
Automated Bone Fracture Detection System Using YOLOv7 with
Secure Email Data Sharing
Swarada Gade
1
, Vashita Nukala
1
, Tanaya Sutar
1
, Shravani Walunj
1
, Avinash Golande
2
,
Amruta Hingmire
2
and
Vinodkumar Bhutnal
2
1
Department of Computer Engineering, JSPM’S Rajarshi Shahu College of Engineering, Pune, India
2
School of Engineering and Technology Pimpri Chinchwad University, Pune, India
Keywords: Short Automated Bone Fracture Detection, Deep Learning Model, X-Ray Imaging, Automated Diagnosis,
Email Data Exchange, DICOM Images, Diagnostic Reporting, Secure Data Transmission, Computer Vision,
Medical Imaging, Healthcare Technology, Image Analysis, Radiology Automation.
Abstract: The demand for more precise and timely bone fractures diagnosis grew resulting in integration of advanced
technologies to medical imaging. This paper describes the development and realization of a deep learning
system for automatic detection of bone fractures in X-ray images, which uses YOLOv7 model for improved
diagnostic accuracy and efficiency. A secure mechanism to transmit medical images and reports through
email is included in the system. Furthermore, this system supports direct downloading of DICOM images as
well as creating simple diagnostic reports automatically thus taking out serial steps that increase delay time
for patient feedback. This innovative approach leverages cutting-edge deep learning techniques to address
critical healthcare needs, streamline diagnostic workflows, and enhance patient outcomes. Real-time
fracture detection in the YOLOv7 model is possible because of its use of data augmentation methods during
the process of training, which assures robustness and reliability in different scenarios. Through the
systematic methodology of data preparation, model training, and evaluation defined in this framework, the
potential of the system as a reliable asset in clinical applications is demonstrated. The proposed model
minimized the dependency on manual procedures, maximized the speed of clinical decisions, and reinstated
decision processes through standardized results. Hence, this framework serves as a noble contribution to
modern medical diagnostics.
1 INTRODUCTION
Providing accurate diagnosis of bone fractures is an
integral part of optimal patient care, with a
significant role of X-ray technology in the
identification and management of injuries.
Historically, the diagnostic phase of X-ray analysis
requires engagement and expertise from a
radiologist who scans each film to assess for
fractures. Human observation likely varies in
reliability depending on factors such as fatigue and
cognitive load, which can impact the interpretation
of diagnostic images.
Given these challenges, the motivation behind
this systems design is to establish a bone fracture
detection system for X-ray images using the model
YOLOv7, which is a real-time object detection
model. The model is established in detection of
fractures in diagnostic images, thus improving
accuracy and consequently efficiency in the clinical
decision-making process.
The system’s motive is to reduce the manual
processing with neuro-scientifically expert-grounded
design choices to ensure effective fracture
detection.This system allows for collaborative care
by ensuring that patient information can be shared
effectively, which is necessary for timely
intervention. The primary benefit of the system is
the ability at improving the diagnostic accuracy.
By the application of deep learning, the system
will then be able to process the minute detail in X-
rays to detect even very small bone fractures that
would otherwise be missed by the human eye. Early
detection followed by prompt intervention could,
therefore, lead to better outcomes for patient care. It
will be used for standardization across various
538
Gade, S., Nukala, V., Sutar, T., Walunj, S., Golande, A., Hingmire, A. and Bhutnal, V.
Automated Bone Fracture Detection System Using YOLOv7 with Secure Email Data Sharing.
DOI: 10.5220/0013624300004664
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 3rd International Conference on Futuristic Technology (INCOFT 2025) - Volume 3, pages 538-549
ISBN: 978-989-758-763-4
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
health care facilities since one human interpretation
may vary from another, thus reducing this
variability.
This technology plays an important role in
advancing medical research. It can support large
studies over X-ray imaging that may advance the
understanding of fracture epidemiology, risk factors,
and treatment methods. Such data may also
contribute to the development of new and even
newer technologies for treating bone fractures that
will reduce the number of occurrences and make
patients recover even better.
The integration of emerging diagnostic
technologies, such as explainable AI, will further
enhance transparency and trust in the decision-
making process, so that healthcare providers are able
to understand and validate the system's conclusions.
Also, the system aligns with the green Internet of
Things framework, that enables secure data
exchange between devices while maintaining the
integrity of existing healthcare infrastructure. The
accuracy of medical imaging is also supported by
ongoing technological advancements in the field.
Studies combining traditional diagnostic methods
with modern technological integrations bring
advancements to evolving models of investigation
and data management. This system’s approach to
detecting bone fractures, combining deep learning
algorithms with secure data management, offers an
original progression in enhancing diagnostic
methods. By stimulating diagnostic accuracy,
improving workflow, and promoting positive patient
outcomes, this project aims to integrate cutting-edge
technology into clinical practice. As healthcare
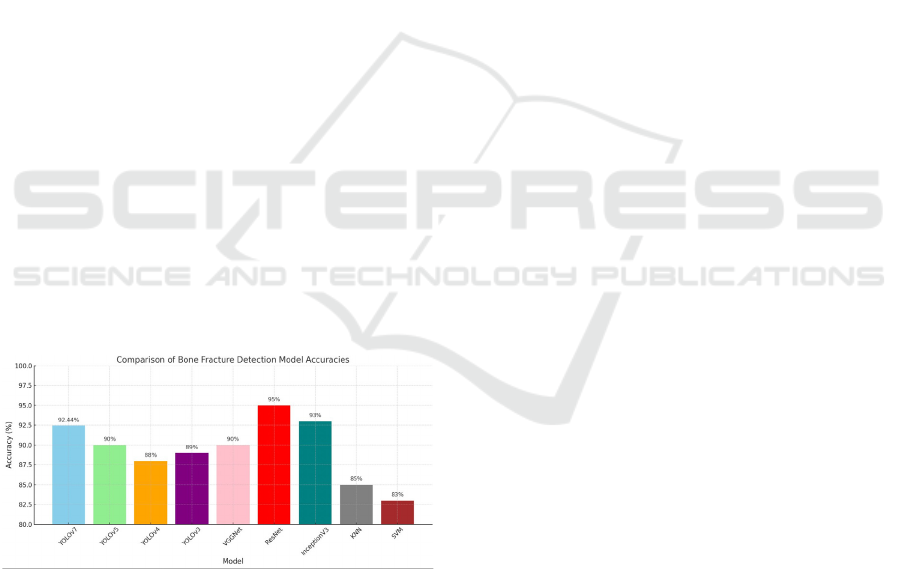
Figure 1: Accuracy comparison.
professionals adopt this modern approach, they can
support the generation of informed decisions,
leading to enhanced healthcare delivery and
improved patient care.
