
Medical Image Classification Using Deep Neural Networks: An X-Ray
Classification
Addagatla Prashanth, Marripalepu Srihimamshu, Somla and Adavi Satya Vinay Aghamarsha
Department of CSE (AI & ML), Institute of Aeronautical Engineering, Hyderabad, India
Keywords:
X-ray Image Classification, Deep Learning, Convolutional Neural Networks (CNN), VGG16, VGG19,
ResNet, DenseNet, Inception Model, Medical Imaging.
Abstract:
Lung X-rays are among the most important for making medical diagnoses. In this work, we identify lung X-
rays as either Normal or Pneumonia using a range of deep learning models, such as VGG16, VGG19, ResNet,
DenseNet, and Inception. Before sending the images into the dense neural network, we suggest integrating a
new pooling layer. Both healthy and pneumonia-affected lung X-rays are included in our collection. Convo-
lutional neural networks (CNNs) are used to identify the X-rays’ condition and classify them appropriately.
By comparing our findings with those of other models, we analyzed our models using a confusion matrix and
quantified measures like precision and recall. We examine the CNN algorithm and provide the following ex-
amples: (I) With enough training data, deep learning algorithms can correctly categorize X-ray images of sick
lungs. (II) Compared to many typical models, the design may perform better by including an average pooling
layer at the end. (III) Optimizing hyperparameters improves the performance and accuracy of the model. (IV)
Our models outperform several existing CNN models with less trainable parameters when properly trained,
hyperparameter adjusted, and data augmented. With the accurate automation of X-ray image interpretation
made possible by this method, there may be less need for invasive MRI and CT scans, which subject patients
to high radiation doses.
1 INTRODUCTION
Humans effortlessly recognize and distinguish fea-
tures in images due to our brains constantly and sub-
consciously training on familiar data. In contrast,
computers perceive the world as arrays of numeri-
cal values representing critical aspects of images or
videos they attempt to identify. The interpretation
process for computers relies on image recognition al-
gorithms to analyze and understand visual content.
For example, identifying pedestrians and vehicles is
achievable through the categorization and sorting of
millions of images provided by users. Medicine is
a prime field requiring reliable image identification
systems, as it generates vast amounts of data that can
be used to train these systems. The main challenge
lies in effectively analyzing and processing this data
for practical use. Various methods exist for organiz-
ing medical data, with classification being a widely
used technique to detect disease symptoms. In im-
age classification, a computer analyzes an image and
assigns it to a specific class, a task that is straightfor-
ward for humans but exemplifies the Moravec para-
dox—simple for humans yet difficult for artificial in-
telligence. Early image classification methods relied
on analyzing pure pixels, which proved problematic
due to variations in backgrounds, angles, and other
factors. Deep learning, particularly through neural
networks, addresses this issue by enabling more so-
phisticated image recognition. However, classifica-
tion remains resource-intensive, often requiring op-
timization or enhanced computational resources to
achieve timely results. This study aims to use deep
learning models, including VGG16, VGG19, ResNet,
DenseNet, and Inception, to classify X-ray images for
pneumonia detection. We propose a novel approach
by integrating a pooling layer before the dense neural
network and evaluate the performance of our mod-
els using precision, recall, and confusion matrix met-
rics. Our goal is to demonstrate that, with adequate
training data and hyperparameter tuning, deep learn-
ing techniques can significantly improve the accuracy
and efficiency of medical image classification.
Prashanth, A., Srihimamshu, M., Somla, and Vinay Aghamarsha, A. S.
Medical Image Classification Using Deep Neural Networks: An X-Ray Classification.
DOI: 10.5220/0013659500004664
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 3rd International Conference on Futuristic Technology (INCOFT 2025) - Volume 3, pages 763-769
ISBN: 978-989-758-763-4
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
763

2 OBJECTIVES
• To use deep learning models to classify lung X-
ray pictures as either normal or pneumonia.
• To optimize model performance by implementing
a novel pooling layer and hyperparameter tuning
techniques.
• To assess and contrast the several deep learning
models (ResNet, DenseNet, VGG19, Inception,
and VGG16) for the categorization of X-ray im-
ages.
3 EXISTING SYSTEMS
Current image recognition systems leverage various
advanced methodologies to process and analyze vi-
sual data. Early systems relied on pixel-based anal-
ysis, which faced significant challenges due to varia-
tions in image backgrounds, angles, and lighting con-
ditions (Klette, 2014).
These limitations led to the development of more
sophisticated techniques involving deep learning,
which allows computers to recognize patterns and
features within images with greater accuracy (Krishna
et al., 2021). In the field of medicine, deep learn-
ing has been particularly transformative. Machine
learning models, such as convolutional neural net-
works (CNNs), have been widely used to classify
medical images, providing critical support in diag-
nostic processes (Borad, 2020). These models can
analyze vast amounts of data efficiently, offering re-
liable identification of diseases from X-ray images,
among other medical imaging modalities (Mochu-
rad & Yatskiv, 2020). Despite their effectiveness,
deep learning models are computationally intensive
and often require significant resources for training
and deployment. Advances in parallel computing and
optimization techniques have enabled more efficient
training of these models, reducing the time and re-
sources needed while improving performance (Shal-
lue et al., 2019). These advancements have made it
possible to implement deep learning models in real-
time diagnostic applications, thereby enhancing their
practical utility in clinical settings (Moujahid et al.,
2020).
