
Layer-Wise Relevance Propagation for Classifying Brain MRI Images
Ganesh Naik, Shivyogi Bendegerimath, Vijeth Kawari,
Gautam Narajji and Prashant Narayankar
School of Computer Science and Engineering, KLE Technological University, Hubli, Karnataka, India
Keywords:
Brain Tumor Classification, Explainable AI (XAI), Medical Imaging, Layer-Wise Relevance Propagation
(LRP), Automated Medical Report Generation.
Abstract:
Accurate diagnosis and explainable predictions are important in effective planning and monitoring treatment in
brain tumor analysis using medical imaging. To enhance the capabilities of tumor detection and interpretation
in Brain MRI scans, the proposed work presents a comprehensive framework that combines Explainable AI
(XAI) with brain tumor classification. The framework, based on ResNet18, a deep learning model, classifies
MRI images into four categories: glioma tumor, meningioma tumor, pituitary tumor, and no tumor. The
system incorporates Layer-wise Relevance Propagation (LRP) to highlight regions influencing predictions,
providing richer interpretability and visual explanations of the decision-making process. The proposed work
has demonstration of 3 approaches for explainable decision making process 1) LRP with heatmaps 2) LRP
using overlayed heatmaps 3) Pixel-wise Relevance of presence of tumor. Additionally, the proposed approach
includes Automated Medical Report Generation, summarizing categorization results and presenting visual
explanations to assist physicians effectively. The proposed model has reached 85% accuracy with strong
prediction capabilities and superior explainability in performance to adequately fulfill the fundamental demand
of AI-based health solutions to provide more transparent and reliable performance.
1 INTRODUCTION
Brain tumors are among the most serious and danger-
ous types of cancer; they disturb the delicate balance
of the brain and impair vital physical and cognitive
functions. The diagnosis is especially challenging be-
cause of the complex anatomy of the brain and be-
cause the tumors have a tendency to simulate healthy
tissue in imaging studies (Litjens et al., 2017a).
In Magnetic Resonance Imaging (MRI), despite ad-
vancements, diagnoses remain time-consuming and
heavily reliant on human judgment. This reliance
delays critical treatments, particularly for aggressive
cancers. Chemotherapy, radiation, and surgery also
carry significant risks, making surgical precision cru-
cial to avoid damaging surrounding tissues. These
challenges prolong the emotional and mental stress
experienced by patients and their families, emphasiz-
ing the need for timely and precise diagnostic solu-
tions.
Conventional MRI analysis requires interpretation
by radiologists, which is subject to human error and
delays the early diagnosis and treatment of brain tu-
mors (Ronneberger et al., 2015). Furthermore, many
existing AI algorithms function as “black boxes,” pro-
viding predictions without explaining their rationale.
This lack of transparency hampers medical profes-
sionals, who require clear, interpretable information
to make informed decisions. Addressing these limita-
tions is critical to advancing diagnostic accuracy and
building trust in AI-powered tools.
The proposed work aims to address these chal-
lenges using advanced machine learning techniques,
such as Deep Learning and Layer-wise Relevance
Propagation (LRP) (Bach et al., 2015a). By lever-
aging the ResNet architecture, the model effectively
processes high-dimensional MRI datasets and han-
dles complex analysis tasks, such as identifying ir-
regular shapes and varying intensities in tumors (He
et al., 2016). The integration of LRP provides inter-
pretable heatmaps that highlight critical MRI regions
influencing classification decisions, fostering trans-
parency and trust among medical professionals. The
approach further reduces dependence on traditional
manual feature engineering, making it suitable for
low-resource settings where expert radiologists may
be scarce. Additionally, the system supports accu-
rate tumor subtype characterization and visual expla-
Naik, G., Bendegerimath, S., Kawari, V., Narajji, G. and Narayankar, P.
Layer-Wise Relevance Propagation for Classifying Brain MRI Images.
DOI: 10.5220/0013607800004664
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 3rd International Conference on Futuristic Technology (INCOFT 2025) - Volume 3, pages 5-11
ISBN: 978-989-758-763-4
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
5

nations of tumor-specific properties, such as hetero-
geneity and border irregularity, enabling personalized
therapeutic interventions (Isensee et al., 2021).
