
Early Detection of Diabetic Retinopathy Using ResNet-18
Rohan Doggalli, Aakash Deep, Rohith Naik V, Vinay B Achari, Vijaykumar Muttagi
and Uday Kulkarni
School of Computer Science and Engineering, KLE Technological University, Hubballi, India
Keywords: Diabetic Retinopathy, Deep Learning, ResNet-18, Automated Screening, Retinal Image Analysis, Clinical
Decision Support System.
Abstract: Diabetic retinopathy (DR) is a leading cause of preventable blindness, especially among diabetic patients.
Early diagnosis is critical to halt its progression and prevent vision loss. This work leverages deep learning,
specifically the ResNet-18 model, to detect DR from retinal images. Using a Kaggle dataset divided into
training and validation sets, the model achieved a training accuracy of 98.57% and a validation ac- curacy of
83.49%. These findings underscore the efficacy of ResNet-18 in automating DR detection. Integrating such
technology into clinical workflows has the potential to enhance early screening and treatment strategies,
improving patient outcomes while optimizing healthcare re- sources.
1 INTRODUCTION
Diabetic retinopathy is a severe complication of
diabetes that affects the fragile blood vessels of the
retina and can cause vision loss or even blindness if
left untreated. DR progresses through stages: the first
one being nonproliferative di- abetic retinopathy, the
earliest stage characterized by leaking and swelling
blood vessels. The more advanced stage, proliferative
diabetic retinopathy (PDR), is characterized by the
abnormal proliferation of blood vessels, leading to
detach- ment of the retina, bleeding, and irreversible
vision loss.
According to the World Health Organization,
more than 420 million people worldwide suffer from
diabetes, and this number is expected to surge
exponen- tially in the near future (Nirgude, Revathi,
et al. , 2024). Diabetic retinopathy remains one of the
leading causes of preventable blindness globally. In
2010 alone, it caused 0.8 million cases of blindness
and 3.7 million cases of visual impairment worldwide
(Bourne, Price, et al. , 2012), (Solomon, Chew, et al.
, 2017). By 2030, the number of DR patients is
projected to rise to 191 million, with a prevalence rate
of 27% globally from 2015 to 2019 (Teo, Tham, et al.
, 2021), (Yau, Rogers, et al. , 2012). These statistics
underscore the urgent need for effective early
detection and timely treatment to prevent vision loss.
Early diagnosis of DR is essential because the
disease process can be pre- vented in its early stages
if timely intervention is performed. Early DR can be
controlled with laser treatment, anti-VEGF
injections, and vitrectomy that stops the advancement
of the disease. Traditional screening of DR has
proven to be a very tedious process, laborious, and
prone to human errors. The current scenario among
ophthalmologists is retinal images analysis, and due
to this, there have been delayed diagnoses, cases left
behind, and overloads in healthcare resources. With
an increase in the incidence of diabetes and the
prevalence of diabetic retinopathy projected to
increase, there is a dire need for automated systems
that improve the screening process to give faster and
more accurate diagnoses in support of early detection
efforts(Abràmoff, 2020), (Cheung, Ikram, et al. ,
2015).
Machine learning (ML) and deep learning (DL)
have emerged as revolution- ary tools in the medical
field, particularly in the automatic detection of DR.
Con- volutional Neural Networks (CNNs) are a class
of deep learning models that have shown impressive
performance in the analysis of retinal images by
autonomously learning complex features from large
datasets. These models can identify even the subtlest
signs of DR in its early stages, far outperforming
traditional meth- ods in terms of accuracy and
efficiency (Gulshan, Peng, et al. , 2016), (Krizhevsky,
Doggalli, R., Deep, A., Naik V, R., B Achari, V., Muttagi, V. and Kulkarni, U.
Early Detection of Diabetic Retinopathy Using ResNet-18.
DOI: 10.5220/0013609200004664
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 3rd International Conference on Futuristic Technology (INCOFT 2025) - Volume 3, pages 81-90
ISBN: 978-989-758-763-4
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
81

Sutskever, et al. , 2012). Yet, there are still challenges
in applying DL in DR detection: a need for large
annotated datasets to train, over- fitting issues when
trained on limited data, and a lack of interpretability,
which limits clinical adoption (Ward, Maselko, et al.
, 2017).
This work focuses on using ResNet-18, an
efficient deep learning architecture based on the
residual network design, to better identify diabetic
retinopathy early. The architecture of ResNet-18,
better suited to the image classification task because
it prevents the vanishing gradient problem with
residual connec- tions, results in better training of
deep networks. The model’s ability to learn complex
features from medical image datasets makes it a
powerful tool for iden- tifying subtle patterns
indicative of DR, even in its early stages (Zhang, Ren,
et al., 2016). Advanced techniques like image
preprocessing, data augmentation, and
hyperparameter optimization are incorporated into
the proposed system with the aim of enhanc- ing
model robustness and generalization, thus improving
its ability to detect DR across diverse populations and
imaging modalities. It aims at addressing the critical
problem of model transparency besides the limited
annotated data to overcome the challenges this
presents. This means the system develops a model
that, besides providing the correct predictions, also
allows interpretability us- ing techniques like Class
Activation Maps (CAMs), hence gaining trust among
healthcare professionals. This approach allows
ophthalmologists to understand how the model comes
to its conclusion, thereby facilitating better
integration of AI-based tools into clinical decision-
making processes (Ward, Maselko, et al. , 2017).
