
Interpretability in AI for Early Lung Cancer Diagnosis: Fostering
Confidence in Healthcare
Sukanya Walishetti
1 a
, Subhasareddy Khot
1 b
, Shreya Marihal
1 c
,Prathmesh Kittur
1 d
,
Shantala Giraddi
2 e
and Prema T. Akkasaligar
1 f
1
Department of Computer Science and Engineering, KLE Technological University’s Dr. M. S. Sheshgiri College of
Engineering and Technology, Belagavi, Karnataka, India
2
KLE Technological University’s B.V.B. College of Engineering and Technology, Hubballi, Karnataka, India
Keywords:
Explainable AI (XAI), Convolutional Neural Networks (CNN), Medical Imaging, Grad-CAM, ResNet-18,
MobileNetv4, Chest X-Ray Analysis.
Abstract:
Lung cancer represents a significant cause of death, making early detection essential for better survival rates.
Lung nodules, which are small tissue masses in the lungs, can be initial indicators of lung cancer but are often
difficult to detect in chest X-rays due to their subtle appearance and potential overlap with normal anatomical
features. Analyzing these images manually is both error-prone and inefficient, often leading to discrepancies.
This research integrates convolutional neural networks (CNNs), specifically ResNet-18 and MobileNetV4,
with explainable AI techniques such as Grad-CAM to overcome these challenges. The ResNet-18 model
demonstrates high accuracy in nodule classification, while MobileNetV4 also shows strong performance, high-
lighting the potential of deep learning in this area. Grad-CAM is used to provide interpretability by visually
highlighting the regions of chest X-rays that influence the model’s predictions. This transparency is essential
for gaining trust from medical professionals, as it addresses the clinical need for accountability and supports
more informed diagnostic decisions.
1 INTRODUCTION
Lung cancer is the one of the primary causes of cancer
as there is increase in number of deaths related to lung
cancer. In majority of cases, lung cancer is detected
during critical stages where treatment is very limited.
Lung cancer is the leading cause of cancer-related
deaths globally, accounting for 1.8 million deaths in
2020 (18% of all cancer-related deaths). Early detec-
tion significantly improves outcomes, with localized
cases achieving a five-year survival rate of 61.2%. In
Malaysia, lung cancer constitutes 10% of all cancer
cases but has a notably low five-year survival rate of
9.0% (95% CI: 8.4–9.7).(Sachithanandan et al., 2024)
LDCT screening overcomes lung cancer mortality by
20–61%, yet its high cost and limited accessibility
a
https://orcid.org/0009-0009-5394-8181
b
https://orcid.org/0009-0003-4366-6718
c
https://orcid.org/0009-0007-0295-4931
d
https://orcid.org/0009-0002-8257-0172
e
https://orcid.org/0000-0003-3929-3819
f
https://orcid.org/0000-0003-2214-9389
hinder widespread adoption .
Timely detection of lung cancer is critically
important as it increases the survival rates but the
drawback is positive decisions cannot be taken as the
results obtained from the X-rays may be sometimes
inaccurate or invasive. The machine learning lan-
guage has created major important opportunities to
detect the lung cancer in early stage through medical
images and patient data. The implementation of AI in
healthcare sector must not only gives accurate data,
but it also gives explanation about how it reached to
the conclusion. In the recent times with the help of
AI and medical imaging has given significant results
in terms of detection of lung cancer in its early stage
using the assistance of deep learning techniques. It is
useful in detecting the features that may be difficult
to analyze by the human radiologists. However,
it is essential for AI systems to not only provide
high accuracy but also offer transparency in their
decision-making processes. This clarity is essential
in building trust among healthcare professionals, as it
ensures that AI-driven conclusions can be understood
and confidently integrated into clinical practice.
Walishetti, S., Khot, S., Marihal, S., Kittur, P., Giraddi, S. and Akkasaligar, P. T.
Interpretability in AI for Early Lung Cancer Diagnosis: Fostering Confidence in Healthcare.
DOI: 10.5220/0013610300004664
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 3rd International Conference on Futuristic Technology (INCOFT 2025) - Volume 3, pages 143-151
ISBN: 978-989-758-763-4
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
143

Deep learning methods, especially convolutional
neural networks (CNNs), are among the most promi-
nent solutions for early lung cancer detection. These
techniques are commonly applied to medical imaging
modalities like chest X-rays and CT scans, achieving
notable accuracy in identifying lung nodules. Ad-
vanced CNN variants, such as ResNet-18, have been
particularly effective in analyzing medical images
for tumor detection. Some models also incorporate
patient-specific data, including medical history
and genetic markers, to improve the precision of
predictions. Early detection frameworks are designed
to pinpoint minute changes in lung tissues that is
signify the early stages of cancer, potentially leading
to better patient prognosis.
A 2024 study (Divya et al., 2024) underscored the
high detection performance of CNN models in cate-
gorizing lung cancer cases. However, these systems
face significant obstacles, such as their reliance on
extensive computational resources and the availabil-
ity of large, annotated datasets for effective scaling.
The challenges in early lung cancer detection with AI
include the need for significant computational power,
large labeled datasets, and handling data imbalance.
Moreover, issues like limited model generalization,
interpretability, and ethical concerns related to patient
data privacy make it difficult to implement these
systems effectively in clinical practice.
The present paper focuses on developing an
accurate AI model for early lung cancer detection,
integrating explainable AI techniques to ensure
transparency. The model aims to improve clinical
decision-making by providing interpretable insights
for healthcare professionals, boosting patient con-
fidence. The model’s effectiveness is assessed in
practical scenarios to ensure its dependability and ef-
ficiency in clinical settings, enhancing early detection
and treatment outcomes.