Furthermore, the integration of CNNs with other
neural network architectures, such as recurrent neu-
ral networks (RNNs) with attention mechanisms, has
shown promising results in specific medical imag-
ing tasks, such as histology image classification for
breast cancer (Yao et al., 2019). These hybrid ap-
proaches leverage the strengths of multiple neural net-
work types to improve accuracy and robustness in
medical diagnostics.
In conclusion, current systems have established a
solid basis for the use of deep learning in medical
imaging, and further study is concentrated on refin-
ing these models to increase their effectiveness and
performance in clinical settings.
4 LITERATURE SURVEY
The literature on biomedical image analysis has seen
significant advancements. Afshar et al. (2018) pro-
posed a new Capsule Network model combined with
Convolutional Neural Networks (CNNs) for segment-
ing biomedical images, specifically applying their ar-
chitecture to MRI images of brain tumors. Their
study, which involved 233 subjects and 3,064 images,
achieved a maximum accuracy of 86.56% using a sin-
gle convolutional layer with 64 feature maps. How-
ever, the findings are limited by the specific dataset
used, which may affect generalizability.
Similarly, Frid-Adar et al. (2018) introduced a
hybrid model that integrates Generative Adversarial
Networks (GANs), CNNs, and synthetic data aug-
mentation to improve the segmentation and classifi-
cation accuracy of liver lesions. Nonetheless, their
reliance on synthetic data may introduce biases, lim-
iting real-world applicability. In a different study,
Cires¸an et al. (2013) used CNN-based deep neural
networks to detect mitosis in breast cancer histology
images, showing a significant improvement in detec-
tion performance. However the performance of the
model could differ for various histology datasets, in-
dicating the necessity for thorough validation..
Litjens et al. (2017) conducted a comprehensive
survey reviewing various architectures and their ap-
plications in this domain. The results of their study
demonstrated how well Convolutional Neural Net-
works (CNNs) and other deep learning models per-
formed image processing tasks. The survey did, how-
ever, also highlight the necessity for standardized
evaluation metrics and point out issues with dataset
variability. In the same line of thought, Shen et al.
(2017) examined the use of deep learning techniques,
concentrating especially on CNNs, and showed how
they might boost medical image processing signifi-
cantly in terms of accuracy and speed. They did, how-
ever, note that there are significant obstacles, includ-
ing the need for huge annotated datasets and high pro-
cessing requirements. Complementing these insights,
Reyes et al. (2018) presented a survey highlighting
the advancements and ongoing challenges in medical
imaging due to deep learning. Their work confirmed
INCOFT 2025 - International Conference on Futuristic Technology
764
the transformative potential of these techniques in im-
proving diagnostic accuracy, while also noting limi-
tations in data availability and the pressing need for
robust validation frameworks.
Deep learning applications in medical imaging are
the subject of a growing corpus of study. In their study
of several deep learning algorithms and architectures,
Shrestha and Mahmood (2019) focused on the appli-
cations in medicine. Convolutional Neural Networks
(CNNs) were found to be the most efficient archi-
tecture for a variety of medical imaging applications;
nevertheless, they also talked about the necessity for
explainable AI models in the healthcare industry and
the difficulties associated with interpretability. Raz-
zak et al. (2018) provided a deeper analysis of the
difficulties and potential applications of deep learn-
ing in medical image processing, stressing the sig-
nificant improvements in segmentation and classifica-
tion. They did, however, highlight the need for more
effective algorithms that can deal with huge medical
datasets.
Mohsen et al. (2018) concentrated on using CNN
architectures and deep learning neural networks for
brain tumor classification. They achieved high clas-
sification accuracy and proved that deep learning is
useful for tumor detection. However, the extent to
which their results can be applied to other tumor types
may be restricted because of their dependence on par-
ticular datasets. Lastly, Shin et al. (2016) verified
CNNs’ superiority in improving system performance
by looking into CNN designs, dataset properties, and
transfer learning for computer-aided detection sys-
tems. However, they highlighted that big, annotated
datasets—which can be resource-intensive—are nec-
essary for training good models.
5 PROPOSED METHODOLOGY
The proposed methodology uses advanced deep-
learning techniques to improve the classification
of lung X-ray images for diagnosing pneumonia.