Deep learning models suffer from several major
challenges. One major issue is their dependence on
large annotated datasets for training. Annotated data
are often either unavailable or scarce in many
healthcare settings, thereby preventing such models
from generalizing well to different populations and
imaging modal- ities. This problem leads to
overfitting, mainly when the models are trained on
less or biased datasets, resulting in poor accuracy
when tested with new or varied data. Further, the
issue of uninterpretability is another huge challenge
for deep learning models to be used in clinical
practice at large scales. For medical professionals to
trust and appropriately use AI-based systems, they
need to be aware of how the models generate their
predictions (Ward, Maselko, et al. , 2017).
Over the years, research studies have been
conducted that have explored the possibility of deep
learning in diabetic retinopathy detection and have
shown promising results. Gulshan et al. (2016)
developed a deep learning algorithm that gained
diagnostic accuracy equal to an expert
ophthalmologist in DR identifica- tion from retinal
images (Abràmoff, 2020). Leibig et al. demonstrated
in 2017 the superiority of deep learning models
compared to traditional methods for screening DR
(Ward, Maselko, et al. , 2017). The remaining
challenges include dataset variability, high
computational require- ments from deep learning
models, and the lack of model transparency, which
have significantly prevented the wider clinical
applicability of these technologies.
2 LITERATURE WORK:
Diabetic Retinopathy (DR) has become an
extremely active area of research due to its severe
contribution to blindness in diabetic patients.
Timely detec- tion and treatment play a crucial role
in the prevention of permanent blindness in many
patients, which puts emphasis on early diagnosis.
Traditional methods for DR detection involved the
visual inspection of retinal fundus images by us- ing
techniques like thresholding, edge detection, and
region growing. However, these conventional
approaches are unable to deal with intrinsic
variability and complexity in the retinal images.
Some of this variability includes varying illu-
mination, noise, and dimensions of lesions that
become challenging to diagnose accurately (Yau,
Rogers, et al. , 2012).
Figure 1: Healthy eye and Diabetic Retinopathy
Fig 1 presents a normal eye alongside a diabetic
retinopathy-affected eye. The normal eye blood
vessels look healthy and intact. Consequently, proper
functioning is provided by these vessels. The DR-
afflicted eye presents damaged vessels leaking fluids
to the retina, leading to swelling and resulting loss of
vision. These pathological changes are characteristic
of DR and underlie the importance of early detection
and intervention to prevent irreversible damage. This
INCOFT 2025 - International Conference on Futuristic Technology
82

visual representation emphasizes the need for
advanced diagnostic tools, such as deep learning
models, in order to identify the condition at its earliest
stages to provide prompt treatment.
With the advent of ML and DL technologies,
much progress has been made in automating the
detection of DR. CNNs are currently identified as the
best tech- niques to automatically identify DR
because it can learn hierarchies of features from raw
pixel data (Gulshan, Peng, et al. , 2016). Models like
ResNet, Inception, and VGG, which are CNN-based,
have achieved outstanding accuracy in the
classification of fundus images into the different DR
stages (Zhang, Ren, et al. , 2016). These models were
trained and vali- dated on public datasets, including
the Kaggle diabetic retinopathy competition dataset,
thus making them more generalized and reproduce in
real-world settings (Lu, Liu, et al. , 2018).
Among the new developments in the field, there
is also the application of ResNet-18. It is a deep
learning architecture, presented by He et al. (2016)
for residual learning. The success of ResNet-18 has
been achieved in different ap- plications, including
diabetic retinopathy detection from images of the
retina. By applying residual connections, the
architecture is able to avoid the vanishing gradient
problem, and hence train very deep networks. This
innovative archi- tecture has been applied
successfully to the analysis of medical images, and it
is therefore a very powerful tool in the detection of
subtle patterns that can indicate DR at its earliest
stages (Zhang, Ren, et al. , 2016). Success of ResNet-
18 in DR detection and a relatively lightweight
structure of the model make it a good candidate for
healthcare applications where accuracy and
efficiency are equally critical.
Recent advances in transfer learning and attention
mechanisms have further improved the performance
of DR detection systems. Transfer learning, which
fine-tunes pre-trained models on DR datasets, has
been shown to achieve high accuracy even with
limited labeled data (Lu, Liu, et al. , 2018). In
addition, attention mecha- nisms such as self-
attention and saliency maps allow models to focus on
clinically relevant regions of retinal images, thus
improving both interpretability and diag- nostic
accuracy (Jia, Li, et al. , 2019). These advancements
ensure that deep learning models are more capable of
distinguishing between subtle features in retinal
images, making them more suitable for early
detection of DR. Data augmentation and multi-task
learning techniques have also been used to handle
imbalanced datasets and im- prove model robustness.
However, several challenges remain. One major issue
is the generalizability of DR detection models across
different imaging de- vices, populations, and datasets.