The structure of this paper is as follows. Section
2 presents a concise overview of recent research in
the field. Section 3 outlines the problem statement,
background information, and the proposed method-
ology. Section 4 details the implementation process,
followed by the outcomes and an analysis of the re-
sults. Lastly, Section 5 wraps up the paper.
2 LITERATURE SURVEY
Advancements in imaging technologies and machine
learning have revolutionized lung cancer detection.
The survey delves into a variety of innovative
methodologies proposed by researchers for early
detection, classification, and diagnosis of lung cancer.
It highlights the strengths of these computational
models while addressing the challenges faced in
achieving widespread clinical application.
(Praveena et al., 2022) MobileNet V2: Efficient
Lung Cancer Detection Models for Mobile Devices
are discussed. It is an used on a small dataset
efficiently. But to ensure clinical reliability, di-
verse datasets need to be evaluated for it. (Elnakib
et al., 2020) reported that CNN models using archi-
tectures like AlexNet demonstrate high accuracy in
early detection but require significant computational
resources, limiting their real-world application. (Wu-
lan et al., 2021) reported that probabilistic neural net-
works demonstrate notable accuracy using X-rays but
may experience misclassifications due to poor-quality
images.
(Ingle et al., 2021) reported that AdaBoost-based
models demonstrate strong accuracy in predicting
lung cancer types, but their performance can be
impacted by dataset imbalances. (Maalem et al.,
2022) Hybrid models (CNN + Faster R-CNN) out-
perform traditional methods but demand high compu-
tational resources, hindering use in under-resourced
settings.(Mukherjee and Bohra, 2020) CNN models
minimize human intervention for CT scan-based lung
cancer detection, but hardware requirements hinder
real-time applications. (Thallam et al., 2020) Com-
bining SVM, random forest, and ANN outperforms
single models, but handling noisy data remains a lim-
itation.
(Aharonu and Kumar, 2023) stated that CAD
systems using neural networks demonstrate high
accuracy, but challenges remain with noisy (Thaseen
et al., 2022) Artificial neural networks (ANN)
combined with segmentation and noise reduction
show high early detection accuracy.(Prasad et al.,
2023) reported that EfficientNet B3 models effec-
tively classify lung cancer on CT scans, but broader
generalization to varied datasets remains necessary.
(Ravi et al., 2023) AlexNet combined with SVM
achieves high sensitivity but requires large annotated
datasets for general applicability. (Kalaivani et al.,
2020) reported that CNNs for image processing
exhibit strong accuracy in lung cancer detection,
demonstrating significant potential for clinical use.
INCOFT 2025 - International Conference on Futuristic Technology
144

In conclusion, the majority of the studies are con-
cerned with the determinant value, a few have ex-
plored explainable artificial intelligence approaches.
There is a deficiency in determining specific nodule
regions, which is imperative in gaining the confidence
of the medical practitioners. The aim of this research
is to close these gaps through the use of Grad-CAM
and other techniques that not only provide predictions
but also expose the inner workings of the model. It in-
creases the trust in lung nodule detection.
3 PROPOSED METHODOLOGY
Lung cancer is the one of the deadliest disease, where
early detection is vital for improving survival rates.
Chest X-rays are widely used for screening, but man-
ual analysis is complex, often leading to diagnostic in-
accuracies. Deep learning models, such as ResNet-18
and MobileNetV4, show promise for detecting nodule
but face trust issues due to their lack of transparency.
Explainable AI technique, such as Grad-CAM, over-
comes the issue by creating visual representation into
the model predictions. Grad-CAM highlights the re-
gion in X-ray, improving interpretability, fostering
trust among the medical professionals.
Since lung cancer is a major cause of death, early
detection is crucial in today’s world. The primary
method for detecting lung cancer is X-ray imaging;
however, it is often insufficient for identifying tumors
because cancerous tissues are very minute in the early
stages and difficult to detect. The goal of this study
is to create a model that enhances early lung can-
cer detection using deep learning technique and Grad-
CAM, which highlights the exact location of nodule,
thereby increasing the confidence of the medical com-
munity. The objectives of this study are as follows:
– To develop deep learning AI model for early lung
cancer detection
– To implement the explainable AI techniques to
identify minute cancerous tissues in early stage
The system is proposed for automated lung cancer
classification. Figure 1 shows the block diagram
of the proposed methodology. The X-ray images,
sourced from the JSRT dataset, which includes chest
X-ray images, their associated diagnoses, and the
location of lung nodules, are uploaded by the user
through the interface and then undergo the required
transformations to prepare them for future processing.
The JSRT created this dataset to offer a standardized
dataset for the creation and assessment of CAD
systems for identifying lung cancer. This dataset
includes 154 nodule images and their corresponding
locations, and 93 no nodule images. The original
images were in .IMG format with a resolution of
2048×2048 pixels, encoded with 16-bit unassigned
numbers. These images were converted to .png
usable format using “Image J” (Schneider, 2012).
Preprocessing includes resizing the given input X-ray
images to 224×224 pixels and normalizing the pixel
values. By standardizing the input, the model ensures
consistency of the images and provides accurate
results across the system. The data augmentation
module addresses the challenge of the limited dataset
size by expanding the original dataset of 247 X-ray
images to a total of 10,000 samples. This module
applies various augmentation techniques, such as
geometric transformations like flipping, rotation, and
cropping. The augmentation process introduces di-
versity into the dataset, enhancing the model’s ability
to generalize and reducing the risk of overfitting.
