancreatic surgery - a review. Wideochir Inne Tech
Maloinwazyjne; 10(2):141-149; 2015.
doi:10.5114/wiitm.2015.52705.
De Pastena, M., van Bodegraven, E. A., Mungroop, T. H.,
Vissers, F. L., Jones, L. R., Marchegiani, G. et al. Distal
pancreatectomy fistula risk score (D-FRS):
Development of a Control System for an Innovative Parallel Robot Used in Laparoscopic Pancreatic Surgery
249

development and international validation. Annals of
Surgery, 277(5), e1099-e1105, 2023. doi:
10.1097/SLA.0000000000005497.
Rus, G., Andras, I., Vaida, C., Crisan, N., Gherman, B., Radu,
C., Tucan, P., Iakab, S., Hajjar, N.A., & Pisla, D.
Artificial Intelligence-Based Hazard Detection in
Robotic-Assisted Single-Incision Oncologic Surgery.
Cancers, 15, 3387, 2023. doi: 10.3390/cancers15133387.
Haidegger, T., Speidel, S., Stoyanov, D., & Satava, R. M.
Robot-assisted minimally invasive surgery—Surgical
robotics in the data age. Proceedings of the IEEE, 110(7),
835-846, 2022. doi: 10.1109/JPROC.2022.3180350.
Kastelan, Z., Hudolin, T., Kulis, T., Knezevic, N., Penezic,
L., Maric, M., & Zekulic, T. Upper urinary tract surgery
and radical prostatectomy with Senhance® robotic
system: Single center experience—First 100 cases. The
International Journal of Medical Robotics and
Computer Assisted Surgery, 17(4), e2269, 2021. doi:
doi.org/10.1002/rcs.2269.
Khachfe, H.H., Habib, J.R., Harthi, S.A. et al. Robotic
pancreas surgery: an overview of history and update on
technique, outcomes, and financials. J Robotic Surg 16,
483–494, 2022. doi:10.1007/s11701-021-01289-2.
Korayem, M. H., & Vahidifar, V. (2022). Detecting hand's
tremor using leap motion controller in guiding surgical
robot arms and laparoscopic scissors. Measurement,
204, 112133. (a)
Korayem, M. H., Madihi, M. A., & Vahidifar, V. (2021).
Controlling surgical robot arm using leap motion
controller with Kalman filter. Measurement, 178,
109372.
Korayem, M. H., Vosoughi, R., & Vahidifar, V. (2022).
Design, manufacture, and control of a laparoscopic
robot via Leap Motion sensors. Measurement, 205,
112186. (b)
Li, J., et al (2023). Application of Improved Robot-assisted
Laparoscopic Telesurgery with 5G Technology in
Urology. European urology, 83(1), 41–44. doi:
10.1016/j.eururo.2022.06.018.
McGuigan, A., Kelly, P., Turkington, R. C., Jones, C.,
Coleman, H. G.,McCain, R. S. Pancreatic cancer: A
review of clinical diagnosis, epidemiology, treatment
and outcomes. W. J. of Gastroenterology, 24(43),4846–
4861, 2018. doi:10.3748/wjg.v24.i43.4846.
Melvin WS, Needleman BJ, Krause KR, Ellison EC.
Robotic resection of pancreatic neuroendocrine tumor.
J Laparoendosc Adv Surg Tech A.; 13:33–6, 2003. doi:
10.1089/109264203321235449.
Minamimura, K., Aoki, Y., Kaneya, Y., Matsumoto, S.,
Arai, H., Kakinuma, D., & Yoshida, H. Current status
of robotic hepatobiliary and pancreatic surgery. Journal
of Nippon Medical School, 91(1), 10-19, 2024.
Najafinejad, A., & Korayem, M. H. (2023). Detection and
minimizing the error caused by hand tremors using a
leap motion sensor in operating a surgeon robot.
Measurement, 221, 113544.
Nießen, A, Hackert, T. State-of-the-art surgery for pancreatic
cancer. Langenbecks Arch Surg.;407(2):443-450, 2022.
