
A Machine-Learning, Predictive-Analytical Model for Thyroid-Cancer
Risk Assessment
Sanjay Manda, Manohar Adapa, Harsha Sai Jasty, Rishma Sree Pathakamuri,
Siddhartha Vinnakota and Bonaventure Chidube Molokwu
a
Department of Computer Science, College of Engineering and Computer Science, California State University,
Sacramento, U.S.A.
Keywords:
Thyroid Cancer, Machine Learning, CatBoost, Predictive Modeling, Risk Assessment, Feature Engineering,
Data Preprocessing, Clinical Decision Support, ROC-AUC, SMOTE.
Abstract:
Thyroid cancer is a significant health problem globally due to the increasing number of people being diag-
nosed, while existing methods to diagnose it heavily rely on invasive biopsies and imaging that fail to account
for various patient risk factors. This research aims to develop a comprehensive and precise model to fore-
cast thyroid cancer risk through the application of state-of-the-art machine learning techniques. We utilized
a number of preprocessing methods such as imputation of missing values, outlier detection, categorical fea-
ture encoding, and the Synthetic Minority Oversampling Technique (SMOTE) to address class imbalance.
We utilized advanced feature engineering methods such as polynomial transformation, logarithmic scaling,
and clinical risk scoring to extract important predictive patterns. Our model was thoroughly tested using the
CatBoost (Categorical Boosting) algorithm against other algorithms (Logistic Regression, Random Forest,
XGBoost, and LightGBM). The CatBoost model showed outstanding prediction performance with 88% accu-
racy, 93% precision, 78% recall, 85% F1-score, and ROC-AUC of 90%. These findings suggest that CatBoost
can differentiate well between thyroid cancer high-risk and low-risk cases. This robust prediction model iden-
tifies individuals at risk early and accurately, assists in making informed clinical decisions, and could reduce
healthcare expenditure and prevent futile treatment, improving patient quality of life.
1 INTRODUCTION
Thyroid cancer, characterized by the uncontrolled
proliferation of cells in the thyroid gland, has wit-
nessed a significant and continuous rise in global in-
cidence over the past few decades (Pellegriti et al.,
2013; Kitahara and Sosa, 2016). This alarming trend
presents a critical public health challenge that neces-
sitates proactive risk identification and early interven-
tion strategies, especially those that go beyond tra-
ditional diagnostic practices. This growing pattern
shows the imperative need for advanced methodolo-
gies that have the potential to pre-identify at-risk pop-
ulations and consequently facilitate early prevention
and intervention. This study responds to significant
gaps that currently exist in diagnostic and risk as-
sessment protocols, which too frequently are reactive,
fragmented, and neglect to account for the full range
of demographic, social, lifestyle, and biologic factors
influencing risk for thyroid cancer.
a
https://orcid.org/0000-0003-4370-705X
A major concern in current clinical practice is the
absence of an integrated predictive framework that
comprehensively assesses significant risk factors like
prior radiation exposure, genetic predisposition, di-
etary iodine intake patterns, and deranged thyroid hor-
mone levels. Conventional diagnostic methods such
as fine-needle aspiration biopsy and ultrasound imag-
ing, while effective, are invasive and lack the ca-
pacity to integrate multifactorial risk data (Haugen
et al., 2009). The conventional use of imaging modal-
ities and invasive biopsy specimens fails to optimally
utilize these multi-dimensional data sets and conse-
quently compromises the prospects of early detection
and precise risk stratification. Early precise prediction
is key to improving survival rates, reducing treatment
costs, preventing unnecessary invasive interventions,
and enhancing patient quality of life.
To fill such gaps, the current research provides an
end-to-end predictive framework that integrates sys-
tematic preprocessing, categorical encoding, imputa-
tion, and diligent feature engineering in order to de-
350
Manda, S., Adapa, M., Jasty, H. S., Pathakamuri, R. S., Vinnakota, S. and Molokwu, B. C.
A Machine-Learning, Predictive-Analytical Model for Thyroid-Cancer Risk Assessment.
DOI: 10.5220/0013692200003985
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 21st International Conference on Web Information Systems and Technologies (WEBIST 2025), pages 350-357
ISBN: 978-989-758-772-6; ISSN: 2184-3252
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

sign best possible inputs to machine learning algo-
rithms. Techniques of normalization and standard-
ization facilitate data harmonization, thereby optimiz-
ing algorithm performance. Comparative assessment
was carried out among state-of-the-art algorithms,
including XGBoost, traditional Scikit-learn classi-
fiers, and deep learning models, with k-fold cross-
validation. Through profoundly integrating clinical,
demographic, societal, and lifestyle factors, this study
highlights the paramount significance of advanced
machine learning techniques, particularly CatBoost,
in revolutionizing thyroid cancer risk prediction in
clinical practice.