2 RELATED WORK
M. Nandyala, P. Kanumuri, and M. Garlapati
(Nandyala, Kanumuri et al. 2023). This paper
conducts a review and analysis focused on the
automatic diagnosis of fractures with deep learning
application techniques. Here, an attempt has been
made to set up which deep learning models that can
potentially be applied for the automatic fracture
detection and classification from medical images are
the most promising in terms of efficiency and
reliability. For this purpose, authors have
extensively analyzed existing literature and
attempted to present the most important
methodologies used, performance metrics, and
validation processes across a variety of studies. By
synthesizing findings from several studies, one will
be able to note the trends, strengths, and limitations
of current approaches in order to illustrate insights to
best practices in the development and
implementation of deep learning algorithms for
fracture diagnosis.
S. Shelmerdine, H. Liu, O. Arthurs, R. White,
and N. Sebire (Shelmerdine, Liu et al. 2022) AI in
fracture detection has been the dominant line of
research and commercialization in medicine.
Children are the primary victims of the inadvertent
over- or under-diagnosis of injuries. This is as a
result of missed out fractures. The study focuses on
the AI tools’ diagnostic performance for pediatric
fracture detection on imaging. It also aims to
compare its performance with human readers where
possible. Following a systematic review between
2011 and 2021, databases including MEDLINE,
Embase, and Cochrane Library were analyzed. Nine
from 362 identified eligible articles have found
fracture detection being most frequent with the
elbow mostly studied. Most of the studies were done
using data from single institutions, deep learning
algorithms were usually used, and external
validation was lacking. The figures for AI were from
88.8% to 97.9% in terms of accuracy. In those
researches where AI and the readers werecompared,
AI has been discovered to be more sensitive (though
the difference is not significant). The analysis
pointed out AI’s unclear algos and thereby limited
genabilities due to the lack of effective validation
and a heterogeneous dataset. Such future research
would benefit not only from the diverse patient
populations of multi center studies around the world
but also be the cornerstone of real-world
assessments. Hence, it can be argued that such future
research would give a strong ground and potential
for the tools to help doctors in pediatric ways.
Automated Bone Fracture Detection System Using YOLOv7 with Secure Email Data Sharing
539
S. Rathor and D. P. Yadav (Rathor, Yadav et al.
2020) Given the frequency of the fractures and
drawbacks of hand identification with pictures of X-
Ray, the authors of this review addressed the
significance of automated fracture detection. To
differentiate between healthy and damaged bones, a
DNN model is suggested. Overfitting on a tiny
dataset is eliminated using data augmentation
techniques, yielding a dataset of 4000 photos. DNN
outperforms earlier techniques with accuracy of
92.4% in differentiating between broken and healthy
bone.Additionally, certain subgroups yield accuracy
rates of 95% and 93%, demonstrating the model’s
resilience.
S. Yang, W. Cao, B. Yin, C. Feng, S. He and
G.Fan (Yang, Cao et al. 2020) The paper is a
thorough review of diagnostic efficiency of deep
learning technology for orthopedic fractures. It
introduces various researches which have used deep
learning algorithms for medical imaging purposes
including X-rays and CT scans. The authors
examine how these algorithms performed on their
diagnostic tasks and to what accuracy these methods
were compared with the standard ones. The article
addresses the considerations that affect the
diagnostic performance including the models used,
the quality of the datasets and the imaging
modalities.
J. Zou and M. R. Arshad (Zou and Arshad,
2024). The present article studies the customization
and introduction of enhanced YOLOv7 algorithm
for predicting of whole body fractures from medical
images. The authors start with the problem of good
fracture detection since different fractures can be of
different sizes, at different locations and need to
have dependable and efficient tools. They conduct a
complete evaluation of the images using a large
database of medical images containing different
fractures of the bone.
Puttagunta, M., Ravi, S (Puttagunta and Ravi,
2021) This paper examines the numerous DL
architectures, from CNNs, to RNNs, to hybrid
models, and explains how they are effective and
ineffective at dealing with different medical
imaging, whether that be X-rays, MRIs, CT scans, or
ultrasounds. The review is about how models can be
trained on huge datasets to recognize patterns that
might indicate certain diseases or abnormalities, like
tumors or fractures. In this article, the authors
provide cases where deep learning systems have
been used in a clinical setting, not to replace the
radiologist or any other medical personnel, but to
help them make the most accurate and timely
diagnosis possible.
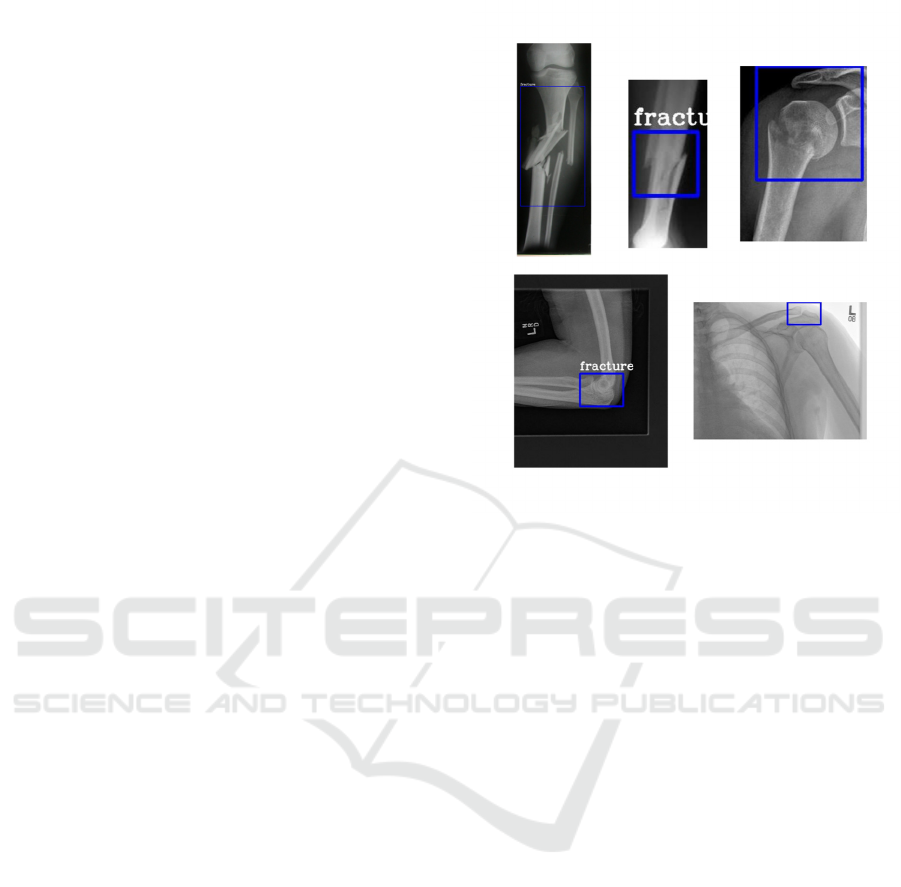
Figure 2: Bone fracture detection output.
W. Kim, S. Kim, Y. Kim, G. Moon, Y. Jeong
and H. S. Choi: (Kim, Kim et al. 2022) In this
study, the researchers propose a novel approach that
leverages advanced object detection algorithms to
automatically identify and classify facial bone
fractures from medical imaging data. It describes the
performance metrics of the system, which compares
the diagnostic accuracy with conventional methods.