Models trained on single-source datasets tend to
overfit and thus perform poorly if exposed to new or
diverse data (Nirgude, Revathi, et al. , 2024). A sec-
ond major problem is that deep learning models lack
interpretability. Healthcare practitioners often do not
embrace AI-driven tools because of opaque decision-
making processes. Efforts have been made to explain
these models better with XAI techniques, like Grad-
CAM and saliency maps, for the sake of increased
transparency of the models in this concept (Ward,
Maselko, et al. , 2017)]. Another issue that still exists
in this field is data imbalance, especially for
underrepresented stages of diabetic retinopathy.
Many datasets lack good representations of samples
from early or severe stages of the disease, which
makes training and evaluation difficult. Advanced
preprocessing and augmentation strategies are being
used to address these issues to improve model
performance across different datasets(Bourne, Price,
et al. , 2012).
These gaps can be filled by focusing on model
generalization using robust training strategies,
including advanced preprocessing, data
augmentation, and hyperparameter optimization
techniques in the proposed methodology.
Using ResNet-18 and transferring its ability into
DR detection with high ac- curacy, it will attempt to
diagnose the disease at its earliest stage. Furthermore,
the incorporation of attention mechanisms is explored
to improve both diagnos- tic accuracy and
interpretability. This work thus contributes to a global
effort aimed at reducing loss of vision due to DR by
proposing an automated solution for efficient and
scalable DR detection at an early stage. In summary,
although deep learning has really revolutionized the
detection of diabetic retinopathy, challenges like
generalizability, interpretability, and data imbalance
still pose se- rious issues. The gaps in these areas are
likely to be bridged in the near future through
improved model generalization using architectures
such as ResNet-18, using explainability techniques,
and enhancing the data strategies so that such models
can be made clinically viable and reliable for large-
scale use in healthcare systems.
3 PROPOSED
WORK:
3.1 Data Collection
In the first important milestone of the project, there
is the provision of data input with the sourcing of
Early Detection of Diabetic Retinopathy Using ResNet-18
83

retinal fundus images ahead of processing by further
stages. For this work, the Aptos 2019 Blindness
Detection dataset(Aptos, 2019) is used. This dataset
includes a collection of high-resolution images of the
retina with labels categorized as five types of diabetic
retinopathy on a spectrum of severity: No DR, Mild
DR, Moderate DR, Severe DR, and Proliferative
DR.in Fig 2.
Fig. 2. Stages of Diabetic Retinopathy
All images are in PNG, ensuring high fidelity and
resolution—the require- ments for a deep learning
model, like ResNet-18, to correctly identify the pat-
terns with the subtle and complex information
indicative of diabetic retinopathy. Once in the system,
the image passes through a series of pre-processing
oper- ations that have improved consistency and
quality in various operations such as standardization,
cleaning of the images, normalization, and
augmentation.
Presenting high-quality and standardized images,
the model can obtain robust learning and generate
accurate predictions for early detection and
classification of diabetic retinopathy.
3.2 Data Preprocessing
Among all these steps toward preparation of retinal
images for training, it is undeniable that
preprocessing is one of the most important ones,
especially when detecting diabetic retinopathy (DR).
It actually depends on the quality of input images -
hence, performance of the model. In order to
optimize the dataset and to ensure that the data
will be very much consistent with high quality
while training, there are several preprocessing
techniques, such as image cleaning, resizing,
normalization, and data augmentation.
Image Cleaning: The pre-processing stage in this
regard is image cleaning. This step cleans the retinal
images of noise, artifacts, and distortions. Because
the raw retinal images can be captured under varying
conditions and on different devices, pixelation,
irrelevant background noises, etc. may confuse the
model. This stage enhances important features like
blood vessels, hemorrhages, and microaneurysms
that are important in DR, but suppresses irrelevant
patterns. This is because the improvement in the
signal-to-noise ratio allows the model to capture the
weak changes in the retina efficiently, which may not
be easy to decide and diagnose cases of early DR.
All the images are resized to the same dimension
of 256x256 pixels. For a deep learning model like
ResNet-18, this fixed input dimension is vital. The
resolution chosen has been a balance between
computational efficiency and preserving de- tails. It is
large enough to preserve important retinal features so
that the model could correctly identify and classify the
stages of DR but not large enough to be
computationally manageable.
Normalization: This step rescales pixel values of
images to be within the range of 0 and 1. That way,
the input to the neural network will become
standardized. Without normalization, big variations
in pixel values would cause instability in the training
process, leading the model to converge at a slow
speed. Normalizing the pixel value means that the
model becomes effective at processing data with
quicker convergence and better overall performance.
Data Augmentation : Techniques of data
augmentation are used to make the model more robust
and avoid overfitting. Techniques of data
augmentation artificially increase the size of a dataset
by applying transformations such as random flipping,
rotation, brightness adjustment, and color jittering.
Augmen- tation introduces variations in the
orientation, lighting, and color of the images in a
manner that it mimics real-world conditions. It helps
diversify the training data but also enhances the
model’s ability to generalize to unseen data, which
will improve the reliability of the model in clinical
settings.
3.3 Data Labeling
Once all the images are pre-processed, it is the
labelling procedure of data, which actually guides the
model training for diabetic retinopathy (DR).