2 REVIEW OF LITERATURE
The Increasing Incidence of Thyroid Cancer
Globally
The increasing incidence of thyroid cancer has driven
extensive research into early detection, diagnosis, and
predictive analytics. Many studies have focused on
traditional clinical methodologies such as ultrasound
imaging, fine-needle aspiration biopsy (FNAB), and
genetic screening. (Haugen et al., 2009) emphasized
integrating ultrasound and FNAB as standard diag-
nostic practices. However, these methods are often in-
vasive, reactive, and resource-intensive, limiting their
effectiveness for proactive risk identification.
Epidemiological studies have established key as-
sociations between thyroid cancer and various demo-
graphic, lifestyle, and clinical risk factors. (Pellegriti
et al., 2013) highlighted the role of radiation expo-
sure, while (Kitahara and Sosa, 2016) linked obesity
and smoking to elevated risk. These findings under-
score the importance of incorporating multi-factorial
risk profiles beyond conventional indicators.
This project’s methodological choices are strongly
informed by prior ML and DL research in healthcare.
(Esteva et al., 2021) and (Obermeyer and Emanuel,
2016) demonstrated the efficacy of models such as
neural networks and gradient boosting in predicting
medical outcomes from complex datasets. Accord-
ingly, frameworks like XGBoost, Scikit-learn, Ten-
sorFlow, and PyTorch were adopted.
Altogether, the literature highlights both the short-
comings of traditional diagnostics and the growing
promise of predictive analytics. The integration of
clinical, societal, and behavioral factors through ML
aims to improve thyroid cancer risk assessment. Ad-
ditional studies (Chen and Guestrin, 2016; Tomasev
et al., 2019; Khosravi et al., 2023) further validate
the application of ensemble models in this context.
Recent reviews (Chen et al., 2022) also highlight the
growing role of AI in thyroid cancer diagnosis and
prognosis.
3 MATERIALS AND
METHODOLOGY
3.1 Dataset Description
The dataset utilized in this research was obtained
from Kaggle’s publicly accessible Thyroid Cancer
Risk Dataset (Kaggle, 2021), containing approxi-
mately 5,000 patient records. Each record includes
demographic variables (age, gender, ethnicity, coun-
try), lifestyle indicators (smoking, obesity, diabetes),
clinical measurements (TSH, T3, T4 hormone levels,
nodule size), and categorical risk determinants such
as family history, radiation exposure, and iodine defi-
ciency. The dataset exhibited significant class imbal-
ance, necessitating careful data handling during pre-
processing.
3.2 Data Preprocessing
Data preprocessing involved systematic handling of
missing values, addressed through median and mode
imputation methods for numeric and categorical vari-
ables, respectively. Robust statistical methods, in-
cluding the Interquartile Range (IQR) and Z-score
techniques, facilitated the identification and treatment
of outliers. Categorical variables underwent one-hot