Nortunen, M., Meriläinen, S., Ylimartimo, A., Peroja, P.,
Karjula, H., Niemelä, J., Saarela, A., Huhta, H.
Evolution of pancreatic surgery over time and effects of
centralizationa single-center retrospective cohort study.
J Gastrointest Oncol. 2023;14(1):366-378.
doi:10.21037/jgo-22-649.
Pisla, D., Carami, D., Gherman, B., Soleti, G., Ulinici, I.,
Vaida, C. A novel control architecture for robotic-
assisted single incision laparoscopic surgery. The
Romanian Journal of Technical Sciences. Applied
Mechanics., 66(2), 141-162, 2021.
Pisla, D., Popa, C., Pusca, A., Ciocan, A., Gherman, B.,
Mois, E., Cailean, A.-D., Vaida, C., Radu, C., Chablat,
D., Al Hajjar, N. On the Control and Validation of the
PARA-SILSROB Surgical Parallel Robot. Appl. Sci.,
14, 7925, 2024. doi: 10.3390/app14177925. (a)
Pisla, D., Chablat, D., Birlescu, I., Vaida, C., Pusca, A.,
Tucan, P. Gherman, B. AUTOMATIC INSTRUMENT
FOR ROBOT-ASSISTED MINIMALLY INVASIVE
SURGERY. Romania, Patent number: RO138293A0.
2024, pp.15. (b)
Pisla, D., Plitea, N., Gherman, B., Pisla, A., Vaida, C.
Kinematical Analysis and Design of a New Surgical
Parallel Robot. In Computational Kinematics;
Kecskeméthy, A., Müller, A., Eds.; Springer:
Berlin/Heidelberg, Germany, 2009.
Tucan, P., Vaida, C., Horvath, D., Caprariu, A., Burz, A.,
Gherman, B., Iakab, S., Pisla, D. Design and
Experimental Setup of a Robotic Medica Instrument for
Brachytherapy in Non-Resectable Liver Tumors.
Cancers, 2022, 14, 5841.
Tucan, P.; Ciocan, A.; Gherman, B.; Radu, C.; Vaida, C.;
Hajjar, N.A.; Chablat, D.; Pisla, D. Design
Optimization of a Parallel Robot for Laparoscopic
Pancreatic Surgery Using a Genetic Algorithm. Appl.
Sci., 15, 4383., 2025. doi:10.3390/app15084383.
Vaida, C., Ciocan, A., Caprariu, A., Radu, C., Al Hajjar, N.,
Pisla, D. A 3D-printed anatomical pancreas model for
Robotic assisted Minimally Invasive Surgery. Journal
of Functional Biomaterials. J. Funct. Biomater. 2025,
16, 207. (a)
Vaida, C.; Birlescu, I.; Gherman, B.; Condurache, D.;
Chablat, D.; Pisla, D. An analysis of higher-order
kinematics formalisms for an innovative surgical
parallel robot. Mech. Mach. Theory, 2025, 209,
105986. (b)
Vaida, C.; Pisla, D.; Schadlbauer, J.; Husty, M.; Plitea, N.
Kinematic analysis of an innovative medical parallel
robot using study parameters. In New Trends in
Medical and Service Robots. Mechanisms and Machine
Science; Wenger, P., Chevallereau, C., Pisla, D.,
Bleuler, H., Rodić, A., Eds.; Springer: Cham,
Switzerland, 2016; Volume 39.
Wong MCS, Jiang JY, Liang M, Fang Y, Yeung MS, Sung
JJY. Global temporal patterns of pancreatic cancer and
association with socioeconomic development. Sci Rep.
2017;7(1):3165. Published 2017 Jun 9.
doi:10.1038/s41598-017-02997-2.
Zhang, W., Wang, Z., Ma, K. et al. State of the art in
movement around a remote point: a review of remote
center of motion in robotics. Front. Mech. Eng. 19, 14
2024. doi:10.1007/s11465-024-0785-3.
ICINCO 2025 - 22nd International Conference on Informatics in Control, Automation and Robotics
250