Labelling in- volves associating the severity of
damage caused due to this disease with the re-
spective image of the retina. Our project uses a pre-
labelled image dataset in the aptos 2019 version.
These pre-labeled images would be important for
supervised learning: they help the model connect
input images with their classifications of severity.
Each image in the dataset is classified into one of
the following severity levels: No DR, Mild DR,
Moderate DR, Severe DR, or Proliferative DR. No
DR is a healthy retina with no signs of diabetic
retinopathy, whereas Proliferative DR is the most
severe stage of the disease, which involves abnormal
INCOFT 2025 - International Conference on Futuristic Technology
84

blood vessel growth and significant retinal damage.
These severity levels are used as ground truth for
training. It can compare its prediction against the
actual labels and iteratively minimize errors.
The learning process of a model heavily relies on
the correctness of labeling. For supervised machine
learning, a good basis is labeled data where the model
learns mapping input data, such as retinal images, into
output labels, which can be considered as severity
levels. Quality labeling ensures the well-definition
and reliability in severity for every image, enabling
effective learning by the model. Poor labeling can
result in lower performance and less accurate detec-
tion and classification of diabetic retinopathy.
Accurate labels are therefore vital in enhancing the
model’s ability to predict, with good performance in
clinical applications.
3.4 Model Architecture
It discusses and presents a deep learning-based
architecture of ResNet-18 for the detection and
classification of diabetic retinopathy from retinal
fundus images. Fig 3 is a representation of such
architecture.
Figure 3: ResNet-18 architecture
As depicted in Fig 3, the model first accepts a
224×224×3 retinal fundus image as input. It goes
through several stages:
Convolutional Layers: The initial convolutional
layers (Conv1 to Conv5) ex- tract hierarchical
features. Low-level features such as edges and
textures are captured in the earlier layers, while
deeper layers extract complex patterns, such as blood
vessels, hemorrhages, and microaneurysms. The
convolution operation is mathematically defined as:
𝑂𝑢𝑡𝑝𝑢𝑡
(
𝑥,𝑦
)
=
∑∑
1
(
𝑥+𝑚,𝑦+
𝑛
)
𝑘
(
𝑚,𝑛
)
(1)
where I(x + m, y + n) is the input pixel value, and
K(m, n) is the convolutional kernel. Residual Blocks:
To better learn residual connections bypass some
layers such that the model is directed to learn the
residual features.
𝑦=𝐹
(
𝑥,𝑊𝑖
)
+𝑥 (2)
This framework ensures efficient training by
mitigating the vanishing gradient problem. Pooling
and Feature Reduction: Pooling layers reduce the
spatial di- mensions, emphasizing important features.
Max-pooling is computed as
𝑀𝑎𝑥𝑃𝑜𝑜𝑙𝑖𝑛𝑔 = max
,
(𝑙(𝑥 + 𝑖, 𝑦 + 𝑗)) (3)
The Final Layers: Fully Connected Layers
aggregate the features that were extracted. Softmax
Classification with activation for the prediction of
diabetic retinopathy levels: No DR, Mild DR,
Moderate DR, Severe DR and Proliferative DR.
Important parts of architecture like ReLU
activation, batch normalization ensure efficient
learning by introducing non-linearity to the ReLU
function :
𝑓
(
𝑥
)
= max
(
0, 𝑥
)
(4)
Batch Normalization normalizes activations to
accelerate training:
x i=
(5)
The model is optimized using the Adam optimizer
with categorical cross- entropy loss. Its equations
ensure adaptive learning rates for each parameter
η
=
(6)
This architecture efficiently extracts features at
multiple levels of abstraction which allows for robust
classification of diabetic retinopathy severity.
3.5 Training the Model
The training phase of the ResNet-18 model initiates
after preprocessing and la- belling the retinal images.
These images are feed into the deep convolutional
net- work, and ResNet-18 automatically extracts
hierarchical features such as blood vessels,
hemorrhages, and microaneurysms that are pertinent
for the detection of DR. The model utilizes the
backpropagation algorithm, which updates its
weights based on the gradients calculated from the
loss function with the aim of minimizing prediction
errors. It uses the Adam optimizer for weight updates,
dynamically adjusting the learning rate during
training, which leads to faster convergence and stable
Early Detection of Diabetic Retinopathy Using ResNet-18
85

optimization. The initial learning rate is set at 0.001
and dynamically adjusted as the training progresses.
The model uses categorical cross-entropy loss,
which is the difference between the actual labels and
the predicted probabilities for each class. Thus, the
loss function can be defined as
Cross Entropy Loss =−
∑
𝑦
log
(
𝑦
)
(7)
where C is the total number of classes, and yi is
the true label, and yˆi is the predicted probability for
every class.
Hyperparameter tuning is crucial for optimizing
the model’s performance. The three main parameters
include the learning rate, dropout rate, and the
number of epochs to be used. All of these are adjusted
accordingly to get the best possible output. A learning
rate scheduler is used to speed up convergence in the
early epochs and to gradually refine the model as it
approaches opti- mum performance. The dropout
rate, set between 0.2 and 0.5, is used to avoid
overfitting and generalization.
The ResNet-18 model was trained for 50 epochs.