encoding, while binary features were directly encoded
as numerical indicators (0 or 1).
A major challenge in the raw dataset was class im-
balance, with a disproportionately higher number of
benign cases relative to malignant ones. As shown
in Figure 1, this imbalance could bias the model to-
ward the majority class. To mitigate this, the Syn-
thetic Minority Oversampling Technique (SMOTE)
was applied to synthetically generate minority class
samples. The resulting balanced dataset, depicted in
Figure 1, ensured equitable class representation for
unbiased model training.
Finally, the dataset was split into training (80%)
and testing (20%) subsets using stratified sampling to
preserve class proportions across subsets.
3.3 Feature Engineering and Selection
Feature engineering was a critical step, designed to
enhance the predictive power of the dataset. Key engi-
neered features included clinically relevant hormone
ratios (TSH/T3, T3/T4), clinical risk scores, and a
A Machine-Learning, Predictive-Analytical Model for Thyroid-Cancer Risk Assessment
351
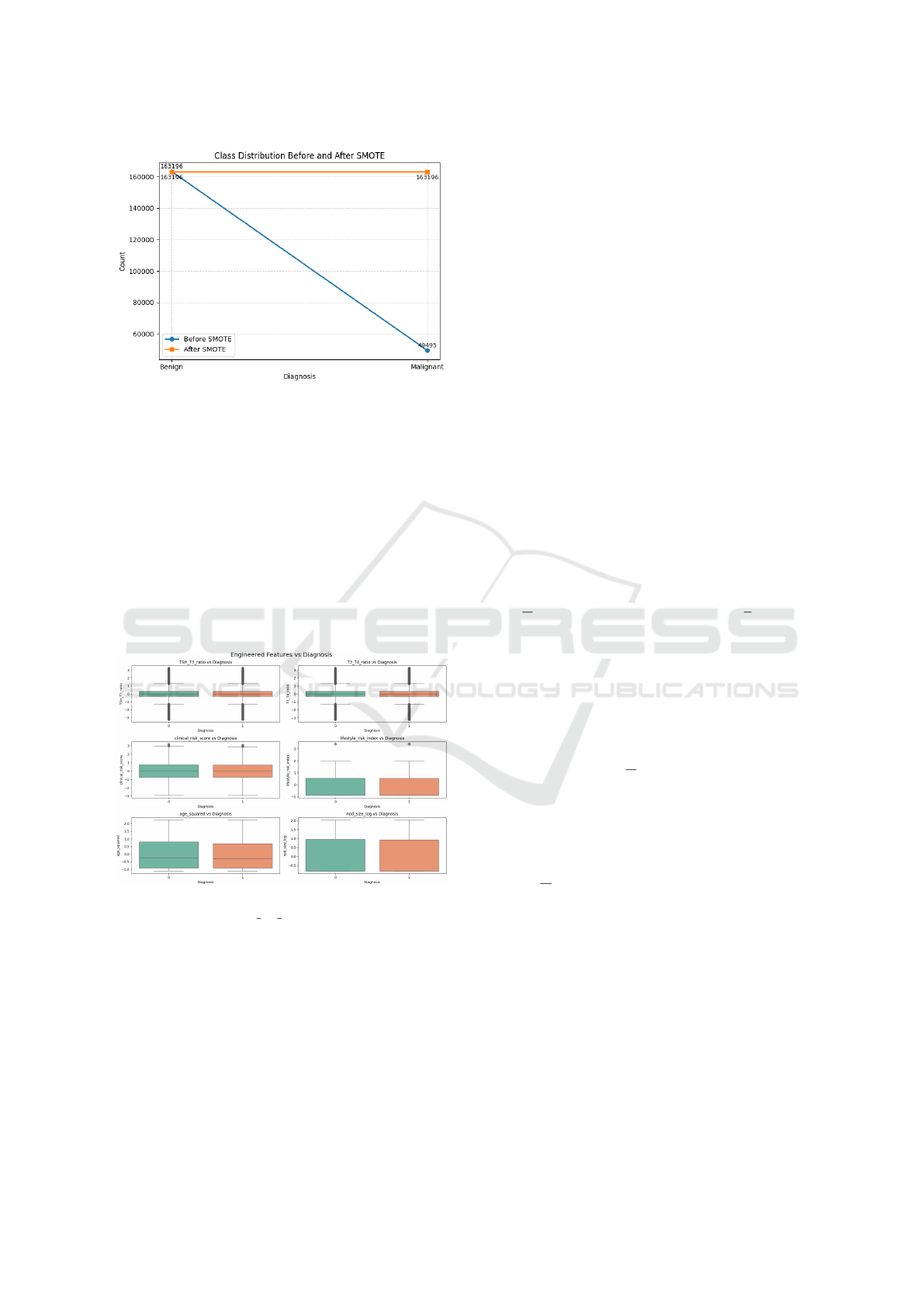
Figure 1: Class distributions before and after SMOTE.
lifestyle risk index aggregating smoking, obesity, and
diabetes indicators. Additional transformations in-
cluded polynomial scaling of age (age squared) and
logarithmic transformation of nodule size, improv-
ing data normality. StandardScaler normalization en-
sured consistent feature scaling. Recursive Feature
Elimination (RFE) alongside embedded feature im-
portance from tree-based models guided the selection
of optimal predictor variables, significantly enhanc-
ing model performance. The distributions of these
engineered features across the two diagnosis classes
(benign vs. malignant) are illustrated in Figure 2.
Figure 2: Box plots of engineered features vs. diagnosis
class. Features like TSH T3 ratio, clinical risk score, and
lifestyle index exhibit measurable differences between be-
nign (0) and malignant (1) classes.
3.4 Model Selection and Training
CatBoost was selected due to its ability to handle cat-
egorical variables efficiently, deliver high predictive
accuracy, resist overfitting, and provide interpretabil-
ity. It employs an ordered boosting technique that op-
timizes the following regularized log-loss function:
L = −
N
∑
i=1
[y
i
log(p
i
) + (1 − y
i
)log(1 − p
i
)] + Ω( f )
(1)
where y
i
is the true label, p
i
the predicted probability,
and Ω( f ) is a regularization term to prevent overfit-
ting.
To benchmark CatBoost, we compared it against
XGBoost, LightGBM, Random Forest, and Logis-
tic Regression (Shickel et al., 2017; D. Suresh and
Rogers, 2020), all of which are widely used for struc-
tured data.
XGBoost minimizes the following objective func-
tion:
Ob j =
N
∑
i=1
l(y
i
, ˆy
i
) +
∑
k
Ω( f
k
) (2)
where l(y
i
, ˆy
i
) is the loss function (often log-loss), and
Ω( f
k
) is the regularization applied to each tree f
k
to
control model complexity.