Brain tumors affect an estimated 40,000 to 50,000
adults annually in India, with children constituting
20% of these cases. This prevalence, combined with
the unique challenges posed by brain tumors, empha-
sizes the urgent need for improved diagnostic tools.
Many existing algorithms remain opaque, operating
as “black boxes” and providing predictions without
explaining the rationale. The lack of transparency
hampers medical professionals, who rely on clear and
precise information for making informed decisions.
Addressing this gap is crucial to advancing patient
care and fostering confidence in AI-powered solu-
tions.
The rest of the paper is organized as follows:
Section II reviews the relevant literature and high-
lights existing gaps in the domain. Section III de-
tails the methodology, including the ResNet architec-
ture, LRP integration along with Report Generation.
Section IV presents experimental results and analysis,
showcasing the model’s effectiveness in addressing
diagnostic challenges. Section V discusses the clin-
ical applicability of the proposed approach and its po-
tential impact along with future research directions.
2 BACKGROUND STUDY
2.1 Related Work and Prior Studies
Recent advancements in medical imaging have fo-
cused on multi-class classification of brain MRI im-
ages, with deep learning models achieving significant
breakthroughs in accuracy and efficiency. Traditional
machine learning techniques, such as Support Vector
Machines (SVMs) and K-Nearest Neighbors (KNNs),
relied heavily on handcrafted features like Gray-
Level Co-occurrence Matrices (GLCM) and Princi-
pal Component Analysis (PCA) (Bach et al., 2015b).
These methods often struggled with the complexity
and variability inherent in medical imaging datasets.
In contrast, Convolutional Neural Networks (CNNs)
and transfer learning frameworks, including ResNet,
AlexNet, and GoogLeNet, have demonstrated supe-
rior robustness and scalability in classifying MRI im-
ages into multiple classes (Vankdothu and Hameed,
2022). The incorporation of preprocessing techniques
such as data augmentation, skull stripping, and mor-
phological operations further enhances the effective-
ness of these models, showcasing their potential for
clinical applications (Kulkarni and Sundari, 2020).
Despite their success in achieving high classifi-
cation accuracy, deep learning models often suffer
from a black-box nature, which hinders their inter-
pretability and transparency in medical imaging. Ex-
plainability is critical in multi-class classification, as
understanding the reasoning behind predictions fos-
ters reliability and trust among clinicians. Visu-
alization methods like Grad-CAM have been em-
ployed to highlight tumor regions in MRI images,
adding a layer of interpretability to these models
(Pang et al., 2023). Additionally, techniques such
as Layer-wise Relevance Propagation (LRP) have
emerged as powerful tools for explaining classifier
decisions by providing pixel-wise decomposition of
predictions. LRP generates heatmaps that highlight
regions most relevant to a given class prediction, en-
hancing transparency and interpretability (Bach et al.,
2015b). Studies have validated LRP’s utility in multi-
class medical imaging tasks by confirming predic-
tions and identifying biologically meaningful fea-
tures, reinforcing its value in AI-driven diagnostic
systems (Babu Vimala et al., ).
2.2 Gaps in Current Research and How
proposed work Addresses Them
While existing deep learning models have achieved
remarkable performance in classifying brain MRI im-
ages, they often lack adequate interpretability. Meth-
ods like Grad-CAM, though widely used, focus pri-
marily on high-level feature activations and lack the
precision required for fine-grained analysis. Addi-
tionally, they may fail to distinguish subtle differ-
ences among multiple classes, a critical need in med-
ical imaging (Pang et al., 2023). Furthermore, tech-
niques like SHAP (SHapley Additive exPlanations),
which emphasize feature importance, are computa-
tionally expensive and do not provide the spatial vi-
sualizations necessary for medical diagnostics.
These limitations highlight the need for more ad-
vanced explainability methods, such as Layer-wise
Relevance Propagation (LRP), which combines com-
putational efficiency with detailed interpretability.