In this period, training and validation performance is
monitored at regular time steps. After training the
model obtained a training accuracy of 98.57% with
the corresponding loss for training as 0.0322. Still,
the validation accuracy stood at 83.49%. This implies
that though the model has picked the features of
interest from the training data, it underperforms a
little bit on the unseen data and hence calls for further
improvements in generalization. These can be
achieved through methods like data augmentation,
regularization, and fine-tuning.
Early stopping was applied in order to avoid
overfitting and maximize the efficiency of
computation. Training stopped once validation
performance did not improve further, saving some
computation resources and ensuring that it would not
overfit on the training data.
3.6 Evaluation
The performance of the ResNet-18 model in detecting
diabetic retinopathy is evaluated by considering the
validation set. In the evaluation, metrics like ac-
curacy, precision, recall, and F1 score are used to
judge the performance of the model. These metrics
provide an overview of the model’s performance in
detect- ing diabetic retinopathy at different stages.
Accuracy is among the evaluation measures, a
ratio of correctly predicted hits to total predictions.
As such, this is essentially an overall performance
measure from the model:
Accuracy =
(8)
Precision refers to accuracy about the positive
cases, i.e., true classification of images from the
retinal images which is diabetic retinopathy in reality
belongs to class DR. Precision is the calculation of:
Precision =
(9)
where TP denotes true positives (correctly
classified DR images), and FP denotes false positives
(non-DR images misclassified as DR).
Recall, in contrast, measures how well the model
detects all true diabetic retinopathy cases, even those
that are more challenging to detect. It is defined as
𝑅𝑒𝑐𝑎𝑙𝑙 =
(10)
where FN stands for false negatives, i.e., DR
images mistakenly classified as non-DR.
The F1 score is the harmonic mean of precision
and recall, hence a single number that reflects both. It
is also useful in case of datasets with class imbal-
ance since it takes into account both the false
positives and false negatives. The formula for
calculating the F1 score is:
𝐹1 − 𝑆𝑐𝑜𝑟𝑒 = 2.
.
(11)
During the evaluation, it passes unseen data to
evaluate real-time perfor- mance. Here also,
validation accuracy turned out to be 83.49%whereas
training accuracy was at a whopping 98.57% which
shows very effective model perfor- mance on the train
set though results from the validation part depict
overfitting. Precision, recall, and F1 score are
calculated as well to test this model in diag- nosing
diabetic retinopathy stages completely.
Hyperparameter tuning: Assuming that
performance isn’t as it should, the learning rate,
possibly the batch size, number of epochs is to be
adjusted. Yet another necessary architectural
adjustment would be putting more layers in or some
other activation function that enhances the generality
of your model. Meth- ods like cross-validation,
increased diversity of the data maybe achieved using
augmentation of training data to work on to improve
on the validation.
By making use of these metrics, it ensures that the
developed model is ac- curate and strong, thereby
being capable to detect diabetic retinopathy in real-
world applications precisely.
INCOFT 2025 - International Conference on Futuristic Technology
86
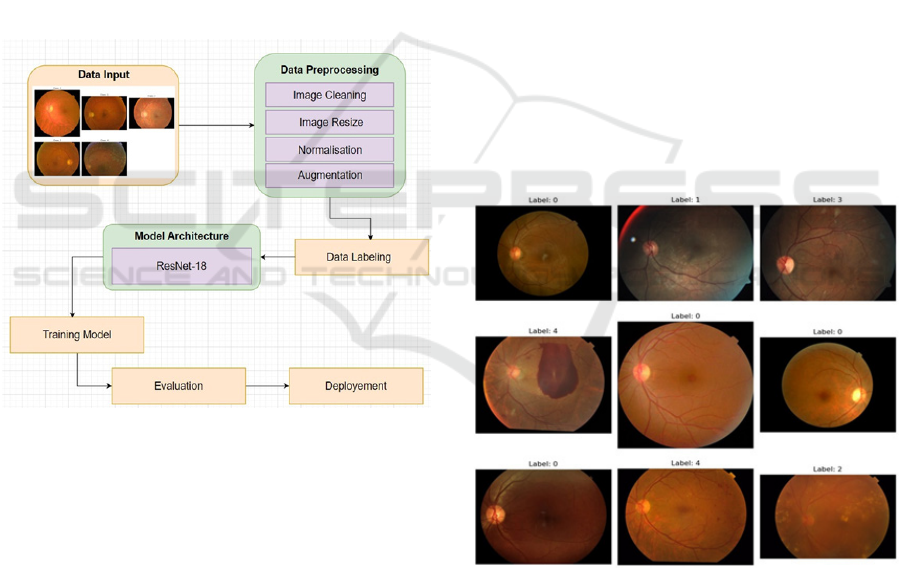
3.7 Deployment
Following satisfactory performance in training and
evaluation, the ResNet-18 model is adopted into a
clinical decision support system to detect DR through
the automatic analysis of images of retinas, further
classifying them on levels of DR severity and guiding
healthcare providers with quicker and more accurate
diagnoses. Reduction in manual assessment of
images aids fast detection of DR, which further leads
to better patient care through early intervention.