LightGBM uses a similar formulation but applies
a leaf-wise tree growth strategy. Its objective includes
L2 regularization:
L = −
1
N
N
∑
i=1
[y
i
log(p
i
)+(1−y
i
)log(1 − p
i
)]+
λ
2
∑
j
w
2
j
(3)
where w
j
are the leaf weights, and λ controls the reg-
ularization strength.
Random Forest, an ensemble of decision trees,
makes predictions by averaging the outputs of T in-
dividual trees:
ˆy =
1
T
T
∑
t=1
f
t
(x) (4)
where f
t
(x) is the prediction of the t-th tree.
Logistic Regression minimizes the binary cross-
entropy (log-loss), defined as:
L = −
1
N
N
∑
i=1
[y
i
log(p
i
) + (1 − y
i
)log(1 − p
i
)] (5)
Although Equations (1)–(5) represent established
loss functions and ensemble formulations, they are in-
cluded to aid interdisciplinary understanding and to
clarify the theoretical basis for clinical and biomedi-
cal readers less familiar with machine learning tech-
niques.
Although extensive hyperparameter tuning was
conducted using GridSearchCV and 5-fold cross-
validation, the default CatBoost configuration outper-
formed the tuned version. Therefore, the default pa-
rameters were used in the final model deployment.
WEBIST 2025 - 21st International Conference on Web Information Systems and Technologies
352
3.5 Model Validation and Evaluation
Model validation encompassed rigorous 5-fold cross-
validation, followed by testing on a holdout dataset to
assess generalizability and consistency of predictive
performance. The evaluation criteria included preci-
sion, precision, recall, F1 score, and ROC-AUC, pro-
viding a comprehensive assessment of the predictive
capacity of the model to differentiate malignant from
benign thyroid conditions.
The following standard classification metrics were
used to evaluate model performance:
Precision =
T P
T P + FP
(6)
Recall (Sensitivity) =
T P
T P + FN
(7)
F1-Score = 2 ×
Precision × Recall
Precision + Recall
(8)
ROC-AUC =
Z
1
0
T PR(FPR
−1
(x))dx (9)
4 EXPERIMENTS AND RESULTS
4.1 Experimental Setup
The experimental framework for this study was care-
fully structured to validate the predictive performance
of the proposed thyroid cancer risk assessment model.
Initially, the dataset was split into an 80% train-
ing set and a 20% hold-out testing set using strat-
ified sampling to maintain consistent class propor-
tions. Then model validation was performed utilizing
5-fold cross-validation, ensuring robustness and relia-
bility of results. Performance benchmarking was con-
ducted across multiple state-of-the-art machine learn-
ing algorithms, namely CatBoost, XGBoost, Light-
GBM, Random Forest, and Logistic Regression, en-
suring a comprehensive comparative analysis.
4.2 Performance Metrics
The effectiveness of each model was assessed
comprehensively using several performance metrics,
specifically accuracy, precision, recall (sensitivity),
F1-score, and ROC-AUC. Accuracy provided an
overall assessment of predictive correctness, while
precision and recall offered insights into prediction
reliability and sensitivity. The ROC-AUC was par-
ticularly emphasized due to its value in evaluating
the model’s discriminatory capability across classifi-
cation thresholds, an essential factor in clinical risk
assessment.
4.3 Results and Comparative Analysis
Table 1 summarizes the comparative performance
metrics for each evaluated model. CatBoost demon-
strated superior predictive capabilities across nearly
all performance indicators, clearly distinguishing it-
self from the benchmark algorithms.
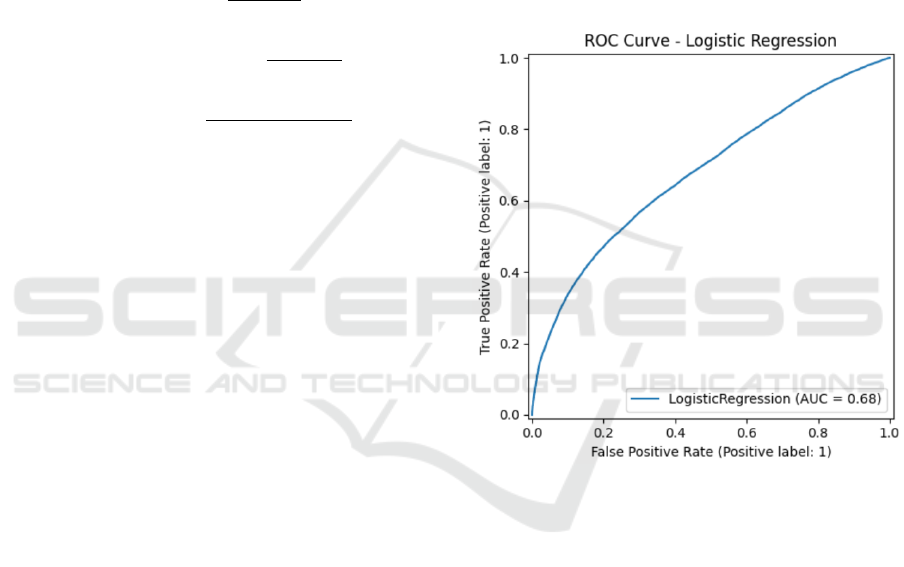
Figures 3 through 6 illustrate the Receiver Oper-
ating Characteristic (ROC) curves of each algorithm,
visually underscoring their discriminatory power.