We implement five renowned convolutional neu-
ral network (CNN) architectures—VGG16, VGG19,
ResNet, DenseNet, and Inception. Each model is fine-
tuned on a specialized dataset of lung X-ray images
to optimize their performance for this specific task. A
unique pooling layer is added before the dense layers
for better feature extraction and to eliminate overfit-
ting. Furthermore, we apply data augmentation and
hyperparameter tuning methods to improve the mod-
els’ accuracy and robustness. Medical practitioners
will have a reliable means to identify pneumonia and
the system’s quick processing and classification of X-
ray images. To confirm the proposed system’s perfor-
mance and guarantee its efficacy in a clinical scenario,
evaluation metrics like precision, recall, F1-score, and
confusion matrix are utilized. By using this method,
the system hopes to improve and automate the di-
agnostic process, which might decrease the need for
more intrusive treatments like CT and MRI scans.
6 DATASET INFORMATION
The dataset consists of approximately 15,000 X-ray
images categorized into normal, pneumonia, opaque,
COVID lung conditions, sourced from Kaggle. Each
image is in standard .png format, facilitating straight-
forward analysis. For uniformity, the photos were
scaled to 32 by 32 pixels, and the pixel values were
normalised to fall between 0 and 1. To improve model
robustness, data augmentation was used, which in-
volved rearranging the dataset. Eighty percent of the
photos in the dataset were utilised for training, while
twenty percent were used for testing. In order to expe-
dite the process, the dataset is uploaded from a desig-
nated directory, after which the photographs are anal-
ysed and labelled appropriately, guaranteeing a struc-
tured framework for further research. This dataset is
essential for creating and assessing models meant to
increase medical imaging diagnostic precision.
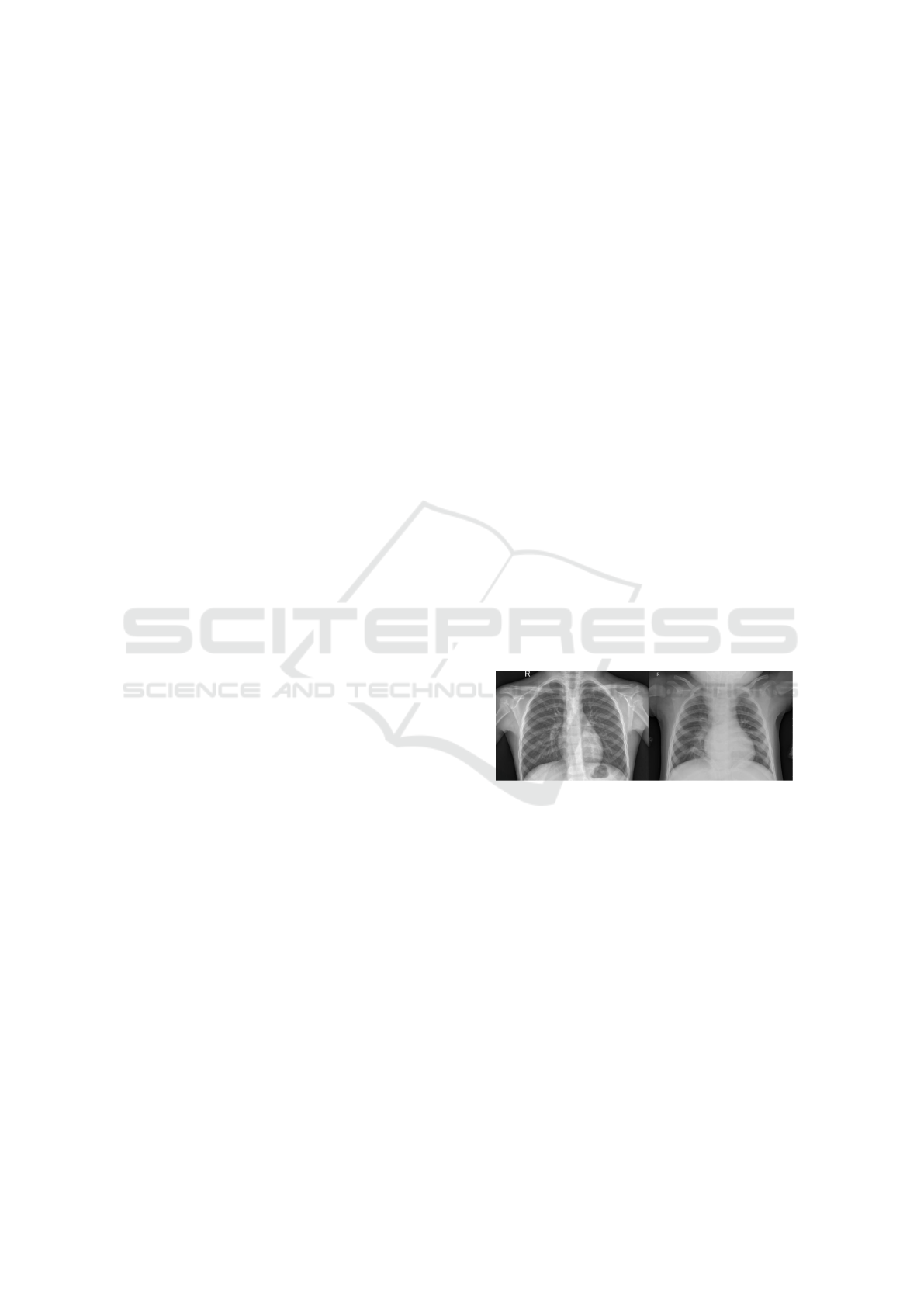
Figure 1: X-rays of the chest: left - without pneumonia,
right – with pneumonia
7 METHODOLOGY AND
IMPLEMENTATION
The study’s methodology section describes in great
detail how several deep learning architectures are
used to the classification of biological images. Con-
volutional neural networks (CNNs), generative ad-
versarial networks (GANs), and transfer learning are
some of the main methods covered. These techniques
are used to improve the precision and effectiveness of
medical image classification, including MRI, CT, and
OCT scans.