This is made accessible through cloud platforms
or even a hospital’s local network, meaning new
retinal images will be processed in real-time. The
model will, therefore, be retrained periodically on
updated data in order to adapt to changes in imaging
techniques and patterns of DR. The model is
monitored in terms of its effectiveness within clinical
settings to ensure continued reliability in DR
diagnosis in the long term.
Figure 4: Flowchart illustrating machine learning workflow
for Early Detection of Dia- betic Retinopathy
The critical challenges of the model are it’s unable
to generalize very well on unseen data; proof for this
can be demonstrated with the difference in terms of
training and validation accuracy. To combat the side
effects, methods involving data augmentation have
been adopted as ways to enhance diversity over the
training set and added further dropout regularization
for cutting the possibil- ity of overfitting. The
learning rate scheduler has also been applied aiming
to improve convergence and the generalization on
both the training and validation sets.
4 RESULTS AND ANALYSIS
This study has applied a dataset labeled with a
train.csv file and created to pre- dict the level of
diabetic retinopathy, which had 3,662 records. Its two
principal attributes include Id_Code and diagnosis.
This is for the sake of Id_Code, an identifying
variable in any given instance for traceability
purposes. The target variable would be the diagnosis
column with five levels of the severity of diabetic
retinopathy: 0 is no DR, 1 is mild, 2 is moderate, 3 is
severe, and 4 is prolifera- tive DR. This dataset serves
as a base for training machine learning models to
classify and predict the severity of diabetic
retinopathy.
Table 1. Initial Records of the Dataset
Id_Code Diagnosis
000c1434d8d7
2
001639a390f0
4
0024cdab0c1e 1
002c21358ce6 0
005b95c28852
0
Displays some sample images from the training
dataset along with their re- spective labels in Fig 5.
Figure 5: Sample images
The diabetic retinopathy dataset is labeled images
extracted from a CSV file that removes irrelevant
columns. The images are preprocessed into grayscale,
resized to 256×256 pixels uniformly, and normalized
pixel values into the range [0, 1]. Some representative
examples are Image 90, 128, and 264, showing in the
Fig 6 and content of the dataset, so one is clear about
what they’re looking at when it comes to analysis.
Early Detection of Diabetic Retinopathy Using ResNet-18
87
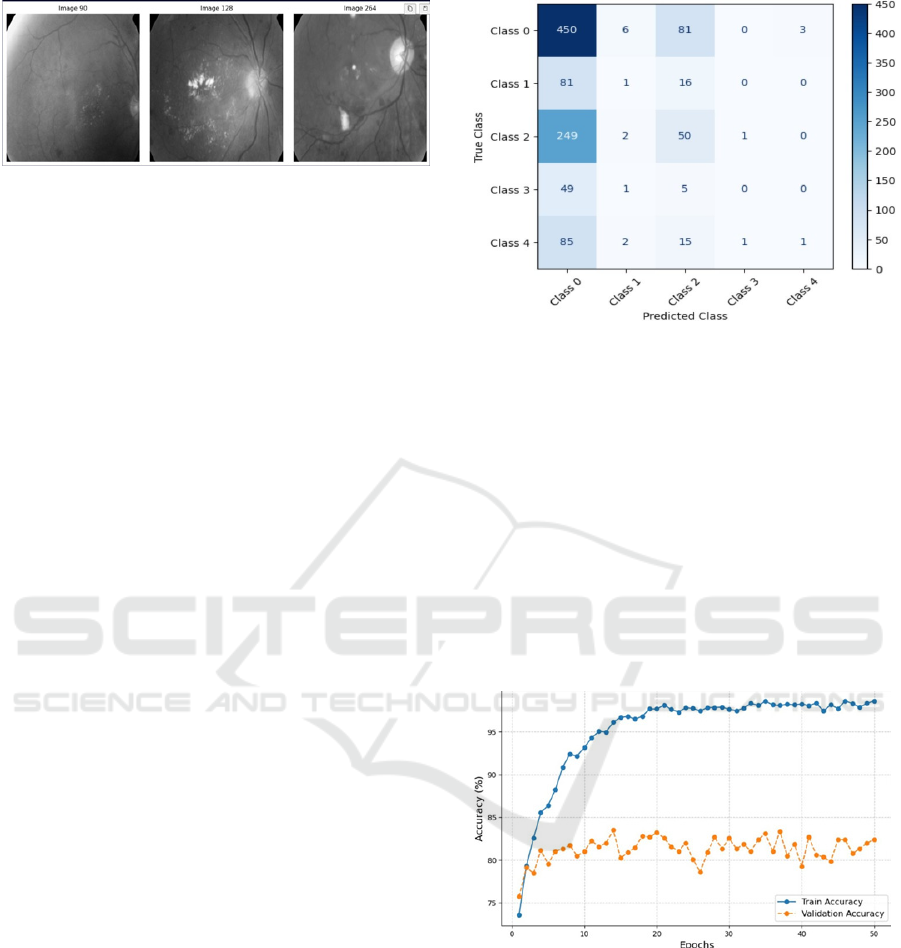
Figure 6: labeled images
As evidenced by the training results, this model’s
performance improves no- tably over 50 epochs. The
training loss begins higher and decreases to 0.0322 by
the last epoch, which means that this model is
effectively minimizing errors in predictions and
learning about patterns in the data set. Similarly, the
accu- racy of training reaches an excellent 98.57%.