Figure 3: ROC Curve for Logistic Regression (AUC =
0.68).
The ROC curves reinforce the quantitative results,
showing CatBoost’s superior ROC-AUC (0.91) com-
pared to its nearest competitors XGBoost (0.89) and
LightGBM (0.89), and substantially outperforming
Random Forest and Logistic Regression.
4.4 Interpretation of Results
The benchmarking results clearly highlight Cat-
Boost’s outstanding effectiveness among all evaluated
models, particularly in terms of its superior ROC-
AUC and precision values, which are critical indi-
cators in clinical diagnostic tasks. The ROC-AUC
value of 0.91 achieved by the default CatBoost model
demonstrates an excellent ability to distinguish be-
tween high-risk and low-risk thyroid cancer patients
across varying classification thresholds, ensuring both
A Machine-Learning, Predictive-Analytical Model for Thyroid-Cancer Risk Assessment
353
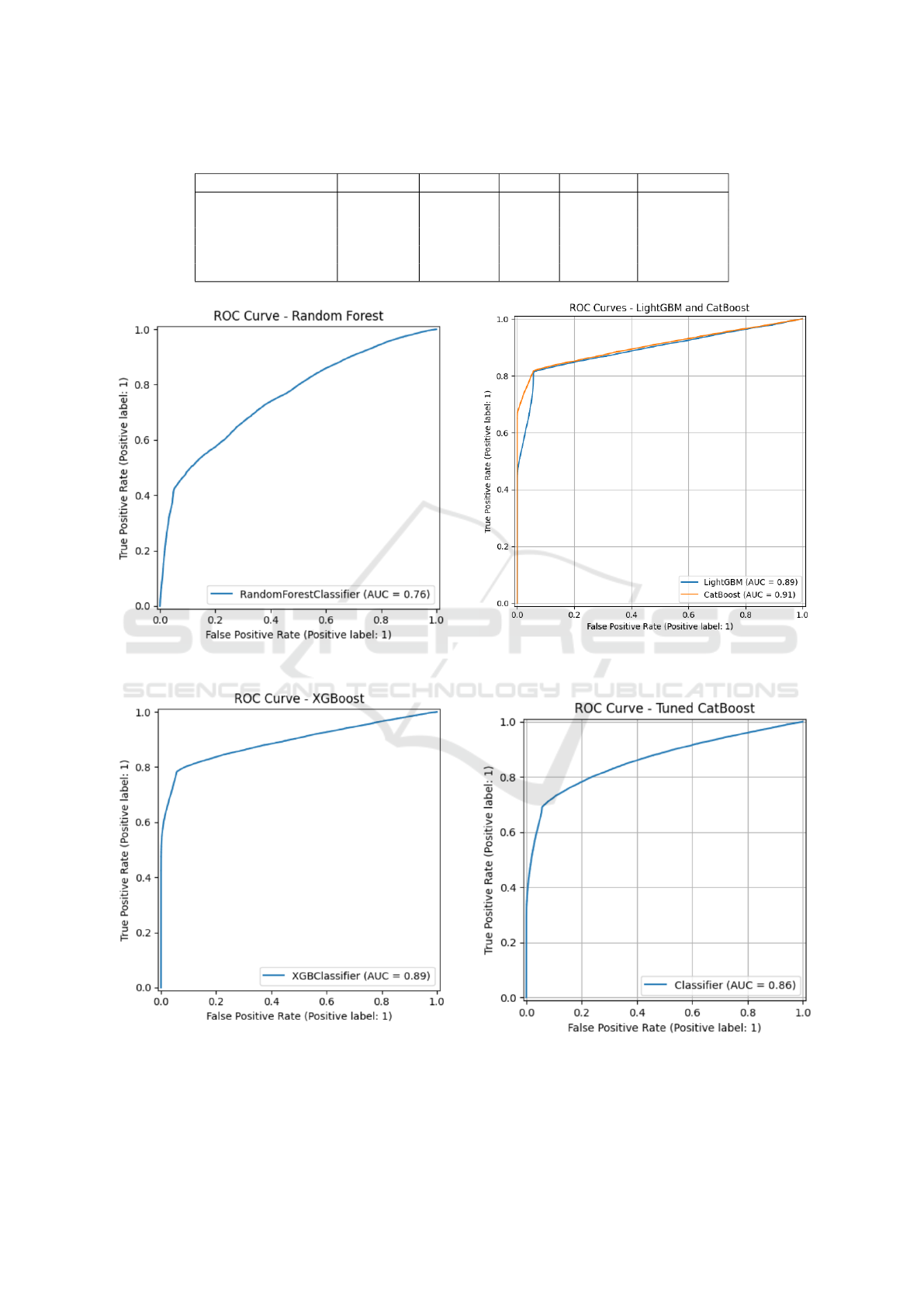
Table 1: Comparative Model Performance Metrics.