The authors point out that their CA-FBFD system
would take less time to diagnose and would have
more consistent fracture identification which would
really help radiologists and other medical
professionals in a clinical setting.
L. T. Martin al. (Martin, Nelson et al. 2022) It is
no doubt that the increasing diversity of the data has
the potential of pointing health decision-making in a
new direction. However, the problems are quite
profound. As such, the difficulties include setting
data sharing and interoperability stan- dards,
developing new methodological and workforce
models, and, more importantly, ensuring strong data
stewardship and governance that will accompany the
data to protect public health data integrity. The
review including literature, environmental scan, and
insights from the National Commission to
Transform Public Health Data Systems inform the
discussion of this article on the major obstacles in
data sharing and reuse. It highlights the sector’s
possibilities to close the gaps by improved
interoperability and better data ownership practices.
INCOFT 2025 - International Conference on Futuristic Technology
540

S. Dash, S. K. Shakyawar , M. Sharma, and
others (Dash, Shakyawar et al. 2019) The challenges
that are associated with using big data in healthcare
are discussed in the study, including issues with data
privacy, interoperability, and the need for skilled
professionals for assessing and interpreting the data.
They go over a variety of big data management
techniques and technologies, such as frameworks for
processing large data sets, data integration tools, and
data storage options. These techniques and
technologies can be applied to enhance patient
outcomes, manage healthcare more effectively, and
ultimately transform the way that healthcare is
delivered.
Aiello, M., Esposito, G., Pagliari, G (Aiello,
Esposito et al. 2021) The authors begin by providing
context on the importance of DICOM as a
foundational standard that governs the storage,
transmission, and sharing of medical imaging data.
The paper highlights DICOM’s structured data
format, which includes not only image data but also
rich metadata. This metadata encompasses essential
information such as patient demographics, imaging
protocols, and clinical notes, facilitating better
organization and retrieval of information. Such
structured data is crucial for effective analysis in big
data applications.
Sun, Y.; al. (Sun, Li et al. 2023) The paper
aimed at improving detection accuracy and
efficiency in various applications by outlining the
limitations of existing YOLO models, particularly in
terms of their performance in complex environments
and under varying conditions. To counter these
challenges, the authors included a number of major
adjustments to the YOLOv7 framework. Key to
these includes the development of the Parallel
Backbone Architecture, known as PBA, to facilitate
better feature extraction at several scales. The
authors carried out extensive experiments against the
baseline YOLOv7 and other state-of-the-art object
detection models to validate the performance of the
PBA-YOLOv7 model. This is accomplished by
using benchmark datasets on the model, hence
assessing performance on metrics such as precision,
recall, and mean Average Precision (mAP). Based
on performance, PBA-YOLOv7 performs much
better compared to its predecessors, especially in
complex scenarios related to occlusion or change of
object size.
A related study was conducted by Shahnaj
Rahman, Parvin, and Abdur (Shahnaj, Rahman et al.
2024), wherein the proposed model was
strengthened by integrating different imaging
modalities like CT scans, X-rays, and MRI scans,
resulting in a more extensive collection of images.
They applied advanced architectures in deep
learning, such as convolutional neural networks
(CNNs), to appropriately analyze these different
types of images. Their paper highlights the
importance of multimodal data in capturing the
intensity of fractures, as the appearance can vary
greatly from one imaging method to another.
Research conducted by Gupta A. (Gupta, 2024)
discusses various optimization algorithms such as
Stochastic Gradient Descent and its variants. The
author also studies optimizers like Adam and
AdaGrad, highlighting their individual advantages
when applied in specific cases. The paper compares
the performance of these optimizers using empirical
data, providing valuable insights into their
effectiveness across different deep learning tasks.
A new loss function was proposed by Zheng Z.
et al. (Zheng, Wang et al. 2019), named Distance-
IoU Loss, which aims to improve the accuracy and
efficiency of bounding boxes. The authors highlight
shortcomings of existing loss functions like
Intersection over Union (IoU) and its variants, which
often encounter convergence issues, speed
limitations, and optimization stability problems. To
support their argument, the authors conducted
extensive experiments using well-known benchmark
datasets such as Pascal VOC and COCO. The results
showed that models utilizing Distance-IoU Loss
performed better than those using conventional IoU-
based loss functions. The paper provides a robust
comparison of detection accuracy and training time,
offering strong evidence to validate the effectiveness
of the proposed method.
Li X. et al. (Li, Wang et al. 2020) introduced a
novel loss function designed to enhance the
performance of dense object detection tasks,
particularly in situations with class imbalance and
overlapping bounding boxes. The authors propose a
"generalized focal loss" that addresses these
challenges through two key innovations: qualified
bounding boxes and a distributed approach to box
regression. The concept of qualified bounding boxes
allows the model to prioritize certain boxes based on
their confidence and relevance, helping to mitigate
the impact of noise from less relevant detections.
Beyaz et al. (Beyaz, Acıcı et al. 2020) reported a
convolutional neural network (CNN) architecture
achieving an accuracy of 97.4%. The study involved
a total of 1,341 femoral neck fracture images,
including 765 non-fracture images. The probability
threshold for the current version process was set at
0.5.
Automated Bone Fracture Detection System Using YOLOv7 with Secure Email Data Sharing
541
In the research conducted by Mutasa et al.
(Mutasa, Varada et al. 2020), the main focus was on
convolutional neural networks to automate the
detection and classification of femoral neck fractures
in medical images. This research aims to overcome
the challenges of accurately diagnosing femoral
neck fractures, which is crucial for determining
appropriate treatment and care for patients. The
model was trained using a dataset of annotated X-
ray images containing both normal and fractured
femoral necks. CNNs enabled the system to learn
spatial hierarchies of features directly from raw
image data, automating feature extraction that
previously required significant expertise and manual
intervention. These deep learning models are
designed not just to detect fractures but also to
determine their type and severity, enabling doctors
to make better decisions for patient care. The results
from the study indicate that these models outperform
conventional diagnostic methods, sometimes even
surpassing human radiologists, providing greater
precision and faster results. Such capabilities enable
consistent and reliable fracture detection, reduce
diagnostic errors, and facilitate quicker decision-
making processes. The integration of these
automated systems into clinical practice could
greatly assist radiologists, particularly in low-
resource settings or high-volume environments, by
facilitating timely and effective patient care. Overall,
the study highlights the transformative potential of
deep learning in medical imaging, particularly in the
diagnosis and classification of femoral neck
fractures, leading to improved health outcomes.
Deep learning methods show great potential to
revolutionize medical imaging and enhance
healthcare outcomes, especially in diagnosing and
classifying femoral neck fractures.
3 METHODOLOGY
We propose a thorough approach to detect objects
using the latest deep learning architectures,
YOLOv7 in particular. To improve detection
accuracy and operating efficiency, our strategy
incorporates multiple components: training
techniques, model architecture, assessment metrics,
and data preparation.