The module ensures that the dataset is well-balanced
between the two classes (nodule present and nodule
absent), enabling the models to learn effectively from
both categories.
The training module is the important system,
where the two advanced deep learning models
like ResNet-18 and MobileNetV4 are trained, The
training features differentiate the chest X-rays into
nodule present and nodule absent categories.
ResNet-18 is a deep convolutional neural network
renowned for its strong effectiveness in image
classification. It utilizes residual learning with skip
connections, which help train deeper networks by
alleviating the vanishing gradient issue. In this study,
a modified ResNet-18 model features an architecture
with multiple convolutional layers, batch normal-
ization, and ReLU activation functions, enhancing
feature extraction and learning capabilities. To tackle
class imbalance in the dataset, a weighted Cross-
entropy loss function is used, ensuring balanced
learning from both classes. This method harnesses
the strengths of ResNet-18’s architecture and adapts
it for effective lung nodule detection.
MobileNetV4 is a lightweight and efficient
neural network architecture specifically designed
for resource-constrained environments, providing a
faster alternative to larger, more complex models.
A custom MobileNetV4 inspired model developed,
incorporating a simpler architecture composed of
convolutional layers, max-pooling, and dropout to
reduce overfitting. To address the issue of class im-
balance in the dataset, a weighted Cross-entropy loss
Interpretability in AI for Early Lung Cancer Diagnosis: Fostering Confidence in Healthcare
145
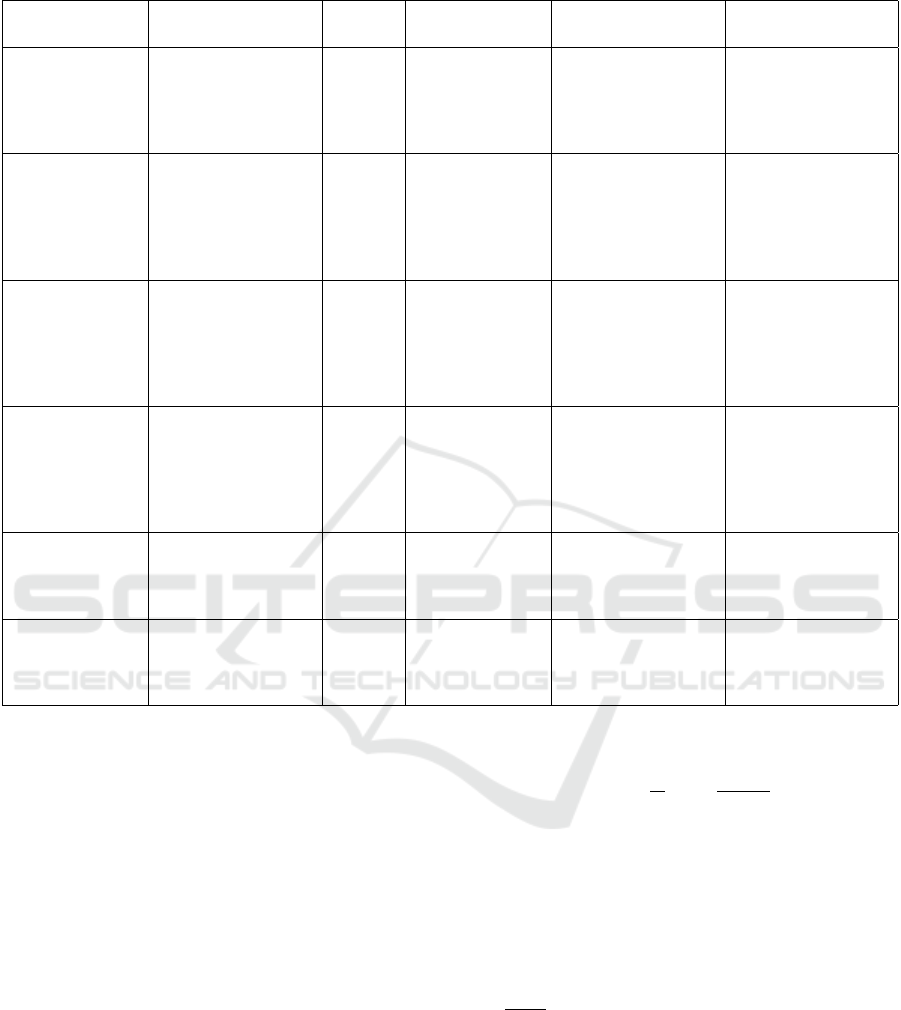
Table 1: Comparative Analysis of Diagnostic Models for Chest X-ray Imaging.
Methodology Dataset Description Year Performance Pa-
rameter
Limitation Gap Identified
DenseNet-121
(Wedisinghe and
Fernando, 2024)
Dataset has 247 im-
ages of frontal chest
X-ray.
2024 Training accuracy:
85%, Validation
accuracy: 73%.
Early detection issues,
lack of diversified
datasets, and technical
challenges in AI
integration.
Challenges in public
education, AI adop-
tion, and follow-up
processes.
Combined AI-
assisted chest
radiography
and LDCT (Sa-
chithanandan
et al., 2024)
Dataset of 16,551 X-
rays; 389 indetermi-
nate pulmonary nod-
ules (IPNs) detected
(2.35% yield).
2024 Sensitivity: 96%,
Specificity: 100%,
High diagnostic
accuracy: 96%.