The validation accuracy stands at 83.49% and shows
the ability of the model to generalize for unseen data.
Eval- uation metrics provide a precision of 0.70,
recall of 0.52, and an F1 score of 0.58 to understand
the model’s efficiency in balancing false positives
with false negatives.
However, the gap between training and validation
accuracy still leaves room for improvement in
generalization. Techniques such as further
hyperparameter tuning, advanced data augmentation,
or the integration of more complex archi- tectures like
ResNet could be used to enhance performance.
Overall, the training process highlights the model’s
potential but also shows areas for optimization to
achieve even better results.
The confusion matrix evaluates the performance
of the model on five diabetic retinopathy classes,
ranging from Class 0 to Class 4. Class 0 performs well
with high accuracy, correctly identifying 450 out of
540 samples as normal, effectively classifying
healthy retinal images. However, it frequently
confuses Class 1 and Class 2 with Class 0,
highlighting challenges with class balance and
overlapping features. For Class 3 and Class 4, correct
predictions are sparse, suggesting the model struggles
to differentiate higher severity levels due to
insufficient data or distinctive features. This
emphasizes the need for further model refinement.
shown in Fig 7.
Figure 7: Normalized Confusion Matrix
The "Accuracy vs Epoch" plot shows that the
model is training to a huge accuracy, well over 95%,
meaning that the model is learning the patterns in the
data very effectively. The steady rise in validation
accuracy in the initial epochs also shows that the
model is not overfitting but is rather generalizing very
well for unseen data. These trends show that the
model is capable of learning the data patterns
successfully and has a good generalization ability,
hence showing it to be able to give very good
predictions on both training and validation datasets.
This makes it a robust and potentially excellent
model.
Figure 8: Accuracy vs Epoch
The "Loss vs Epoch" plot suggests that the
training loss stays relatively flat, indicating proper
learning and optimization of the model. Validation
loss goes along a straight trend up to the first few
epochs (about three to five), which again is very good
generalization and stability. This pattern suggests that
the model successfully captures the patterns in the
training set and validation sets, as it generalizes well
and maintains consistent performance.
INCOFT 2025 - International Conference on Futuristic Technology
88
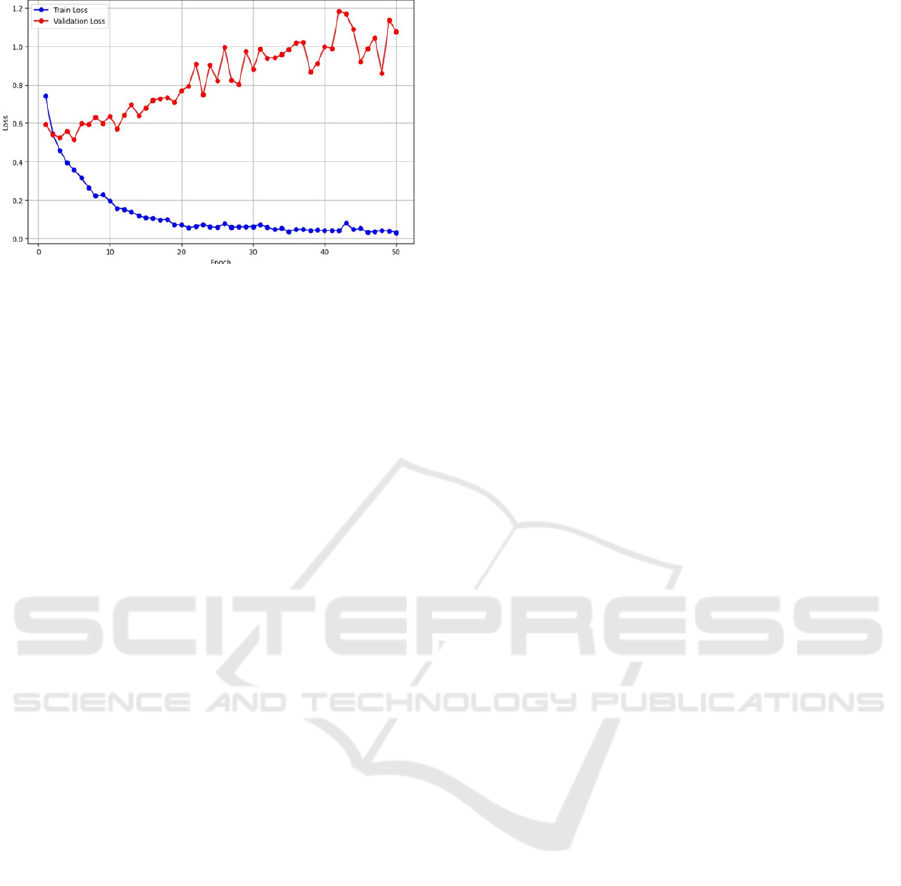
Figure 9: Loss vs Epoch
5 CONCULSION AND FEATURES
This project successfully applies deep learning in the
detection of diabetic retinopa- thy using the Aptos
2019 dataset by focusing on the class-based
classification of DR from the severity level in the
images of the retina. All preprocessing tech- niques
applied—namely, image cleaning, resizing,
normalization, and augmen- tation—proved useful
for improving the quality and consistency of the
dataset for enhanced model performance. The
ResNet-18-based model achieved a very high training
accuracy of 98.57% and a validation accuracy of
83.49% after 50 epochs, demonstrating strong
performance but also some room for improvement in
terms of generalization.