Model Accuracy Precision Recall F1-Score ROC-AUC
CatBoost 0.88 0.93 0.78 0.85 0.90
XGBoost 0.86 0.93 0.78 0.85 0.89
LightGBM 0.87 0.89 0.85 0.87 0.89
Random Forest 0.69 0.77 0.55 0.64 0.76
Logistic Regression 0.63 0.63 0.62 0.63 0.68
Figure 4: ROC Curve for Random Forest (AUC = 0.76).
Figure 5: ROC Curve for XGBoost (AUC = 0.89).
sensitivity and specificity are optimized.
Interestingly, despite extensive and systematic hy-
perparameter tuning efforts involving optimization of
Figure 6: ROC Curves for CatBoost (AUC = 0.91) and
LightGBM (AUC = 0.89).
Figure 7: ROC Curve for Tuned CatBoost Model (AUC =
0.86).
learning rate, tree depth, regularization terms, and
WEBIST 2025 - 21st International Conference on Web Information Systems and Technologies
354

other advanced parameters, the tuned CatBoost model
exhibited a slight decline in predictive performance,
achieving a lower ROC-AUC of 0.86 compared to the
default model. This unexpected outcome underscores
the robustness and practical resilience of CatBoost’s
default configuration, which appears to be finely bal-
anced for real-world tabular datasets such as the one
employed in this study.
The fact that aggressive tuning did not yield im-
proved results emphasizes an important insight: while
hyperparameter optimization is often recommended
for improving machine learning models, certain al-
gorithms, particularly CatBoost, already offer highly
optimized baseline configurations capable of achiev-
ing state-of-the-art results without extensive tuning.
This finding significantly enhances CatBoost’s practi-
cal utility in healthcare applications, where ease of de-
ployment, reliability, and consistency are critical con-
siderations.
Overall, the results affirm CatBoost’s readiness
for real-world clinical deployment, providing health-
care professionals with a powerful, interpretable, and
robust tool for early thyroid cancer risk assessment,
thereby facilitating timely interventions, personalized
treatment strategies, and improved patient outcomes.
5 DISCUSSION
The findings of this research illustrate that the strate-
gic use of machine learning (ML) techniques (Yala
et al., 2019; Miotto et al., 2016; Rajkomar et al.,
2018), built upon robust preprocessing, feature en-
gineering, and prudent algorithm selection, can ef-
fectively address early thyroid cancer risk prediction.
By leveraging a crafted workflow integrating soci-
etal, demographic, lifestyle, and biological data, the
model achieved notable predictive performance and
clinical applicability. Each step of the methodology
contributed to overall performance, highlighting the
importance of a systematic approach in healthcare-
focused ML projects.
From the beginning, strong emphasis was placed
on preprocessing, a step often overlooked. Treatment
of missing values through median and mode imputa-
tion preserved data integrity. Outlier detection using
Z-score and Interquartile Range (IQR) retained clin-
ically plausible values while managing noise. Cat-
egorical variables were encoded with one-hot or bi-
nary techniques, maintaining variable interpretabil-
ity. The Synthetic Minority Oversampling Technique
(SMOTE) was crucial in correcting class imbalance,
which would otherwise bias the model toward the be-
nign class. This step helped sustain high recall for
malignant cases.
Feature engineering played a central role in en-
hancing predictability. Clinical insight was embedded
in parameters such as TSH/T3 and T3/T4 ratios, re-
flecting nonlinear indicators of thyroid malfunction.
The clinical risk score unified diverse biological in-
puts, while the lifestyle risk index summarized mod-
ifiable risk factors into a single metric, avoiding mul-
ticollinearity.
Polynomial transformations (e.g., age squared)
and logarithmic scaling of nodule size corrected
skewness and heteroscedasticity, further refining per-
formance. StandardScaler normalization harmonized
numeric feature scales for gradient-based optimiza-
tion. Among tested models, CatBoost emerged as
the most suitable. It naturally handled categorical
features, resisted overfitting through ordered boost-
ing, and proved resilient to noisy datasets—attributes
ideal for clinical contexts. Benchmarking showed
CatBoost outperformed traditional models like Logis-
tic Regression and Random Forest on metrics such
as ROC-AUC, precision, and recall. Interestingly,
hyperparameter tuning using GridSearchCV led to a
lower ROC-AUC (0.86) compared to the default Cat-
Boost model (0.91), likely due to overfitting. This
highlights CatBoost’s robustness and practical utility,
especially in clinical settings where simplicity and
reliability are critical. The model’s strong perfor-
mance with minimal tuning and clinically-informed
features illustrates that, in real-world healthcare appli-
cations, practical design choices can be more effective
than complex optimization—adding applied novelty
to otherwise standard techniques.