Medical Image Classification Using Deep Neural Networks: An X-Ray Classification
765

7.1 CNN Architecture
Using CNNs, the paper thoroughly examines layers
including convolutional, pooling, and fully connected
layers. Activation functions, specifically ReLU, and
dropout layers are used to prevent overfitting. The
method emphasises how important these layers are for
feature extraction and dimensionality reduction of the
input images as a requirement for accurate classifica-
tion.
7.2 Data Augmentation and
Preprocessing
In order to reduce overfitting and boost the range of
the training dataset, data augmentation techniques are
used. The dataset requires being set up for deep learn-
ing model training using preprocessing procedures
like image scaling and normalization. These stages
ensure that the input data is in a proper format for the
neural networks to process
7.3 Transfer Learning
It is noted that transfer learning is a useful method
for utilizing pre-trained models on sizable datasets
and optimizing them for particular biomedical image
classification applications. This method expedites the
training process and lessens the requirement for large
labeled datasets.
7.4 Algorithm
The implementation section details the practical ap-
plication of the discussed methodologies. The
deep learning models are implemented using popular
frameworks such as TensorFlow and PyTorch. The
implementation involves setting up the neural net-
work architectures, defining the loss functions, and
configuring the optimization algorithms for training
the models.
7.4.1 VGG16 and VGG19
Deep learning models from the Visual Geometry
Group, such as VGG16 and VGG19, are well known
for their ease of integration and efficiency in image
classification applications. What distinguishes these
architectures from one another is their homogene-
ity—13 or 16 convolutional layers for VGG16 and
VGG19, respectively. Small 3x3 filters with stride 1
are used in each convolutional layer to preserve spa-
tial resolution throughout the layers. Max-pooling
layers employing a 2x2 filter with stride 2 come after
the convolutional layers, hence lowering the computa-
tional cost and spatial dimensions. A softmax classi-
fier, which outputs the probability distribution across
the target classes, is the final fully connected layer in
both models. Each of the model’s initial two layers
has 4096 neurones. Following each convolutional and
fully connected layer, Rectified Linear Unit (ReLU)
activation functions are applied to give the model non-
linearity. With a focus on simplicity and depth, our ar-
chitecture produces significant performance gains on
picture classification benchmarks while maintaining a
neat, consistent design.
7.4.2 ResNet
ResNet, or Residual Networks, represents a break-
through in the design of deep neural networks by ad-
dressing the degradation problem that occurs when
adding more layers to a network. This degradation
problem leads to a decrease in accuracy as the net-
work depth increases, not due to overfitting but be-
cause of the inability to effectively train deeper net-
works. By using skip connections, also known as
identity shortcuts, which let the gradient pass straight
through the network layers, ResNet introduces resid-
ual learning. In order to accomplish this, residual
blocks are introduced, in which the input to a small
number of stacked layers is likewise added straight
to the output, so ”skipping” these levels. With archi-
tectures like ResNet50, which has 50 layers and can
learn exceedingly complicated features while remain-
ing easy to train, this method enables the creation of
incredibly deep networks. To improve convergence,
the network makes use of batch normalisation and
ReLU activations. ResNet can train networks with
hundreds or even thousands of layers because to its
creative architecture, which greatly enhances perfor-
mance on a range of computer vision tasks.
7.4.3 DenseNet
DenseNet, or Densely Connected Convolutional Net-
works, is an architecture that connects each layer to
the following one in a forward method. Each layer
in a DenseNet architecture receives as inputs the fea-
ture maps of all layers that came before it, creat-
ing a dense network of connectedness. Stronger gra-
dients during training, more effective parameter us-
age, and enhanced feature propagation are some of
the main benefits of this approach. DenseNet is di-
vided into dense blocks, each of which is made up of
several convolutional layers that carry out 3x3 con-
volutions. Between dense blocks, transition layers
are used to manage the complexity of the network
and lower the size of the feature maps. These lay-
INCOFT 2025 - International Conference on Futuristic Technology
766
ers consist of a 2x2 average pooling layer and a 1x1
convolution. ReLU activation functions are used in
the model, and average pooling rather than max pool-
ing is used for downsampling, which increases overall
efficiency. Compared to conventional convolutional
networks, DenseNet performs better with fewer pa-
rameters because it directly connects all layers, which
also helps to reduce the vanishing gradient issue.