The trained deep learning models assist in extracting
observations/assessments from pictures. Much use
of CNN-based techniques is utilized in this research
to recognize and categorize images according to
their characteristics. This section will highlight
preparation procedures of the data for model
architecture and evaluation of the model
performance. This section incorporates all the steps
of methodology including training processes,
evaluation metrics, model architecture, and data pre-
processing to ensure maximized predictability of the
learnt model.
𝜎=
∑
(𝑥
−𝜇)
(1)
𝑟=
()()
√
(2)
𝑡=
√
(3)
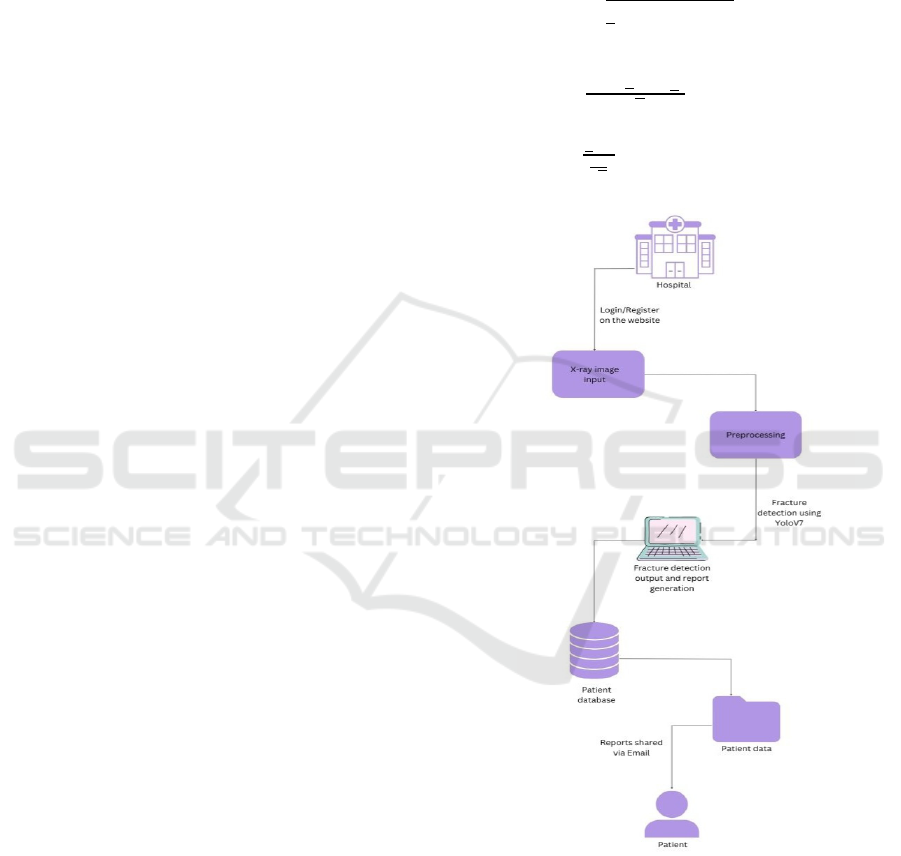
Figure 3: System architecture.
YOLOv7: This architecture is optimized for
performance and efficiency, making use of a new
backbone network and tuned anchor box settings. It
achieves a great mAP while keeping the inference
time small, so it allows real-time detection. The first
task was to generate an appropriately diverse dataset
of representative objects of interest. The collected
INCOFT 2025 - International Conference on Futuristic Technology
542

data was then preprocessed to have the same size;
each image was scaled to 640 x 640 pixels for
YOLOv7 model input. Among the various data
augmentation techniques applied were random
rotations, flips, and color variations, ensuring
diversity in the training dataset. This was done to
counter overfitting and make the model robust with
regard to changes.
Our proposed system employs the highly
accurate and fast YOLOv7 architecture. The
architecture
detection cases. While maintaining the positive traits
of earlier YOLO versions, it adds numerous features
to enhance its performance.
3.1 Training Deep Learning Model
Data preparation constitutes one of the critical
components in any deep learning project. The
quality and quantity of data directly influence the
model's ability to generalize from the data in training
to unseen data.
Dataset Collection: For this study, we collected a
dataset containing images labeled either fractured or
normal. The dataset is taken from public medical
imaging databases, supplemented with synthetically
generated images using image transformation
techniques. The diversity of this dataset is essential
for strong model performance in real-time
applications across various domains.
Data Augmentation: Data augmentation is a
statistical methodology used to artificially enhance
the training dataset size by implementing various
transformations on the images. This includes
rotations, scaling, flipping, and color alterations.
These transformations help augment the data,
thereby addressing overfitting and making the model
robust. Augmentations were applied dynamically
during training, with each epoch exposing the model
to different image variations.
𝛴(𝑧
)=
∑
(4)
𝑙=
∑∑
𝑦
,
𝑙𝑜𝑔(𝑦
,
^)
(5)
𝑤
()
=𝑤
()
−𝜂
(6)
𝐽
(
𝜃
)
=
∑
𝑙(𝑦
−𝑦
)
^
+ 𝜆||𝜃
(7)
𝑅𝑀𝑆𝐸 =
∑
(𝑇𝑟𝑢𝑒
−𝑃𝑟𝑒𝑑
)
(8)
𝛼=
(9)
𝛽=
(10)
𝛾=2.
.
(11)
where:
𝛼 represents prescision
𝛽 represents Recall
𝛾 represents F1-Score
Data Splitting: The dataset was divided into training,
validation, and test sets with proportions of 80%,
10%, and 10% respectively. This division facilitates
effective model training, smooth hyperparameter
tuning, and performance evaluation.
In this application, we use a combination of
CNNs and YOLOv7 architecture to effectively
classify and detect bone fractures in X-ray images.
Together, these technologies enable image
classification and object detection, with high
sensitivity to subtle differences in bone fractures.
CNNs are effective for image-related tasks due
to their hierarchical feature extraction capabilities.
The architecture consists of multiple layers that
process the input image:
Convolutional Layers: These layers apply
convolutional operations using filters (kernels) to
learn image features such as edges, textures, and
shapes.
Activation Functions: Applied after
convolutional layers to introduce non-linearity,
enabling the network to learn complex patterns.
Pooling Layers: Pooling layers (e.g., max
pooling) reduce the spatial dimensions of feature
maps, decreasing computational load and mitigating
overfitting while preserving key features.
Fully Connected Layers: These final layers take
the flattened output from previous layers to perform
classification tasks, outputting probabilities for each
class (healthy or fractured).
YOLOv7 is an advanced object detection
framework providing real-time detection
capabilities, ideal for medical image analysis:
Single-Stage Detector: Processes the entire
image in a single forward pass, enhancing speed and
efficiency.
Grid System: Divides the input image into a grid
to predict bounding boxes and class probabilities for
each cell, enabling multi-object detection.
Automated Bone Fracture Detection System Using YOLOv7 with Secure Email Data Sharing
543
Feature Pyramid Networks (FPN): Extracts
features at multiple scales, crucial for detecting both
small and large fractures.