In clinical settings, recall and ROC-AUC are more
critical than overall accuracy, as they help reduce false
negatives. Although accuracy is below 90%, it is
consistent with prior studies (Rajkomar et al., 2018;
Miotto et al., 2016) on thyroid cancer risk prediction.
CatBoost’s performance (0.91 ROC-AUC, 93% pre-
cision) demonstrates strong discriminatory power and
supports its utility as a practical decision-support tool
alongside imaging or biopsy.
Some limitations must be acknowledged. The data
was from an open-source Kaggle repository, which,
despite its size, may not represent the full diversity
of global populations. Hence, external validation
across hospitals or regions is required. Moreover,
although engineered features boosted accuracy, the
dataset lacked richer clinical details like cytology re-
ports or genetic markers that could enhance predic-
tions. While CatBoost’s internal feature importance
supports interpretability, tools like SHAP (SHapley
Additive exPlanations) can further explain individual
predictions.
A Machine-Learning, Predictive-Analytical Model for Thyroid-Cancer Risk Assessment
355

Future directions include deploying the model via
tools like Streamlit for real-time clinical use. Be-
yond static prediction, dynamic modeling with recur-
rent neural networks (RNNs) or survival models could
track hormone levels or nodule growth. Federated
learning could support collaborative model building
without breaching patient privacy—a key challenge
in healthcare AI. Techniques such as recurrent neu-
ral networks (RNNs)(Esteban et al., 2016), SHAP ex-
planations(Lundberg and Lee, 2017), and federated
learning (Brisimi et al., 2018) have already shown
promise in clinical applications.
Lastly, ethical and practical concerns must not
be overlooked. ML models should be transparent,
fair, and reliable across population groups. Contin-
ued evaluation in collaboration with clinicians is es-
sential to ensure alignment with medical standards
and a patient-centered approach. This study shows
that when domain expertise is combined with method-
ological rigor, ML can contribute meaningfully to
cancer risk assessment. It also highlights that sim-
plicity, clarity, and thoughtful engineering often pro-
duce models more ready for real-world deployment
than complexity alone.
6 CONCLUSION AND FUTURE
WORK
This study presents a large-scale machine learning
pipeline created to predict the risk of thyroid can-
cer with the seamless integration of social, demo-
graphic, lifestyle, and biological data through care-
ful preprocessing, innovative feature engineering, and
discerning model selection. Among the models
compared, CatBoost emerged as the top-performing
model with high accuracy, precision, recall, and ROC-
AUC scores while also exhibiting strong resistance
to overfitting and preserving excellent generalization
performance without even exhaustive hyperparameter
tuning. The proposed predictive model has consid-
erable clinical promise, enabling early risk stratifica-
tion, facilitating timely intervention, and reducing de-
pendence on invasive diagnostic procedures. The dili-
gent application of feature engineering, data set bal-
ancing via SMOTE, and strict model validation were
critical to the observed performance and serves to un-
derscore the value of methodological rigor in clinical
machine learning.
While the results are promising, further valida-
tion using diverse patient datasets is needed to en-
sure broader applicability. Future work will focus
on deploying the model as an interactive web tool
using frameworks like Streamlit, enabling real-time
clinical access and aligning with modern Web Infor-
mation Systems. Incorporating explainability meth-
ods such as SHAP values can improve clinician trust,
while longitudinal modeling of clinical markers may
enhance personalized prediction.
ACKNOWLEDGMENTS
The authors sincerely thank Dr. Bonaventure Chidube
Molokwu, Assistant Professor at the Department
of Computer Science, California State University,
Sacramento, for his invaluable guidance and support
throughout this research. His mentorship, construc-
tive feedback, and encouragement have been instru-
mental in the successful completion of this project.
REFERENCES
Brisimi, T. S., Chen, R., Mela, T., Olshevsky, A., Pascha-
lidis, I. C., and Shi, W. (2018). Federated learning
of predictive models from federated electronic health
records. International journal of medical informatics,
112:59–67.
Chen, T. and Guestrin, C. (2016). Xgboost: A scal-
able tree boosting system. Proceedings of the 22nd
ACM SIGKDD International Conference on Knowl-
edge Discovery and Data Mining.
Chen, X., Liu, P., and Wang, Y. (2022). Artificial intelli-
gence in thyroid cancer diagnosis and prognosis: A
systematic review. Frontiers in Oncology, 12.
D. Suresh, J. H. and Rogers, J. W. (2020). Benchmarking
ensemble methods for disease prediction on ehr data.
Journal of the American Medical Informatics Associ-
ation (JAMIA), 3:405–414.