7.4.4 Inception
The Inception architecture, known as GoogLeNet,
uses a multi-scale strategy at the network layers to
manage the variation in feature scale between images.
Each inception module in the network executes con-
volutions at several scales (1x1, 3x3, and 5x5) and an
overlapping max-pooling operation simultaneously.
The network is thus able to collect a large variety of
features at various scales by concatenating these con-
volutions along the depth dimension. To further re-
duce the computational stress on the following layers,
the architecture incorporates 1x1 convolutions. This
reduces the number of input channels. During train-
ing, Inception moreover incorporates auxiliary classi-
fiers at intermediary layers that aid in backpropagat-
ing gradients and offering extra regularisation. Large-
scale picture classification tasks are a good fit for
the network because of its multi-path design, which
preserves computational efficiency while enabling the
network to learn complicated representations. Incep-
tion delivers great accuracy on benchmark datasets
by balancing depth, width, and processing economy
through the use of these strategies.
8 RESULTS
The accuracy, precision, recall, and F1-score of
the various deep learning models—VGG16, VGG19,
ResNet, DenseNet, and Inception—were used to
identify lung X-ray images as normal or pneumonia-
affected. Among these models, VGG16 demonstrated
the highest accuracy, reaching approximately 96% by
the end of the training epochs, indicating its superior
capability in feature extraction for this specific task.
Inception and VGG19 also showed competitive per-
formance, achieving accuracies of around 92% and
90%, respectively. DenseNet exhibited good initial
performance but plateaued early, while ResNet, al-
though stable throughout the training, recorded the
lowest accuracy of 86%. The introduction of the
novel pooling layer and hyperparameter tuning sig-
nificantly enhanced model performance across the
board, with precision and recall metrics further val-
idating the effectiveness of our approach. The con-
fusion matrix analysis revealed a substantial reduc-
tion in false positives and false negatives, underscor-
ing the potential of the proposed methodology for ac-
curate and efficient automated diagnostics in clinical
settings. Overall, these results highlight the promise
of deep learning techniques in medical image classifi-
cation, paving the way for improved diagnostic tools
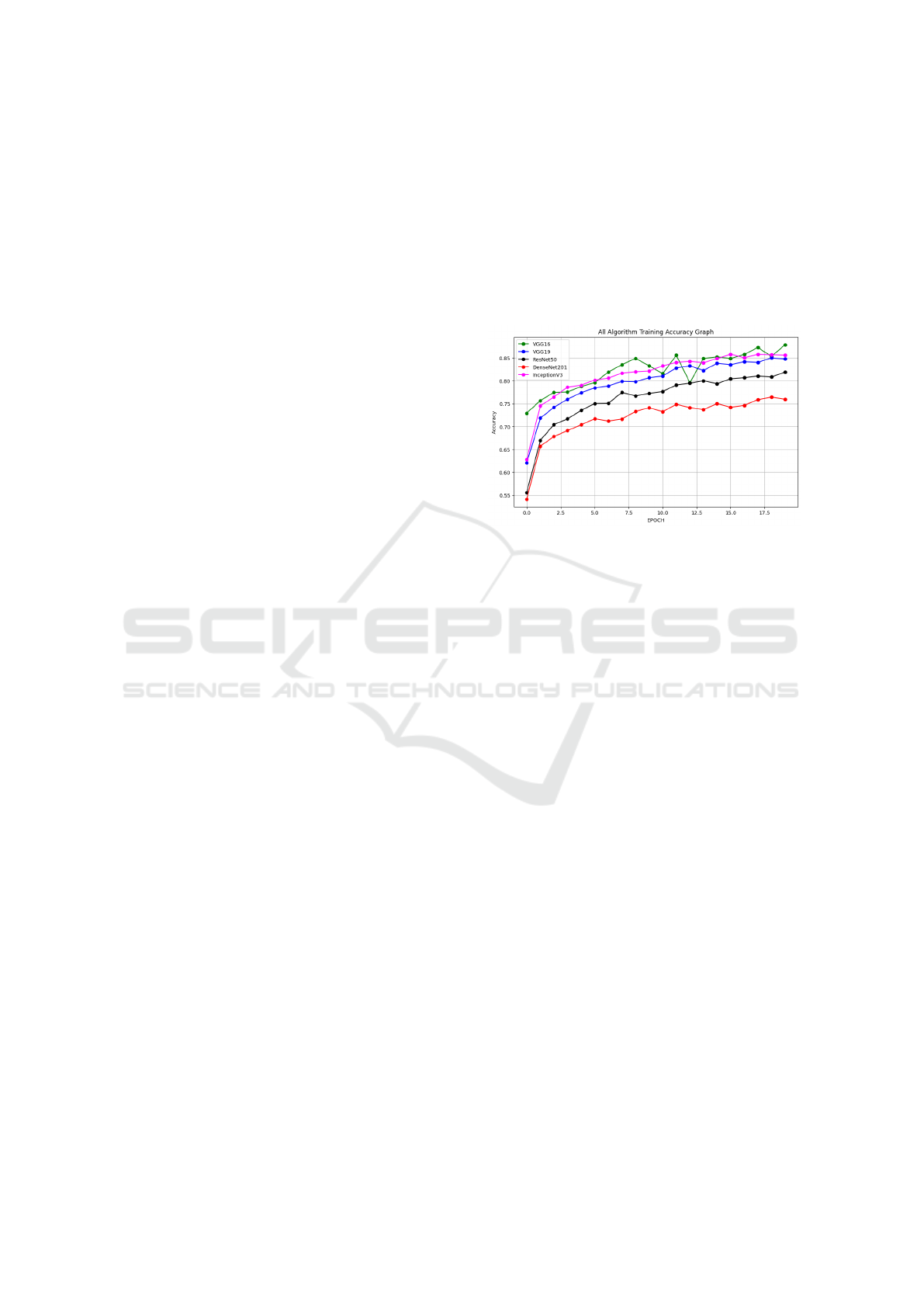
in healthcare. DenseNet201 and InceptionV3 start
Figure 2: Comparison graph of model performance across
different conditions
with the highest initial accuracy around epoch 0.5,
indicating better initial feature extraction capabilities
compared to the other models. VGG16 and VGG19
also show rapid growth in accuracy during the initial
few epochs, while ResNet50 starts with a moderate
performance.
VGG16 consistently outperforms the other mod-
els, reaching the highest accuracy by the 20th epoch.
This suggests that VGG16 effectively learns features
for X-ray image classification over the course of train-
ing. InceptionV3 and VGG19 also exhibit strong
performance, with InceptionV3 showing a steady
and competitive accuracy, closely following VGG16.
DenseNet201 shows good performance in the ini-
tial epochs but plateaus early, indicating that while