LRP addresses the black-box challenge by generat-
ing pixel-wise heatmaps that pinpoint the regions con-
tributing most to model predictions, providing a fine-
grained understanding of decision-making processes
(Bach et al., 2015b). Unlike Grad-CAM, LRP en-
sures granularity in analyzing multi-class predictions,
making it suitable for distinguishing subtle differ-
ences in tumor characteristics. Additionally, the pro-
posed integration of LRP with advanced architectures
like ResNet leverages the strengths of deep learn-
ing for robust multi-class classification while enhanc-
ing transparency. By addressing these gaps, the cur-
INCOFT 2025 - International Conference on Futuristic Technology
6
rent work bridges the divide between high-performing
deep learning models and the clinical need for ex-
plainable AI solutions, promoting trust and adoption
among medical professionals.
3 PROPOSED WORK
The proposed model for brain tumor classification
uses ResNet-18 while for explaining the decision-
making process, it uses Layer-wise Relevance
Propagation, a technique used in Explainable Artifi-
cial Intelligence (XAI) to make deep learning models,
especially neural networks, more interpretable. For
making the classification and explanation process
more readable, the model uses a report generation
module that summarizes the intricate details of
tumor detection and analysis. The workflow for the
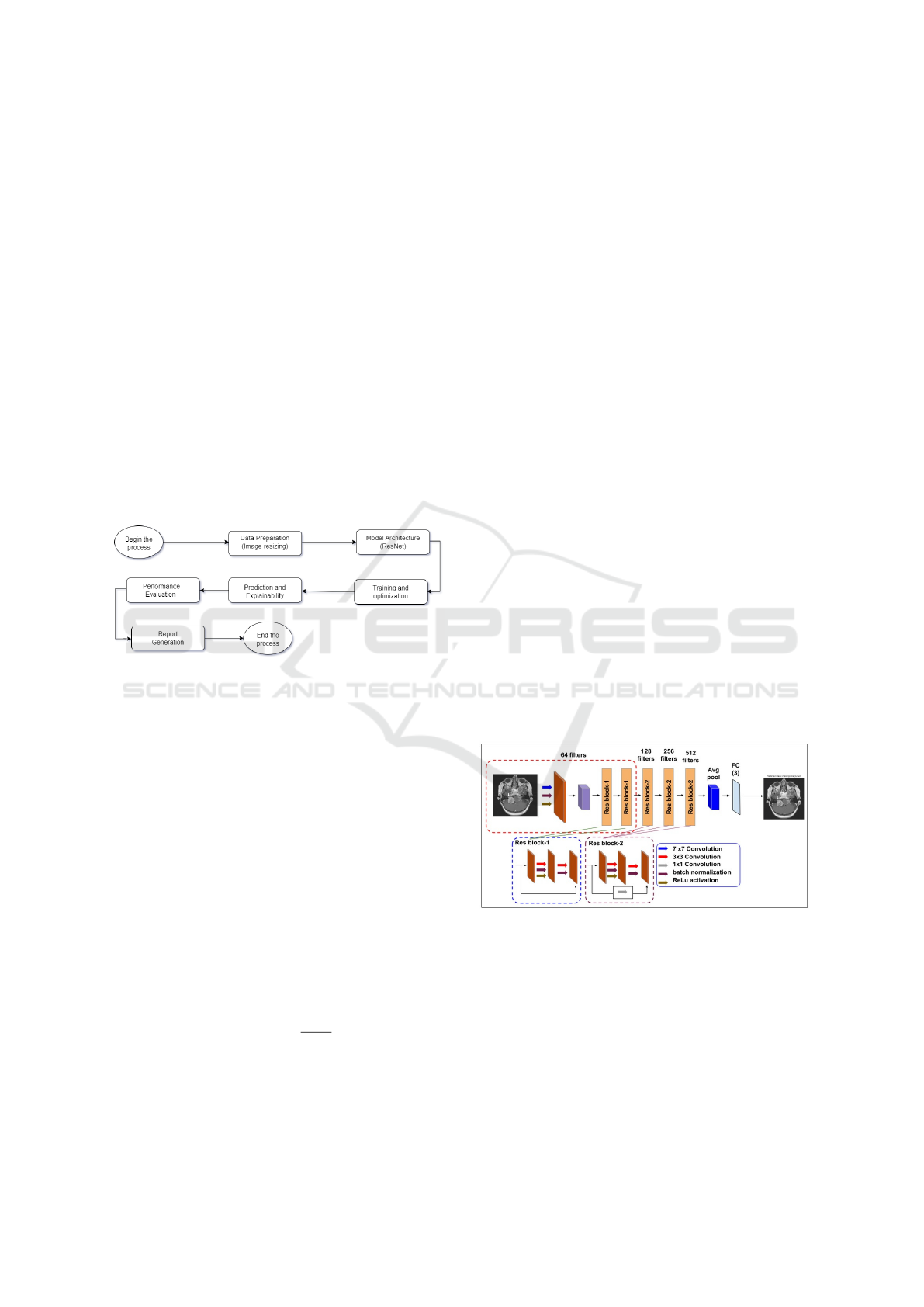
proposed model is shown in Figure 1.