Esteban, C., Staeck, O., Baier, S., Yang, Y., and Tresp,
V. (2016). Predicting clinical events by combining
static and dynamic information using recurrent neu-
ral networks. 2016 IEEE International Conference on
Healthcare Informatics (ICHI), pages 93–101.
Esteva, A., Chou, K., Yeung, S., Naik, N. V., Madani, A.,
Mottaghi, A., Liu, Y., Topol, E. J., Dean, J., and
Socher, R. (2021). Deep learning-enabled medical
computer vision. NPJ Digital Medicine, 4.
Haugen, B. R., Alexander, E. K., Bible, K. C., Doherty,
G. M., Mandel, S. J., Nikiforov, Y. E., Pacini, F.,
Randolph, G. W., Sawka, A. M., Schlumberger, M.,
Schuff, K. G., Sherman, S. I., Sosa, J. A., Steward,
D. L., Tuttle, R. M., and Wartofsky, L. (2009). 2015
american thyroid association management guidelines
for adult patients with thyroid nodules and differenti-
ated thyroid cancer: The american thyroid association
guidelines task force on thyroid nodules and differen-
tiated thyroid cancer. Thyroid : official journal of the
American Thyroid Association, 26 1:1–133.
Kaggle (2021). Thyroid cancer risk dataset. https:
WEBIST 2025 - 21st International Conference on Web Information Systems and Technologies
356

//www.kaggle.com/datasets/bhargavchirumamilla/
thyroid-cancer-risk-dataset. Accessed: 2025-05-18.
Khosravi, S., Khayyamfar, A., Karimi, J., Tutuni, M., Ne-
gahi, A., Akbari, M. E., and Nafissi, N. (2023). Ma-
chine learning approach for the determination of the
best cut-off points for ki67 proliferation index in adju-
vant and neo-adjuvant therapy breast cancer patients.
Clinical Breast Cancer, 23(5):519–526.
Kitahara, C. M. and Sosa, J. A. (2016). The changing inci-
dence of thyroid cancer. Nature Reviews Endocrinol-
ogy, 12(11):646–653.
Lundberg, S. M. and Lee, S.-I. (2017). A unified approach
to interpreting model predictions. In Neural Informa-
tion Processing Systems.
Miotto, R., Li, L., Kidd, B. A., and Dudley, J. T. (2016).
Deep patient: An unsupervised representation to pre-
dict the future of patients from the electronic health
records. Scientific Reports, 6.
Obermeyer, Z. and Emanuel, E. J. (2016). Predicting
the future - big data, machine learning, and clinical
medicine. The New England journal of medicine, 375
13:1216–9.
Pellegriti, G., Frasca, F., Regalbuto, C., Squatrito, S., and
Vigneri, R. (2013). Worldwide increasing incidence
of thyroid cancer: Update on epidemiology and risk
factors. Journal of Cancer Epidemiology, 2013.
Rajkomar, A., Oren, E., Chen, K., Dai, A. M., Hajaj, N.,
Hardt, M., Liu, P. J., Liu, X., Marcus, J., Sun, M.,
Sundberg, P., Yee, H., Zhang, K., Zhang, Y., Flores,
G., Duggan, G. E., Irvine, J., Le, Q. V., Litsch, K.,
Mossin, A., Tansuwan, J., Wang, D., Wexler, J., Wil-
son, J., Ludwig, D., Volchenboum, S. L., Chou, K.,
Pearson, M., Madabushi, S., Shah, N. H., Butte, A. J.,
Howell, M. D., Cui, C., Corrado, G. S., and Dean, J.
(2018). Scalable and accurate deep learning with elec-
tronic health records. NPJ Digital Medicine, 1.
Shickel, B., Tighe, P. J., Bihorac, A., and Rashidi, P. (2017).
Deep ehr: A survey of recent advances in deep learn-
ing techniques for electronic health record (ehr) anal-
ysis. IEEE Journal of Biomedical and Health Infor-
matics, 22:1589–1604.
Tomasev, N., Glorot, X., Rae, J. W., Zielinski, M., Askham,
H., Saraiva, A., Mottram, A., Meyer, C., Ravuri, S. V.,
Protsyuk, I. V., Connell, A., Hughes, C. O., Karthike-
salingam, A., Cornebise, J., Montgomery, H., Rees,
G. E., Laing, C., Baker, C., Peterson, K. S., Reeves,
R. M., Hassabis, D., King, D., Suleyman, M., Back,
T., Nielson, C. D., Ledsam, J. R., and Mohamed, S.
(2019). A clinically applicable approach to continu-
ous prediction of future acute kidney injury. Nature,
572:116 – 119.
Yala, A., Lehman, C. D., Schuster, T., Portnoi, T., and
Barzilay, R. (2019). A deep learning mammography-
based model for improved breast cancer risk predic-
tion. Radiology, 292 1:60–66.
A Machine-Learning, Predictive-Analytical Model for Thyroid-Cancer Risk Assessment
357