Anchor Boxes: Handles different aspect ratios
and sizes of fractures effectively, improving
detection accuracy.
Preprocessing steps before model input include
image resizing (to 640x640 pixels), normalization
(scaling pixel values between 0 and 1), and data
augmentation (e.g., rotation, flipping, brightness
adjustment).
The training process involves:
Loss Function: Categorical cross-entropy loss for
classification, with additional localization and
confidence loss for object detection.
Optimizer: Adaptive optimizers like Adam or
SGD are used to minimize loss during training,
enhancing model fit.
Training Stages: The model undergoes multiple
training stages with regular validation checks to
monitor performance and prevent overfitting.
Model Compilation: The model is compiled with
the chosen optimizer, loss functions, and evaluation
metrics. Adam optimizer and binary cross-entropy
loss are commonly used for binary classification
tasks.
The integration of CNNs and YOLOv7 provides
a robust framework for detecting bone fractures
from X-ray images. Leveraging the strengths of both
architectures improves diagnostic accuracy and
supports timely medical interventions. Advanced
image processing techniques and effective training
strategies further enhance model performance for
real-world clinical applications.
3.2 Performance Analysis
Evaluating the model’s performance is crucial to
determine its effectiveness in classifying unseen
data. Evaluation is conducted using the test dataset,
independent of the training and validation datasets.
Evaluation Metrics: Key metrics include
accuracy, precision, recall, F1-score, and Area
Under the Curve (AUC). Accuracy measures the
proportion of correctly classified instances, while
precision and recall provide insights into the
model’s performance on positive cases. The F1-
score balances precision and recall, offering a
comprehensive performance metric.
SYSTEM CONFIGURATION
The software applications used included:
a) Ubuntu 22.04 LTS or Windows 10/11
b) Python 3.9 or higher
c) Django 5.0.6
d) TensorFlow 2.16.2
e) Ultralytics YOLOv7
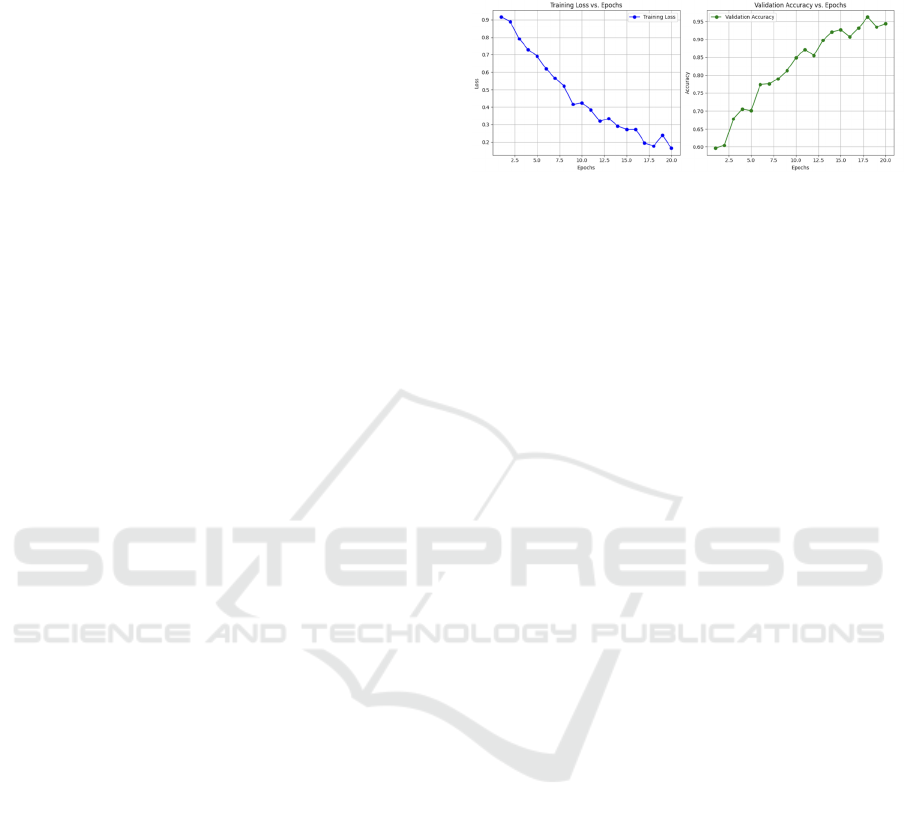
Figure 4: Training vs Epoch and Accuracy vs Epoch.
3.3 Training Parameter Setting
Training was conducted using carefully selected
parameters to optimize performance:
Batch Size: A batch size of 32 balanced memory
utilization and convergence speed, ensuring efficient
GPU resource use while maintaining data diversity
in each iteration.
Learning Rate: An initial learning rate of 0.001
was set, with a scheduler reducing it by a factor of
0.1 after a set number of epochs without validation
loss improvement. This approach refines learning as
the model approaches optimal weights.
Number of Epochs: Models were trained for 50
epochs, providing sufficient learning time without
overfitting. Early stopping was implemented to halt
training when validation loss did not improve for
three consecutive epochs.
Optimizer: Adaptive optimizers like Adam were
used to optimize learning, with binary cross-entropy
loss suitable for binary classification tasks. This
comprehensive methodology supports robust model
training, performance evaluation, and application in
medical image analysis.
4 RESULT ANALY SIS
The system involves new techniques for fracture
detection in bones through advanced deep learning
methods combined with state-of-the-art medical
diagnostic techniques. It achieves up to 92.44%
accuracy in classification using the YOLOv7
architecture, demonstrating high precision and
reliability, making it suitable for healthcare
applications.
A pipeline integrating this network with resizing,
normalization, and advanced data augmentation is in
place. It can adapt to various sizes, orientations, and
imaging conditions of fractures. By employing
INCOFT 2025 - International Conference on Futuristic Technology
544
anchor box methods followed by Feature Pyramid
Networks, the system enhances its ability to
precisely identify fractures, ensuring dependable and
consistent detection across complex datasets. These
capabilities are crucial for minimizing diagnostic
errors and enabling timely intervention for patients.
The YOLOv7 architecture strengthens the
system’s feature extraction, allowing it to detect
even minor cracks that might be missed through
manual analysis. This ability to analyze patterns in
X-ray images is a valuable asset for radiologists, as
it helps reduce mental and decision fatigue. The
system’s real-time detection capabilities, supported
by extensive training data and refined through
adaptive learning rate scheduling and regularization
techniques, make it an effective tool for time-
sensitive and critical situations, such as emergency
cases where rapid diagnosis can be life-saving.
In addition to its diagnostic function, the system
includes a secure email feature for transmitting
clinical images and reports, facilitating easier
communication among healthcare professionals.
This feature ensures strong data protection and
enhances collaborative efforts within medical teams.
The system speeds up patient management
workflows by minimizing delays in sharing
important information, resulting in quicker and more
efficient treatment. These efficiencies are especially
beneficial in resource-limited settings, where prompt
access to diagnostic information is crucial.