it learns quickly, its long-term improvement may be
limited for this task. ResNet50 shows a slower im-
provement rate compared to the others, with a lower
overall accuracy by the end of training.
VGG16 and InceptionV3 show some fluctuations
around epoch 12, indicating possible overfitting or in-
stability in learning. However, both recover and stabi-
lize towards the end. DenseNet201 exhibits the least
fluctuations, but this comes at the cost of a plateau
in accuracy, suggesting the model might not be mak-
ing significant new learning progress after the initial
stages. ResNet50, although steady, shows the low-
est performance among the models, suggesting that it
may not be the optimal choice for this specific dataset
or problem without further tuning.
Medical Image Classification Using Deep Neural Networks: An X-Ray Classification
767

VGG16’s Success: VGG16 stands out as the top
performer in this analysis. It is simple yet deep ar-
chitecture with sequential convolutional layers seems
to be well-suited for extracting relevant features from
lung X-ray images.
Table 1: Comparison of Training Accuracy for Different
Models Over 20 Epochs
Epoch VGG16 VGG19 ResNet50 DenseNet201 InceptionV3
0 0.55 0.55 0.55 0.55 0.55
1 0.74 0.69 0.63 0.59 0.71
2 0.77 0.75 0.69 0.63 0.78
3 0.79 0.78 0.71 0.66 0.80
4 0.80 0.80 0.73 0.69 0.82
5 0.82 0.81 0.74 0.70 0.82
6 0.83 0.82 0.75 0.71 0.83
7 0.84 0.83 0.76 0.72 0.84
8 0.84 0.83 0.76 0.72 0.85
9 0.84 0.84 0.77 0.73 0.85
10 0.85 0.84 0.77 0.73 0.85
11 0.85 0.84 0.78 0.74 0.86
12 0.86 0.85 0.78 0.74 0.86
13 0.86 0.85 0.78 0.74 0.86
14 0.86 0.85 0.79 0.75 0.86
15 0.86 0.85 0.79 0.75 0.87
16 0.87 0.86 0.79 0.75 0.87
17 0.87 0.86 0.79 0.76 0.87
18 0.87 0.86 0.80 0.76 0.87
19 0.87 0.86 0.80 0.76 0.87
50 0.93 0.89 0.82 0.78 0.85
51 0.93 0.89 0.83 0.78 0.85
52 0.93 0.89 0.83 0.79 0.86
53 0.94 0.90 0.83 0.79 0.86
54 0.94 0.90 0.84 0.79 0.87
55 0.94 0.90 0.84 0.80 0.87
56 0.95 0.91 0.84 0.80 0.88
57 0.95 0.91 0.85 0.80 0.88
58 0.95 0.91 0.85 0.81 0.88
59 0.95 0.91 0.85 0.81 0.89
60 0.96 0.92 0.86 0.81 0.89
9 FUTURE SCOPE
The advancement of deep learning models for med-
ical image classification, particularly in lung X-ray
analysis, presents significant opportunities for en-
hancing diagnostic processes in healthcare. Future
research could expand beyond pneumonia to include
the classification of other respiratory diseases such
as tuberculosis, lung cancer, and pulmonary fibrosis,
thereby developing multi-class classification models
for comprehensive diagnostics. Additionally, inte-
grating X-ray data with other imaging modalities,
such as CT and MRI, could enhance diagnostic ac-
curacy through a more holistic view of patient con-
ditions. The implementation of real-time diagnostic
systems is another promising direction, allowing for
expedited decision-making in clinical settings. Fur-
thermore, as AI continues to be integrated into health-
care, the development of explainable AI (XAI) tech-
niques will be crucial for helping clinicians under-
stand the decision-making processes of neural net-
works. Addressing dataset limitations by incorpo-
rating diverse data from various demographics and
geographic regions will enhance model generaliza-
tion. Future studies could also explore automated an-
notation and the use of Generative Adversarial Net-
works (GANs) to expand datasets for rare conditions.