Mixed attitudes to-
wards AI, technical
integration issues,
poor follow-up on
detected nodules.
Research on public
education, follow-up
strategies, and AI
adoption needed.
CNN Model (Jose
et al., 2024)
Dataset of 6034
balanced images
(cancerous and non-
cancerous cases).
2024 Accuracy: 96.4%,
Sensitivity:
83.3%, Speci-
ficity: 91.7%,
AUC: High ROC
performance.
Overfitting risks; re-
liance on augmenta-
tion techniques like
rotations and flips.
Limited clinical
validation and inter-
pretability issues.
CNN for Lung
Cancer Classifica-
tion (Divya et al.,
2024)
Dataset of 16,000 im-
ages covering adeno-
carcinoma, large cell
carcinoma, squamous
cell carcinoma, and
normal cases.
2024 Accuracy: 90%. High data require-
ments; overreliance
on X-rays limits gen-
eralizability.
Need for transfer
learning techniques
and healthcare inte-
gration.
Ante-hoc Concept
Bottleneck Model
(Rafferty et al.,
2024)
Dataset of 2374 chest
X-rays from the
public MIMIC-CXR
database.
2024 Accuracy: 97.1%,
AUC: 0.9495.
Small dataset limits
generalizability.
Validation on larger
datasets needed.
CNN and ResNet-
50 (Sekhar et al.,
2024)
Kaggle dataset:
120,561,416 CT
images (benign, ma-
lignant, normal).
2024 CNN: 95% accu-
racy, ResNet-50:
96%.
Limited to two ar-
chitectures; requires
broader experimenta-
tion.
Results lack gen-
eralization across
datasets.
function is utilized, ensuring the model effectively
learned from two classes. This approach maintained
the efficiency of the MobileNetV4 design while
tailoring it to the specific requirements of the lung
nodule detection.
The explainability module enhances the system
transparency by using Grad-CAM. Grad-CAM
generates heatmaps that takes input X-ray images, by
highlighting the region which is the most influenced
the models predictions. These visual feedback allows
the medical professionals to check whether the model
is focusing on clinically relevant areas such as the
nodule, are present in the lung rather than irrelevant
features.
The first step involves calculating the gradients of
the target class score (x
c
) concerning the feature maps
(B
m
) in the final convolutional layer. The important
feature map weights are calculated using Equation(1):
α
c
m
=
1
N
∑
a
∑
b
∂x
c
∂B
m,ab
(1)
Here:
• α
c
m
represents Importance weight of the m-th fea-
ture map.
• N represents the overall count of pixels in the
feature map.
•
∂x
c
∂B
m,ab
is the gradient of the target score in relation
to the activation B
m,ab
.
Further class activation map (CAM)is generated
through a weighted combination of feature maps,
as described in Equation(2):
L
ab
CAM
= ReLU
∑
m
α
c
m
B
m,ab
(2)
The ReLU activation ensures that only positive
sample contributions to the target class are
INCOFT 2025 - International Conference on Futuristic Technology
146
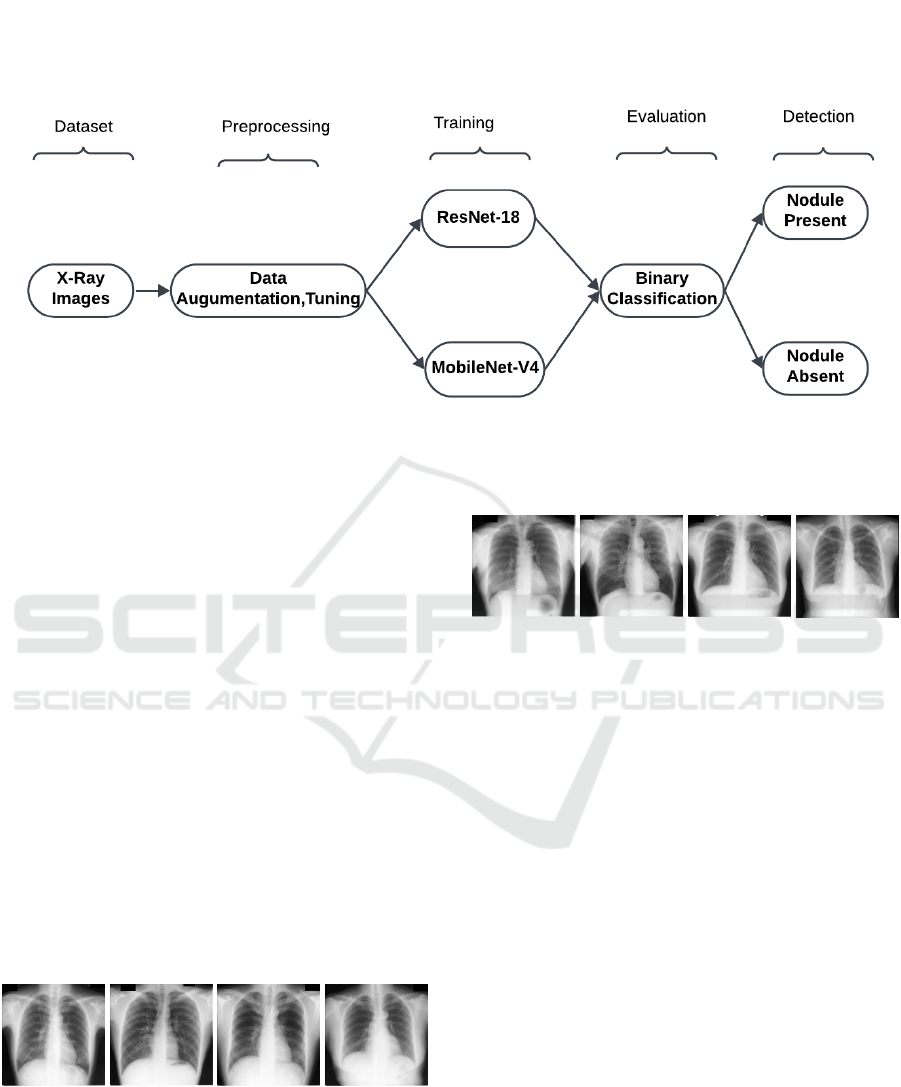
Figure 1: Proposed methodology for lung nodule detection
retained, effectively highlighting regions in the
input X-ray image that play a significant role
in the model’s predictions. By focusing on
these regions, the model generates heatmaps that
provide interpretable visual feedback.