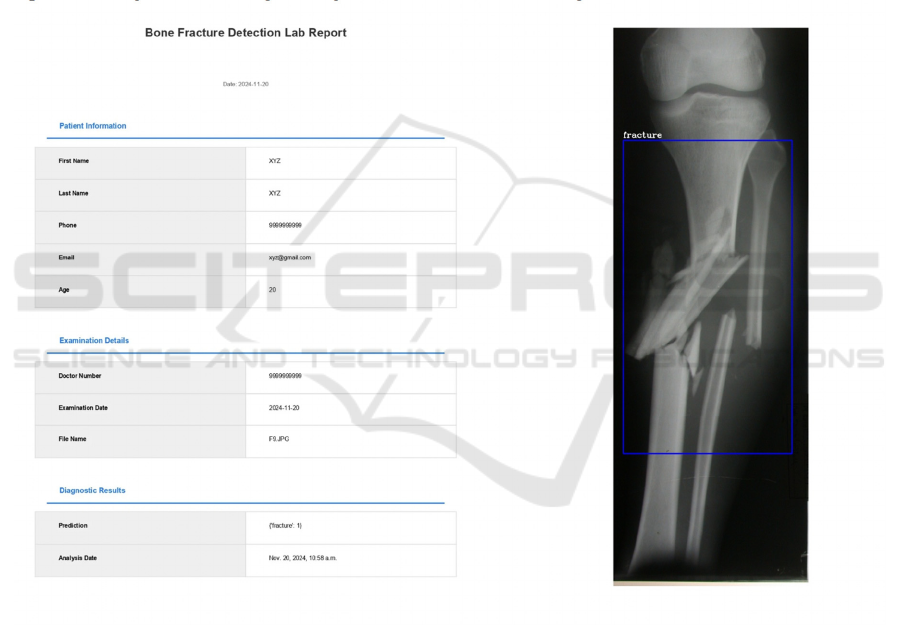
Figure 5: Generated report.
Automated Bone Fracture Detection System Using YOLOv7 with Secure Email Data Sharing
545
Table 1: Comparison with other papers.
Aspect Details
Tas
k
Bone Fracture Classification
Model Used YOLOv7 (You Only Look Once v7)
Fracture Types Classified Normal vs. Fractured bones
Image Modality X-ray Images
Detection Approach Object detection (real-time, single-stage)
Preprocessing - Resizing images to 640x640
- Normalization of pixel values to [0, 1]
- Data augmentation (rotation, flipping, scaling, color
variations)
Training Data 500 X-ray images (fractured and normal)
Model Evaluation Metrics Accuracy, Precision, Recall, F1-Score
Accuracy 92.44%
Precision 93%
Recall 95%
F1 score 93.44%
Performance High accuracy for detecting bone fractures
Real time application Yes
Training duration 50 epochs with Adam optimize
r
Loss function Categorical Cross-Entropy
5 CONCLUSIONS
It has been established that a YOLOv7-based
framework works efficiently in line with the
requirements proposed, aiming at real-time detection
of bone fractures with a high accuracy of 92.44%.
The system, by combining fracture detection
features with CNN-based feature extraction
capabilities, effectively handles fractures of various
sizes and complexities. Strong preprocessing
techniques and optimized training convergence
enhance the model’s reliability, making it highly
deployable from a clinical perspective.
Real-time detection, automated report generation,
and secure data sharing improve diagnostic
workflows, ensure patient data privacy, and facilitate
communication among medical personnel. The
architecture is scalable and flexible, adaptable to
broader healthcare settings, thereby transforming
diagnostic practices. In essence, deep learning has
significantly contributed to advancing medical
imaging processes, leading to better patient
outcomes and fostering innovations in healthcare
technology solutions.
REFERENCES
Swapna, N., & Malge, R. (2022). Classification and
detection of bone fracture using machine learning.
International Journal for Research in Applied Science
& Engineering Technology (IJRASET), 10(2), 45523.
https://doi.org/10.22214/ijraset.2022.45523
Thian, Y. L., Li, Y., Jagmohan, P., Sia, D., Chan, V. E. Y.,
Tan, R. T., & Tan, R. T. (2019). Convolutional neural
networks for automated fracture detection and
localization on wrist radiographs. Radiology: Artificial
INCOFT 2025 - International Conference on Futuristic Technology
546

Intelligence, 1(1), e180001.
https://doi.org/10.1148/ryai.2019180001
Yadav, D. P., & Rathor, S. (2020). Bone fracture detection
and classification using deep learning approach. In
2020 International Conference on Power Electronics
& IoT Applications in Renewable Energy and its
Control (PARC) (pp. 282-285). IEEE.
https://doi.org/10.1109/PARC49193.2020.236611
Yamamoto, N., et al. (2020). An automated fracture
detection from pelvic CT images with 3-D
convolutional neural networks. In 2020 International
Symposium on Community-centric Systems (CcS) (pp.
1-6). IEEE.
https://doi.org/10.1109/CcS49175.2020.9231453
Karimunnisa, S., Savarapu, P. R., Madupu, R. K., Basha,
C. Z., & Neelakanteswara, P. (2020). Detection of
bone fractures automatically with enhanced
performance with better combination of filtering and
neural networks. In 2020 Second International
Conference on Inventive Research in Computing
Applications (ICIRCA) (pp. 189-193). IEEE.
https://doi.org/10.1109/ICIRCA48905.2020.9183085
Savaris, A., Gimenes Marquez Filho, A. A., Rodrigues
Pires de Mello, R., Colonetti, G. B., Von
Wangenheim, A., & Krechel, D. (2017). Integrating a
PACS network to a statewide telemedicine system: A
case study of the Santa Catarina state integrated
telemedicine and telehealth system. In 2017 IEEE 30th
International Symposium on Computer-Based Medical
Systems (CBMS) (pp. 356-357). IEEE.
https://doi.org/10.1109/CBMS.2017.128
Basha, C. Z., Reddy, M. R. K., Nikhil, K. H. S.,
Venkatesh, P. S. M., & Asish, A. V. (2020). Enhanced
computer aided bone fracture detection employing X-
ray images by Harris corner technique. In 2020 Fourth
International Conference on Computing
Methodologies and Communication (ICCMC) (pp.
991-995). IEEE.
https://doi.org/10.1109/ICCMC48092.2020.ICCMC-
000184
Rao, K., Srinivas, Y., & Chakravarty, S. (2022). A
systematic approach to diagnosis and categorization of
bone fractures in X-ray imagery. International Journal
of Healthcare Management, 1(1), 1-12.
https://doi.org/10.1080/20479700.2022.2097765
Widyaningrum, R., Sela, E. I., Pulungan, R., & Septiarini,
A. (2023). Automatic segmentation of periapical
radiograph using color histogram and machine
learning for osteoporosis detection. International
Journal of Dentistry, Article ID 6662911, 9.
https://doi.org/10.1155/2023/6662911
Antani, S., Long, L. R., Thoma, G. R., et al. (2003).