Figure 1: Proposed Model Workflow.
The following steps make up the methodology:
3.1 Dataset Preparation
Dataset consists of training and testing folders of
Brain MRI images of namely 4 classes: glioma tumor,
pituitary tumor, meningioma tumor and no tumor.
During the Dataset Preparation process, photos
are categorized by their class names and arranged
into distinct training and testing folders. The image
is shrunk to 224 × 224 and normalized using stan-
dard ImageNet mean and standard deviation data. Py-
Torch’s ImageFolder tool is used to load the dataset,
and data loaders are made with a batch size of 32 for
processing efficiency.
The formula for normalization is shown in Equation
1:
x
normalized
=
x −µ
σ
, (1)
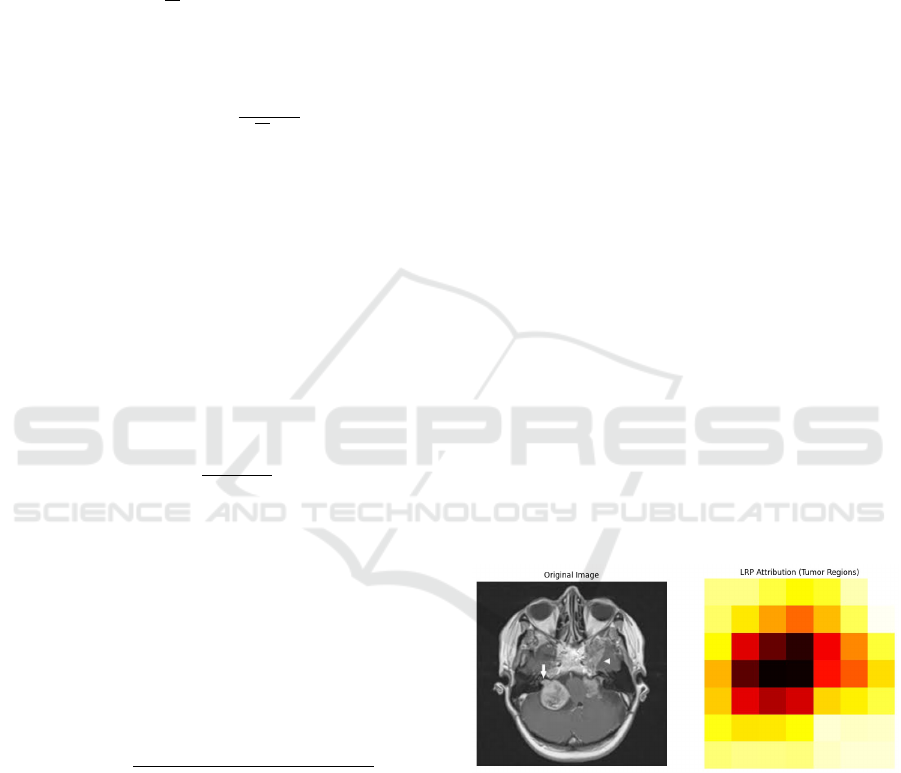
3.2 Resnet-18
Resnet-18 architecture as shown in Figure 2 is a deep
learning architecture that is very powerful in handling
complex image classification tasks. The residual con-
nections make ResNet-18 particularly effective, as
they allow the network to learn deeper and more intri-
cate features without running into problems like van-
ishing gradients. These connections enable the model
to process and understand detailed patterns in images,
which is crucial when working with medical images
like MRIs. ResNet-18 has been used in a number of
image recognition applications, and its ability to cap-
ture simple features such as edges and more complex
patterns that differentiate tumor types makes it a good
choice for this task. Besides, ResNet-18 is computa-
tionally efficient, and it can be fine-tuned for specific
tasks like brain tumor classification without requiring
much more resources. This makes ResNet-18 a good
model for this classification task due to its accuracy,
efficiency, and ability to handle complex data.