4 RESULTS AND DISCUSSIONS
The execution is performed in Python, ensuring seam-
less integration with the PyTorch framework. The
dataset is trained on Google Colab’s GPU environ-
ment, utilizing a high-performance NVIDIA GPU
to accelerate training, particularly for large datasets
and using data augmentation tasks. The training em-
ployed using PyTorch with mixed-precision, enabled
through torch.cuda.amp to enhance computational ef-
ficiency and reduce memory consumption.
Figure 2: Example Chest X-ray Image Illustrating a without
Nodule
The study introduces a lung nodule detection sys-
tem utilizing chest X-ray images, developed with
Python and the PyTorch framework. The JSRT
dataset, comprising 247 chest X-ray images (157 with
Figure 3: Example Chest X-ray Image Illustrating a Nodule
nodules and 93 without nodules), serves as the foun-
dation. Each image has a resolution of 2048x2048
pixels. The preprocessing phase involves resizing the
images to 224x224 pixels and normalizing pixel val-
ues to meet the specifications of pre-trained models.
To improve the model’s robustness and generaliza-
tion, augmentation methods such as horizontal and
vertical flips and random rotations up to 15 degrees
are employed. Figure 2 and Figure 3 illustrate exam-
ples of images with and without nodules.
ResNet-18:
The ResNet-18 model is pretrained on the Ima-
geNet, is replaced by modifying its final fully con-
nected layer to accommodate binary segmentation.
Training is done with learning rate of 0.01 using SGD
of momentum(0.9) and weight decay of(1e-4). A fo-
cal loss function is utilized to handle class imbal-
ance with parameters alpha=0.25 and beta=2.0. The
model trained with the help of augmented dataset in-
cluding 10,000 X-ray images. The model is trained
for 50 epochs. The Figure 4 shows training accu-
racy achieved is 90.84% and the validation accuracy
is 90.36%. The results demonstrates that the data aug-
mentation has a positive impact on the model perfor-
mance. Figure 5 shows the training and validation
loss for ResNet-18, with both decreasing sharply in
Interpretability in AI for Early Lung Cancer Diagnosis: Fostering Confidence in Healthcare
147
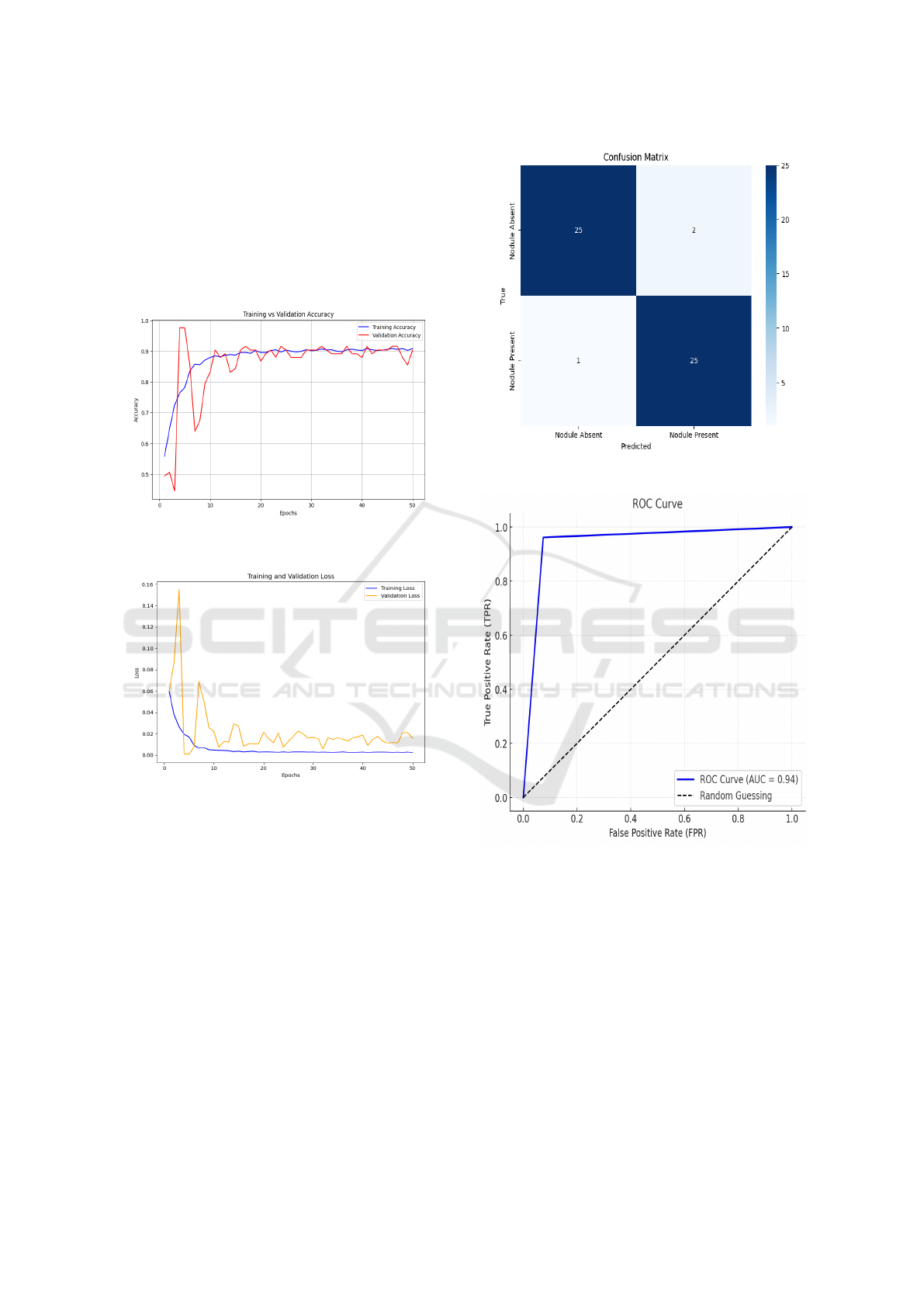
the initial epochs and stabilizing thereafter. The close
alignment of the curves indicates effective learning
with minimal overfitting. Figure 6 presents the con-
fusion matrix, where the model correctly classifies 25
nodule absent images as nodule absent and 25 nodule
present images as nodule present, with only 2 false
positives and 1 false negative. This indicates high
classification accuracy with minimal errors.