Anatomical shape representation in spine X-ray
images. In VIIP 2003: Proceedings of the 3rd IASTED
International Conference on Visualization, Imaging
and Image Processing (pp. 1-6). IASTED.
Abbas, W., et al. (2020). Lower leg bone fracture
detection and classification using Faster R-CNN for
X-ray images. In 2020 IEEE 23rd International
Multitopic Conference (INMIC) (pp. 1-6). IEEE.
https://doi.org/10.1109/INMIC50486.2020.9318052
Yang, L., Gao, S., Li, P., Shi, J., & Zhou, F. (2022).
Recognition and segmentation of individual bone
fragments with a deep learning approach in CT scans
of complex intertrochanteric fractures: A retrospective
study. Journal of Digital Imaging,
35(6), 1681-1689.
https://doi.org/10.1007/s10278-022-00669-w
Ma, Y., & Luo, Y. (2021). Bone fracture detection through
the two-stage system of Crack-Sensitive
Convolutional Neural Network. Informatics in
Medicine Unlocked, 22, 100452.
https://doi.org/10.1016/j.imu.2020.100452
Wang, M., Yao, J., Zhang, G., et al. (2021). ParallelNet:
Multiple backbone network for detection tasks on
thigh bone fracture. Multimedia Systems, 27, 1091–
1100. https://doi.org/10.1007/s00530-021-00783-9
Abbas, W., Adnan, S., Dr., J., & Ahmad, W. (2021).
Analysis of tibia-fibula bone fracture using deep
learning technique of X-ray images. International
Journal for Multiscale Computational Engineering,
19, 1-11.
https://doi.org/10.1615/IntJMultCompEng.202103613
7
Beyaz, S., Acıcı, K., & Sümer, E. (2020). Femoral neck
fracture detection in X-ray images using deep learning
and genetic algorithm approaches. Joint Diseases and
Related Surgery, 31(2), 175-183.
https://doi.org/10.5606/ehc.2020.72163
Jones, R. M., Sharma, A., Hotchkiss, R., et al. (2020).
Assessment of a deep-learning system for fracture
detection in musculoskeletal radiographs. npj Digital
Medicine, 3, 144. https://doi.org/10.1038/s41746-020-
00352-w
Dupuis, M., Delbos, L., Veil, R., & Adamsbaum, C.
(2022). External validation of a commercially
available deep learning algorithm for fracture
detection in children. Diagnostic and Interventional
Imaging, 103(3), 151–159.
https://doi.org/10.1016/j.diii.2021.10.007
Hardalaç, F., Uysal, F., Peker, O., et al. (2022). Fracture
detection in wrist X-ray images using deep learning-
based object detection models. Sensors, 22(3), 1285.
https://doi.org/10.3390/s22031285
Pranata, Y., Wang, K.-C., Wang, J.-C., et al. (2019). Deep
learning and SURF for automated classification and
detection of calcaneus fractures in CT images.
Computers in Biology and Medicine, 171, 1-8.
https://doi.org/10.1016/j.cmpb.2019.02.006
Mutasa, S., Varada, S., Goel, A., Wong, T. T., & Rasiej,
M. J. (2020). Advanced deep learning techniques
applied to automated femoral neck fracture detection
and classification. Journal of Digital Imaging, 33(5),
1209–1217. https://doi.org/10.1007/s10278-020-
00364-8
Nandyala, M., Kanumuri, P., & Garlapati, M. (2023).
Automatic diagnosis of fracture using deep learning
and external validation: A systematic review and
meta-analysis. International Journal of Intelligent
Automated Bone Fracture Detection System Using YOLOv7 with Secure Email Data Sharing
547

Systems and Applications Engineering, 11(2), 192–
200. https://doi.org/10.46328/ijisae.4781
Tigade, A. V., & Shaikh, S. M. (2021). A hybrid approach
for classification of bone fracture using DWT, GLCM,
and KNN. Journal of Computational and Theoretical
Nanoscience, 18(4), 1419–1425.
https://www.naturalspublishing.com/files/published/1r
886bfj97787b.pdf
Shelmerdine, S., White, R., Liu, H., Arthurs, O., & Sebire,
N. (2022). Artificial intelligence for radiological
paediatric fracture assessment: A systematic review.
Insights into Imaging, 13, 10.
https://doi.org/10.1186/s13244-022-01234-3
Yang, S., Yin, B., Cao, W., Feng, C., Fan, G., & He, S.
(2020). Diagnostic accuracy of deep learning in
orthopaedic fractures: A systematic review and meta-
analysis. Clinical Radiology, 75, 1–10.
https://doi.org/10.1016/j.crad.2020.05.021
Zou, J., & Arshad, M. R. (2024). Detection of whole body
bone fractures based on improved YOLOv7.
Biomedical Signal Processing and Control, 91,
105995. https://doi.org/10.1016/j.bspc.2024.105995
Puttagunta, M., & Ravi, S. (2021). Medical image analysis
based on deep learning approach. Multimedia Tools
and Applications, 80, 24365–24398.
https://doi.org/10.1007/s11042-021-10707-4
Zhang, H., & Qie, Y. (2023). Applying deep learning to
medical imaging: A review. Applied Sciences, 13,
10521. https://doi.org/10.3390/app131810521
Tanzi, L., Vezzetti, E., Aprato, A., Audisio, A., & Massè,
A. (2020). Computer-aided diagnosis system for bone
fracture detection and classification: A review on deep
learning techniques.
Moon, G., Kim, S., Kim, W., Jeong, Y., & Choi, H.-S.
(2022). Computer-aided facial bone fracture diagnosis
(CA-FBFD) system based on object detection model.
IEEE Access, 10, 79061–79070.
https://doi.org/10.1109/ACCESS.2022.3192389
Tan, H., Xu, H., Yu, N., Yu, Y., Duan, H., Fan, Q., & Z.
T. (2023). The value of deep learning-based computer
aided diagnostic system in improving diagnostic
performance of rib fractures in acute blunt trauma.
BMC Medical Imaging, 23(1), 55.
https://doi.org/10.1186/s12880-023-01012-7
Martin, L. T., Nelson, C., Yeung, D., Acosta, J. D.,
Qureshi, N., Blagg, T., & Chandra, A. (2022). The
issues of interoperability and data connectedness for
public health. Big Data, 10(S1), S19-S24.
https://doi.org/10.1089/big.2022.0207
Dash, S., Shakyawar, S. K., Sharma, M., et al. (2019). Big
data in healthcare: Management, analysis, and future
prospects. Journal of Big Data, 6, 54.
https://doi.org/10.1186/s40537-019-0217-0
Aiello, M., Esposito, G., Pagliari, G., et al. (2021). How
does DICOM support big data management?
Investigating its use in the medical imaging
community. Insights into Imaging, 12, 164.
https://doi.org/10.1186/s13244-021-01081-8
Sun, Y., Li, Y., Li, S., Duan, Z., Ning, H., & Zhang, Y.