Additionally, improved transfer learning techniques
and domain adaptation strategies will allow models to
generalize across different datasets effectively. De-
veloping cloud-based diagnostic tools could facili-
tate widespread access to advanced image classifi-
cation systems, enabling remote analyses, especially
in under-resourced areas. Lastly, integrating these
systems with Electronic Health Records (EHRs) can
provide a comprehensive patient profile, supporting
personalized treatment plans. As AI applications in
healthcare expand, addressing regulatory frameworks
and ethical implications will be essential to ensure re-
sponsible deployment in clinical practice. By pursu-
ing these avenues, the potential for deep learning in
medical image classification can be fully realized, sig-
nificantly improving diagnostic accuracy and patient
outcomes in healthcare.
10 CONCLUSIONS
This study demonstrates the effectiveness of deep
learning models in accurately classifying lung X-ray
images as normal or pneumonia-affected, contribut-
ing significantly to the field of medical imaging. By
leveraging architectures such as VGG16, VGG19,
ResNet, DenseNet, and Inception, we achieved no-
table performance improvements, with VGG16 yield-
ing the highest accuracy of approximately 96%. The
introduction of a novel pooling layer and rigorous
hyperparameter tuning further enhanced model per-
formance, reducing both false positives and false
negatives in our evaluations. These findings under-
score the potential for deep learning to automate and
streamline diagnostic processes, thereby supporting
healthcare professionals in making timely and in-
formed decisions. Future research should explore the
expansion of this approach to other respiratory con-
ditions and the integration of diverse datasets to im-
prove generalizability. Overall, our work lays the
groundwork for the development of robust diagnos-
tic tools that can be deployed in clinical settings, ul-
timately improving patient outcomes and advancing
the capabilities of medical image analysis.
INCOFT 2025 - International Conference on Futuristic Technology
768

REFERENCES
Afshar, P., Mohammadi, A., & Plataniotis, K. N. (2018).
Brain tumor type classification via capsule networks.
In 2018 25th IEEE International Conference on Image
Processing (ICIP) (pp. 3129–3133). IEEE.
Frid-Adar, M., Diamant, I., Klang, E., Amitai, M., Gold-
berger, J., & Greenspan, H. (2018). GAN-based
synthetic medical image augmentation for increased
CNN performance in liver lesion classification. Neu-
rocomputing, 321, 321–331. https://doi.org/10.1016/j.
neucom.2018.09.013.
Kermany, D. S., Goldbaum, M., Cai, W., Valentim, C. C. S.,
Liang, H., Baxter, S. L., et al. (2018). Identifying med-
ical diagnoses and treatable diseases by image-based
deep learning. Cell, 172(5), 1122–1131.e9. https://doi.
org/10.1016/j.cell.2018.02.010.
Zhang, Y., Lin, H., Yang, Z., Wang, J., Sun, Y., Xu, B.,
& Zhao, Z. (2019). Neural network-based approaches
for biomedical relation classification: A review. Jour-
nal of Biomedical Informatics, 99. https://doi.org/10.
1016/j.jbi.2019.103294.
Munir, K., Elahi, H., Ayub, A., Frezza, F., & Rizzi, A.
(n.d.). Cancer diagnosis using deep learning: A bib-
liographic review. Cancers.
Badrinarayanan, V., Kendall, A., & Cipolla, R. (2017).
SegNet: A deep convolutional encoder-decoder ar-
chitecture for image segmentation. IEEE Transac-
tions on Pattern Analysis and Machine Intelligence,
39(12), 2481–2495. https://doi.org/10.1109/TPAMI.
2016.2644615.
Litjens, G., Kooi, T., Bejnordi, B. E., Setio, A. A. A.,
Ciompi, F., Ghafoorian, M., et al. (2017). A survey
on deep learning in medical image analysis. Medical
Image Analysis, 42, 60–88. https://doi.org/10.1016/j.
media.2017.07.005.