Figure 4: Training vs. Validation Accuracy for ResNet-18
Figure 5: Training vs. Validation Loss for ResNet-18
The Figure 7 shows the ROC curve for the
ResNet-18. The curve moves steeply upward, which
indicates great performance in separating positive
and negative classes. The score for AUC (area under
the curve) is 0.94, is quite high, indicating that the
model has good classification ability.
MobileNetV4: The MobileNetV4 offers the ad-
vantage of faster inference or lower computational
cost. Linear SGD with 0.001 and momentum of 0.9
is employed for training with a batch size of 8 and
StepLR is a learning rate scheduler that decreases the
learning rate by a factor of 0.1 after every 5 epochs.
Loss function is Cross-entropy loss with balanced
class weights, and training is carried on for 50 epochs.
Figure 8 shows training accuracy is 75% and valida-
tion accuracy is 74%. Figure 9 shows a steady de-
Figure 6: Confusion Matrix for ResNet-18
Figure 7: ROC Curve for ResNet-18
cline in training and validation loss, stabilizing after
20 epochs, indicating effective convergence and mini-
mal overfitting. Figure 10 presents the confusion ma-
trix, where the model correctly classifies 22 nodule
absent images as nodule absent and 20 nodule present
images as nodule present, with only a few misclassi-
fications, demonstrating its reliability in lung nodule
detection.
The Figure 11 shows ROC curve representing the
true positive rate (TPR) versus false positive rate
(FPR) using their respective range of thresholds. This
graph shows an AUC value of 0.78, meaning that the
model is better than a random classifier. The curve
INCOFT 2025 - International Conference on Futuristic Technology
148
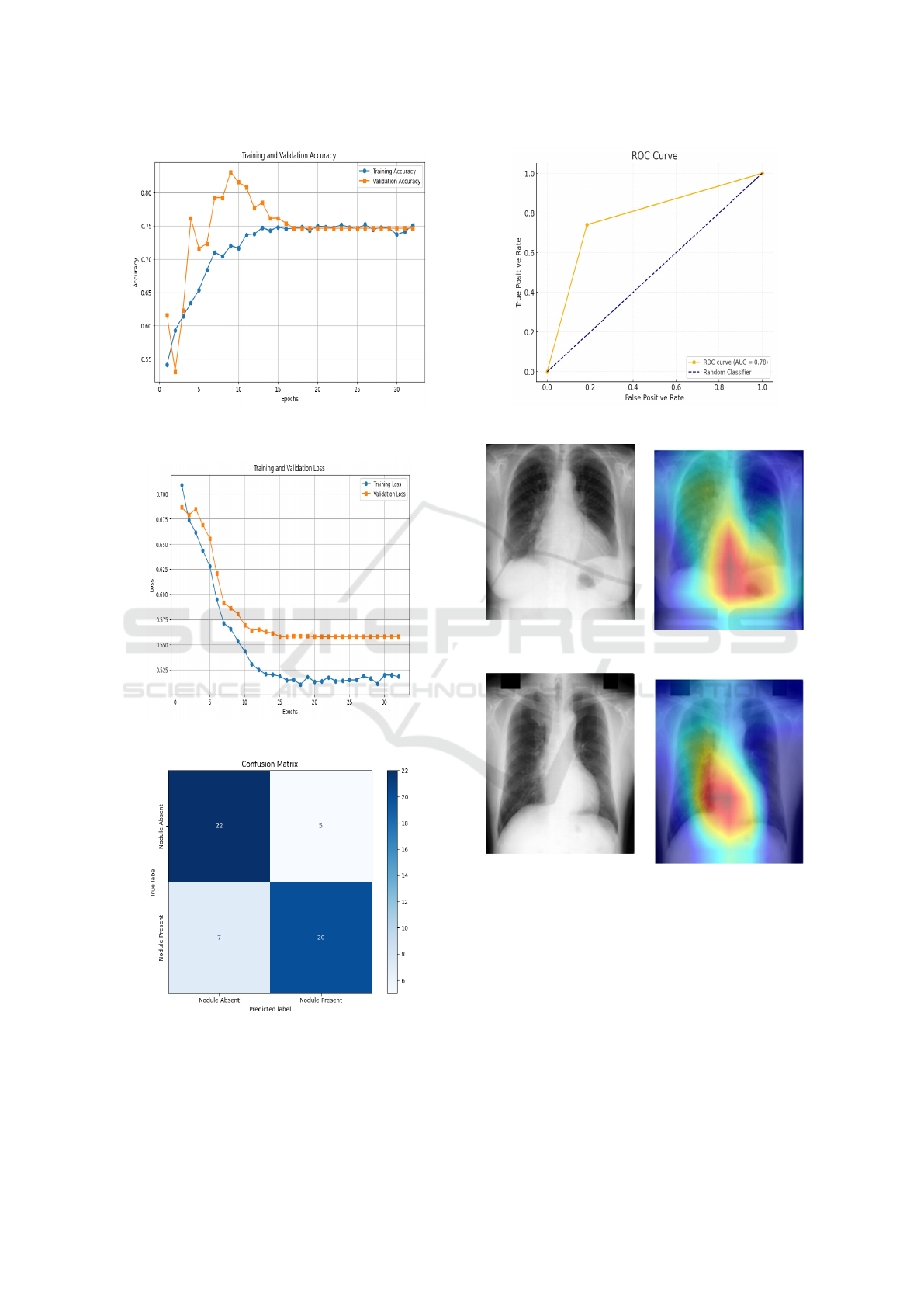
Figure 8: Training vs. Validation Accuracy for Mo-
bileNetV4
Figure 9: Training vs. Validation Loss for MobileNetV4
Figure 10: Confusion Matrix for MobileNetV4
indicates that the model has generally good perfor-
mance, though not perfect in terms of distinguishing
true positives and false positives across all thresholds.
The Table 2 shows the comparison of the perfor-
Figure 11: ROC Curve for MobileNetV4
Figure 12: Lung Nodule
Detection (a)
Figure 13: Grad-CAM (b)
Figure 14: Lung Nodule
Detection(c)
Figure 15: Grad-CAM (d)
mance between models. From Table 2, it is observed
that ResNet-18 provides high accuracy and reliability
for detecting lung cancer nodules. As shown in Ta-
ble 2, ResNet-18 outperforms MobileNetV4 in terms
of accuracy. Moreover, both models surpass the per-
formance of the existing paper (Wedisinghe and Fer-
nando, 2024), where DenseNet21 attained a training