(2023). PBA-YOLOv7: An object detection method
based on an improved YOLOv7 network. Applied
Sciences, 13, 10436.
https://doi.org/10.3390/app131810436
Zha, K. Y., Yan, L., & Zeng, G. (2022). A comprehensive
review of AI techniques for bone fracture detection.
arXiv preprint. https://arxiv.org/abs/2207.02696
Wang, C.-Y., Bochkovskiy, A., & Liao, H.-Y. M. (2023).
YOLOv7: Trainable bag-of-freebies sets new state-of-
the-art for real-time object detectors. In Proceedings
of the IEEE/CVF Conference on Computer Vision and
Pattern Recognition (CVPR) (pp. 7464-7475).
https://doi.org/10.1109/CVPR52729.2023.00721
Medaramatla, S., Samhitha, C., Pande, S., & Vinta, S.
(2024). Detection of hand bone fractures in X-ray
images using hybrid YOLO NAS. IEEE Access, 1.
https://doi.org/10.1109/ACCESS.2024.3379760
Kheaksong, A., Sanguansat, P., Samothai, P., Dindam, T.,
Srisomboon, K., & Lee, W. (2022). Analysis of
modern image classification platforms for bone
fracture detection. In Proceedings of the 6th
International Conference on Information Technology
(InCIT) (pp. 471-474).
https://doi.org/10.1109/InCIT56086.2022.10067836
Yigzaw, K. Y., Olabarriaga, S., Michalas, A., Marco-Ruiz,
L., Hillen, C., Verginadis, Y., Oliveira, M. T.,
Krefting, D., Penzel, T., Bowden, J., Bellika, J., &
Chomutare, T. (2022). Health data security and
privacy: Challenges and solutions for the future.
Computer Systems Science and Engineering.
https://doi.org/10.1016/B978-0-12-823413-6.00014-8
Kumar, K. P., Prathap, B. R., Thiruthuvanathan, M. M.,
Murthy, H., & Pillai, V. J. (2024). Secure approach to
sharing digitized medical data in a cloud environment.
Data Science and Management, 7(2), 108-118.
https://doi.org/10.1016/j.dsm.2023.12.001
Zukarnain, Z., Muneer, A., Atirah, N., & Almohammedi,
A. (2022). Medi-Block record secure data sharing in
healthcare system: Issues, solutions, and challenges.
Computer Systems Science and Engineering.
https://doi.org/10.32604/csse.2023.034448
Guo, H., Wang, Y., Ding, H., & Chen, J. (2023). Bone
fracture detection based on an improved YOLOv7
model. IEEE Access, 11, 7635-7645.
https://doi.org/10.1109/ACCESS.2023.10476607
Parvin, S., & Rahman, A. (2024). A real-time human bone
fracture detection and classification from multi-modal
images using deep learning technique. Applied
Intelligence, 54, 1-17. https://doi.org/10.1007/s10489-
024-05588-7
Gupta, A. (2024). A comprehensive guide on optimizers in
deep learning. Analytics Vidhya.
https://www.analyticsvidhya.com/blog/2021/10/a-
comprehensive-guide-on-deep-learning-optimizers/
Kingma, D., & Ba, J. (2015). Adam: A method for
stochastic optimization. In Proceedings of the
International Conference on Learning Representations
(ICLR). https://doi.org/10.48550/arXiv.1412.6980
Ruder, S. (2016). An overview of gradient descent
optimization algorithms.
https://doi.org/10.48550/arXiv.1609.04747
INCOFT 2025 - International Conference on Futuristic Technology
548

Wu, T., & Dong, Y. (2023). YOLO-SE: Improved
YOLOv8 for remote sensing object detection and
recognition. Applied Sciences, 13(24), Article 12977.
https://doi.org/10.3390/app132412977
Das, P., Chakraborty, A., Sankar, R., Singh, O. K., Ray,
H., & Ghosh, A. (2023). Deep learning-based object
detection algorithms on image and video. In
Proceedings of the 2023 3rd International Conference
on Intelligent Technologies (CONIT), 1–6.
https://doi.org/10.1109/CONIT59222.2023.10205601
Lou, H., Duan, X., Guo, J., Liu, H., Gu, J., Bi, L., & Chen,
H. (2023). DC-YOLOv8: Small size object detection
algorithm based on camera sensor.
https://doi.org/10.20944/preprints202304.0124.v1
Hussain, M. (2023). YOLO-v1 to YOLO-v8, the rise of
YOLO and its complementary nature toward digital
manufacturing and industrial defect detection.
Machines and Tooling, 11(7), Article 677.
https://doi.org/10.3390/machines11070677
Deo, G., Totlani, J., & Mahamuni, C. (2023). A survey on
bone fracture detection methods using image
processing and artificial intelligence (AI) approaches.
https://doi.org/10.1063/5.0188460
Meena, T., & Roy, S. (2022). Bone fracture detection
using deep supervised learning from radiological
images: A paradigm shift. Diagnostics, 12, Article
2420. https://doi.org/10.3390/diagnostics12102420
Redmon, J., & Farhadi, A. (2018). YOLOv3: An
incremental improvement. CoRR.
https://doi.org/10.48550/arXiv.1804.02767
Mehra, A. (2023). Understanding YOLOv8 architecture,
applications, and features. Labellerr.
https://www.labellerr.com/blog/understanding-yolov8-
architecture-applications-features/
He, K., Zhang, X., Ren, S., & Sun, J. (2014). Spatial
pyramid pooling in deep convolutional networks for
visual recognition. In Proceedings of the European
Conference on Computer Vision (ECCV), 346-361.
https://doi.org/10.1007/978-3-319-10578-9_23
Zheng, Z., Wang, P., Liu, W., Li, J., Ye, R., & Ren, D.
(2019). Distance-IoU loss: Faster and better learning
for bounding box regression. CoRR.
http://arxiv.org/abs/1911.08287
Li, X., Wang, W., Wu, L., Chen, S., Hu, X., Li, J., Tang,
J., & Yang, J. (2020). Generalized focal loss: Learning
qualified and distributed bounding boxes for dense
object detection.
https://doi.org/10.48550/arXiv.2006.04388
Yang, G., Wang, J., Nie, Z., Yang, H., & Yu, S. (2023). A
lightweight YOLOv8 tomato detection algorithm
combining feature enhancement and attention.
Agronomy, 13(7), Article 1824.
https://doi.org/10.3390/agronomy13071824
Li, Y., Fan, Q., Huang, H., Han, Z., & Gu, Q. (2023). A
modified YOLOv8 detection network for UAV aerial
image recognition. Drones, 7(5), Article 304.
https://doi.org/10.3390/drones7050304
Saxena, S. (2021). Binary cross entropy/log loss for binary
classification. Analytics Vidhya.
https://www.analyticsvidhya.com/blog/2021/03/binary
-cross-entropy-log-loss-for-binary-classification/
https://doi.org/10.22214/ijraset.2022.45523
Automated Bone Fracture Detection System Using YOLOv7 with Secure Email Data Sharing
549