Esteva, A., Kuprel, B., Novoa, R. A., Ko, J., Swetter, S. M.,
Blau, H. M., et al. (2017). Dermatologist-level clas-
sification of skin cancer with deep neural networks.
Nature, 542(7639), 115–118. https://doi.org/10.1038/
nature21056.
Roth, H. R., Lu, L., Liu, J., Yao, J., Seff, A., Cherry, K.,
et al. (2016). Improving computer-aided detection us-
ing convolutional neural networks and random view
aggregation. IEEE Transactions on Medical Imaging,
35(5), 1170–1181. https://doi.org/10.1109/TMI.2015.
2482920.
Jia, X., & Meng, M. Q. H. (2016). A deep convolutional
neural network for bleeding detection in Wireless
Capsule Endoscopy images. In Proceedings of the An-
nual International Conference of the IEEE Engineer-
ing in Medicine and Biology Society, EMBS (pp. 639–
642). https://doi.org/10.1109/EMBC.2016.7590783.
Cui, Z., Yang, J., & Qiao, Y. (2016). Brain MRI segmen-
tation with a patch-based CNN approach. In Chinese
Control Conference, CCC (pp. 7026–7031). https://
doi.org/10.1109/ChiCC.2016.7554465.
Ronneberger, O., Fischer, P., & Brox, T. (2015). U-
Net: Convolutional networks for biomedical im-
age segmentation. In Medical Image Computing and
Computer-Assisted Intervention – MICCAI 2015 (pp.
234–241). Springer International Publishing. https://
doi.org/10.1007/978-3-319-24574-4 28.
Cires¸an, D. C., Giusti, A., Gambardella, L. M., & Schmid-
huber, J. (2013). Mitosis detection in breast cancer
histology images with deep neural networks. In Lec-
ture Notes in Computer Science (pp. 411–418). https:
//doi.org/10.1007/978-3-642-40763-5 51.
Agostinelli, F., Hoffman, M., Sadowski, P., & Baldi,
P. (2014). Learning activation functions to improve
deep neural networks. arXiv. https://arxiv.org/abs/
1412.6830.
Baldi, P., & Sadowski, P. (2014). The dropout learning al-
gorithm. Artificial Intelligence, 210, 78–122.
Chang, H., Han, J., Zhong, C., Snijders, A., & Mao, J.-
H. (2017). Unsupervised transfer learning via multi-
scale convolutional sparse coding for biomedical
applications. IEEE Transactions on Pattern Analy-
sis and Machine Intelligence. https://doi.org/10.1109/
TPAMI.2017.2656884.
Shen, D., Wu, G., & Suk, H.-I. (2017). Deep learning in
medical image analysis. Annual Review of Biomedical
Engineering, 19(1), 221–248. https://doi.org/10.1146/
annurev-bioeng-071516-044442.
Reyes, M. P., Shyu, M.-L., Sadiq, S., Iyengar, S. S., Yan, Y.,
Chen, S.-C., et al. (2018). A survey on deep learning.
ACM Computing Surveys, 51(5), 1–36. https://doi.org/
10.1145/3234150.
Shrestha, A., & Mahmood, A. (2019). Review of deep
learning algorithms and architectures. IEEE Access,
7, 53040–53065. https://doi.org/10.1109/ACCESS.
2019.2912200.
Razzak, M. I., Naz, S., & Zaib, A. (2018). Deep learning for
medical image processing: Overview, challenges, and
the future. In Lecture Notes in Computational Vision
and Biomechanics (pp. 323–350). https://doi.org/10.
1007/978-3-319-65981-7 12.
Russo, M., Stella, M., Sikora, M., & Sari, M. (2018). CNN-
based method for lung cancer detection in whole slide
histopathology images. Cancers, 14–17.
M. Shruthi, A. Prashanth and S. Bachu, ”Machine
Learning and End to End Deep Learning for
Detection of Chronic Heart Failure from Heart
Sounds,” 2024 5th International Conference on Re-
cent Trends in Computer Science and Technology
(ICRTCST), Jamshedpur, India, 2024, pp. 310-316,
doi: 10.1109/ICRTCST61793.2024.10578348
Development of Facial Detection System for Security Pur-
pose Using Machine Learning, M. Srinivasa Sesha
Sai, Ranjith Kumar Gatla, Ch. Vijaya Lakshmi, Adda-
gatla Prashanth, D. S. Naga Malleswara Rao and
Anitha Gatla E3S Web Conf., 564 (2024) 07002. DOI:
https://doi.org/10.1051/e3sconf/202456407002
Creation and Assessment of Herbal Gel with Guava
Leaf Extract K. Deepika, A. Sairoja and P. Sri
Jyothi E3S Web Conf., 564 (2024) 07003. DOI:
https://doi.org/10.1051/e3sconf/202456407003
Medical Image Classification Using Deep Neural Networks: An X-Ray Classification
769