accuracy of 85% and a validation accuracy of 73%.
The narrow gap between training and validation accu-
racies presented in this paper highlights the model’s
ability to generalize effectively from the augmented
dataset.
Interpretability in AI for Early Lung Cancer Diagnosis: Fostering Confidence in Healthcare
149

Further, Grad-CAM is applied to both ResNet-18
and MobileNetV4 to assess interpretability. Grad-
CAM is a technique that visualizes the important ar-
eas of an input image that have the greatest influence
on the model’s decision. By generating heatmaps,
Grad-CAM highlights areas in the image with high at-
tention (represented in red), indicating regions likely
associated with lung cancer nodules, while blue ar-
eas represent less relevant regions. This allows for a
better understanding of the model’s behavior, making
it more interpretable. Figure 13 and Figure 15 illus-
trate the application of Grad-CAM on chest X-rays to
highlight the regions contributing most to the predic-
tion of lung cancer by ResNet-18 and MobileNetV4
models. Both models successfully identify clinically
significant regions, as shown by the dark areas in the
heatmap. This interpretability helps medical profes-
sionals understand the model’s predictions and aligns
its focus with relevant clinical features, enabling reli-
able and transparent diagnosis.
Table 2: Performance Comparison of ResNet-18,
MobileNet-V4 and DenseNet-121 Classifiers.
Name of Classifier ResNet-18 MobileNet-
V4
DenseNet-
121 (We-
disinghe
and Fer-
nando,
2024)
Training Accuracy 90.84% 75% 85%
Validation Accu-
racy
90.36% 74% 73%
Precision 94.41% 77.93% -
Recall 94.34% 77.78% -
F1 Score 94.34% 77.75% -
5 CONCLUSIONS
This paper introduces lung nodule detection in chest
X-ray images.The problem is approached using deep
learning models such as ResNet-18 and MobileNetV4
trained on an augmented dataset of 10,000 images.
ResNet-18 attained a validation accuracy of 90.36%
while MobileNetV4 gained 74%. The ResNet-18
model outperformed MobileNetV4 in accuracy, mak-
ing it more appropriate for accurate lung nodule de-
tection. The use of Grad-CAM for model explainabil-
ity assures that the systems outcomes are transparent.
Future work will focus on increasing the dataset re-
fining models for real-time use, and integrating the
system into clinical environments.
REFERENCES
Aharonu, M. and Kumar, R. L. (2023). Systematic Review
of Deep Learning Techniques for Lung Cancer De-
tection. International Journal of Advanced Computer
Science and Applications, 14(3).
Divya, N., Dhilip, P., Manish, S., and Abilash, I. (2024).