The model employed the Adam optimizer that led
to efficient training and convergence. Dropout
regularization was applied, which helped prevent
overfit- ting. Cross-entropy loss was used in order to
optimize the model on classification tasks, therefore
leading to effective learning of intricate patterns
within retinal images.
Future work in improving the feature extraction
ability of the model and the overall performance can
be furthered by using more complex architectures
such as ResNet-18. Leveraging pretrained models
through transfer learning, along with hyperparameter
tuning, can improve accuracy and robustness. Also,
multi- modal data integration and explainable AI
techniques will be critical to enhance transparency,
which is paramount in clinical settings where the trust
in model predictions is essential. In addition,
increasing the size of the dataset to reflect different
demographics and using cross-validation methods
will help the model to generalize better with higher
reliability and accuracy over the population
of
patients with diverse backgrounds.
REFERENCES
Abràmoff, M.D.: The autonomous point-of-care diabetic
retinopathy examination pp. 159–178 (2020)
Aptos: Aptos 2019 blindness detection dataset.
https://www.kaggle.com/c/ aptos2019-blindness-
detection (2019), accessed: 2024-12-20
Bourne, R., Price, H., Stevens, G., Group, G.V.L.E., et al.:
Global burden of visual impairment and blindness.
Archives of ophthalmology 130(5), 645–647 (2012)
Cheung, C.Y., Ikram, M.K., Klein, R., Wong, T.Y.: The
clinical implications of recent studies on the structure
and function of the retinal microvasculature in diabetes.
Diabetologia 58, 871–885 (2015)
Gulshan, V., Peng, L., Coram, M., Stumpe, M.C., Wu,
D., Narayanaswamy, A., Venugopalan, S., Widner, K.,
Madams, T., Cuadros, J., et al.: Development and
validation of a deep learning algorithm for detection of
diabetic retinopathy in retinal fundus photographs.
jama 316(22), 2402–2410 (2016)
He, K., Zhang, X., Ren, S., Sun, J.: Deep residual learning
for image recognition pp. 770–778 (2016)
Jia, W., Li, Y., Qu, R., Baranowski, T., Burke, L.E., Zhang,
H., Bai, Y., Mancino, J.M., Xu, G., Mao, Z.H., et al.:
Automatic food detection in egocentric images using
artificial intelligence technology. Public health
nutrition 22(7), 1168–1179 (2019)
Krizhevsky, A., Sutskever, I., Hinton, G.E.: Imagenet
classification with deep convolutional neural networks.
Advances in neural information processing systems 25
(2012)
Leibig, C., Allken, V., Ayhan, M.S., Berens, P., Wahl, S.:
Leveraging uncertainty information from deep neural
networks for disease detection. Scientific reports 7(1),
1–14 (2017)
Lu, X., Liu, J., Wu, M., Zhang, X.: Deep learning for
diabetic retinopathy: A review of recent advancements.
Computers in Biology and Medicine 101, 126–133
(2018)
Nirgude, A.S., Revathi, T., Navya, N., Naik, P.R.: “even
though doctor has advised to practice foot care i have
not practiced soaking feet in lukewarm water so far”
self-care practices, enablers, and barriers: A mixed
methods study among individ- uals with diabetes from
a rural area of south india. Indian Journal of
Community Medicine pp. 10–4103 (2024)
Solomon, S.D., Chew, E., Duh, E.J., Sobrin, L., Sun,
J.K., VanderBeek, B.L., Wykoff, C.C., Gardner, T.W.:
Diabetic retinopathy: a position statement by the
american diabetes association. Diabetes care 40(3), 412
(2017)
Teo, Z.L., Tham, Y.C., Yu, M., Chee, M.L., Rim, T.H.,
Cheung, N., Bikbov, M.M.,
Wang, Y.X., Tang, Y., Lu,
Y., et al.: Global prevalence of diabetic retinopathy and
projection of burden through 2045: systematic review
and meta-analysis. Ophthal- mology 128(11), 1580–
1591 (2021)
Ward, C., Maselko, M., Lupfer, C., Prescott, M., Pastey,
M.K.: Interaction of the human respiratory syncytial
virus matrix protein with cellular adaptor protein
Early Detection of Diabetic Retinopathy Using ResNet-18
89

complex 3 plays a critical role in trafficking. PLoS One
12(10), e0184629 (2017)
Yau, J.W., Rogers, S.L., Kawasaki, R., Lamoureux, E.L.,
Kowalski, J.W., Bek, T., Chen, S.J., Dekker, J.M.,
Fletcher, A., Grauslund, J., et al.: Global prevalence and
major risk factors of diabetic retinopathy. Diabetes care
35(3), 556–564 (2012)
INCOFT 2025 - International Conference on Futuristic Technology
90