Deep Learning Based Lung Cancer Prediction Us-
ing CNN. In 2024 International Conference on Sig-
nal Processing, Computation, Electronics, Power and
Telecommunication (IConSCEPT), pages 1–4. IEEE.
Elnakib, A., Amer, H. M., and Abou-Chadi, F. E. (2020).
Early Lung Cancer Detection Using Deep Learning
Optimization.
Hatuwal, B. K. and Thapa, H. C. (2020). Lung can-
cer detection using convolutional neural network on
histopathological images. Int. J. Comput. Trends Tech-
nol, 68(10):21–24.
Ingle, K., Chaskar, U., and Rathod, S. (2021). Lung Can-
cer Types Prediction Using Machine Learning Ap-
proach. In 2021 IEEE International Conference on
Electronics, Computing and Communication Tech-
nologies (CONECCT), pages 01–06. IEEE.
Jose, P. S. H., Sagar, J. E., Abisheik, S., Nelson, R.,
Venkatesh, M., and Keerthana, B. (2024). Leverag-
ing Convolutional Neural Networks and Multimodal
Imaging Data for Accurate and Early Lung Can-
cer Screening. In 2024 International Conference on
Inventive Computation Technologies (ICICT), pages
1258–1264. IEEE.
Kalaivani, N., Manimaran, N., Sophia, S., and Devi, D.
(2020). Deep Learning Based Lung Cancer Detec-
tion and Classification. In IOP conference series:
materials science and engineering, volume 994, page
012026. IOP Publishing.
Maalem, S., Bouhamed, M. M., and Gasmi, M. (2022). A
Deep-Based Compound Model for Lung Cancer De-
tection. In 2022 4th International Conference on Pat-
tern Analysis and Intelligent Systems (PAIS), pages 1–
4. IEEE.
Mukherjee, S. and Bohra, S. (2020). Lung Cancer Dis-
ease Diagnosis Using Machine Learning Approach. In
2020 3rd International Conference on Intelligent Sus-
tainable Systems (ICISS), pages 207–211. IEEE.
Prasad, P. H. S., Daswanth, N. M. V. S., Kumar, C. V. S. P.,
Yeeramally, N., Mohan, V. M., and Satish, T. (2023).
Detection Of Lung Cancer using VGG-16. In 2023 7th
International Conference on Computing Methodolo-
gies and Communication (ICCMC), pages 860–865.
IEEE.
Praveena, M., Ravi, A., Srikanth, T., Praveen, B. H., Kr-
ishna, B. S., and Mallik, A. S. (2022). Lung Cancer
Detection Using Deep Learning Approach CNN. In
2022 7th International Conference on Communication
and Electronics Systems (ICCES), pages 1418–1423.
IEEE.
Rafferty, A., Ramaesh, R., and Rajan, A. (2024). Trans-
parent and Clinically Interpretable AI for Lung Can-
cer Detection in Chest X-Rays. arXiv preprint
arXiv:2403.19444.
INCOFT 2025 - International Conference on Futuristic Technology
150

Ravi, V., Acharya, V., and Alazab, M. (2023). A Mul-
tichannel EfficientNet Deep learning-Based Stack-
ing Ensemble Approach For Lung Disease Detec-
tion Using Chest X-Ray Images. Cluster Computing,
26(2):1181–1203.
Sachithanandan, A., Lockman, H., Azman, R. R., Tho,
L., Ban, E., and Ramon, V. (2024). The Potential
Role of Artificial Intelligence-Assisted Chest X-ray
Imaging in Detecting Early-Stage Lung Cancer In The
Community—A Proposed Algorithm For Lung Can-
cer Screening In Malaysia. The Medical Journal of
Malaysia, 79(1):9–14.
Schneider, C. A. R. W. A. E. K. (2012). Nih image to im-
agej: 25 years of image analysis. Nature Methods, 9.
Sekhar, K. R., Tayaru, G., Chakravarthy, A. K., Gopiraju,
B., Lakshmanarao, A., and Krishna, T. S. (2024). An
Efficient Lung Cancer Detection Model using Con-
vnets and Residual Neural Networks. In 2024 Fourth
International Conference on Advances in Electrical,
Computing, Communication and Sustainable Tech-
nologies (ICAECT), pages 1–5. IEEE.
Thallam, C., Peruboyina, A., Raju, S. S. T., and Sampath,
N. (2020). Early Stage Lung Cancer Prediction Us-
ing Various Machine Learning Techniques. In 2020
4th International Conference on Electronics, Commu-
nication and Aerospace Technology (ICECA), pages
1285–1292. IEEE.
Thaseen, M., UmaMaheswaran, S., Naik, D. A., Aware,
M. S., Pundhir, P., and Pant, B. (2022). A Review
of Using CNN Approach for Lung Cancer Detection
through Machine Learning. In 2022 2nd International
Conference on Advance Computing and Innovative
Technologies in Engineering (ICACITE), pages 1236–
1239. IEEE.
Wedisinghe, H. and Fernando, T. (2024). Explainable AI
for Lung Cancer Detection: A Path to Confidence. In
2024 4th International Conference on Advanced Re-
search in Computing (ICARC), pages 13–18. IEEE.
Wulan, T. D., Kurniastuti, I., and Nerisafitra, P. (2021).
Lung Cancer Classification in X-Ray Images Us-
ing Probabilistic Neural Network. In 2021 Interna-
tional Conference on Computer Science, Information
Technology, and Electrical Engineering (ICOMITEE),
pages 35–39. IEEE.
Interpretability in AI for Early Lung Cancer Diagnosis: Fostering Confidence in Healthcare
151