The fundamental architecture we use is the
ResNet-18 model pretrained on the ImageNet dataset.
The fully connected layer is swapped out for a
new linear layer that generates probabilities for four
classes in order to modify the model for brain tumor
classification. The brain tumor dataset is then used to
refine the model.
The formula for the output of the linear layer is shown
in Equation 2:
y = softmax(Wh + b) (2)
Figure 2: Resnet18 Architecture for Brain Tumor Classifi-
cation.
3.3 Training and Optimization
In Training and Optimization, the Adam optimizer
with a learning rate of 0.001 is used to optimize the
model parameters during the training phase. The error
between the true and anticipated class labels is calcu-
lated using the cross-entropy loss function. Over the
Layer-Wise Relevance Propagation for Classifying Brain MRI Images
7
course of 50 epochs, the training loop iteratively mod-
ifies the model’s parameters while tracking accuracy
and loss, among other performance metrics, through-
out training and validation.
The formula for Cross-Entropy Loss is shown in
Equation 3:
L = −
1
N
N
∑
i=1
C
∑
j=1
y
i j
log( ˆy
i j
) (3)
The formula for Adam Optimizer is shown in
Equation 4:
θ
t
= θ
t−1
−η
m
t
√
v
t
+ ε
(4)
3.4 Predictability and Explainability
The model uses the training weights to predict the
class of a particular MRI picture in this step of pre-
dictability and explainability. Layer-wise Relevance
Propagation (LRP) is used to make the data inter-
pretable. By breaking down the model’s predictions
into pixel-by-pixel relevance scores, LRP highlights
the areas of the MRI that have the most influence on
the outcome.
The formula for relevance propagation is shown in
Equation 5:
R
i
=
∑
j
a
i
w
i j
∑
k
a
k
w
k j
R
j
(5)
3.5 Performance Evaluation
Lastly, accuracy is the primary metric used to measure
the performance of the model during performance
evaluation. Both training and validation accuracy are
monitored throughout the training process to ensure
robustness and generalization.
The formula for accuracy is given by Equation 6:
Accuracy =
Number of correct predictions
Total number of predictions
(6)
3.6 Report Generation
A comprehensive PDF report is generated at the end
of the model evaluation process.The report provides a
summary of the visualizations, explanations, and key
findings in the experiment. It includes patient-specific
information such as whether or not a tumor was de-
tected, the type of tumor, and the region highlighted
by the heatmap corresponding to the tumor. More-
over, the report includes medical advice for the patient
according to the type of tumor; it might include sug-
gestions for periodic monitoring or potential surgical
removal. It also serves as a useful tool for clinicians
and researchers, providing not only visual outputs but
also contextual information to be used in the decision-
making and further analysis.
4 RESULTS AND ANALYSIS
In this section, we present and analyze the results
of the proposed model, which demonstrated high ac-
curacy in classifying brain tumors into glioma tu-
mor, meningioma tumor, pituitary tumor, and non-
tumorous cases. The performance metrics, such as
precision and F1-score, validate the model’s ability to
perform the task effectively. Additionally, Layer-wise
Relevance Propagation (LRP) was utilized to enhance
interpretability, providing heatmaps that highlight the
location of the tumor.
4.1 Presentation of Results through
Visualizations
To provide clear and effective insights into the
model’s performance, we use three different types of
visualizations based on LRP. These visualizations not
only demonstrate the model’s focus but also help as-
sess its interpretability.
The following results are for the type of Meningioma
tumor:
Figure 3: Layer-wise Relevance Propagation with
heatmaps.
The heatmap shown in Figure 3 highlights areas of
high relevance using warm colors, where the darkest
red indicates the strongest attention by the model. The
areas that correspond to the tumor region are clearly
visible, making it evident that the model is focusing
on clinically significant structures.
Figure 4 shows the overlayed heatmap, which
combines the heatmap with the original MRI image.
This allows us to visualize the anatomical structure
along with the relevance information. This technique
INCOFT 2025 - International Conference on Futuristic Technology
8
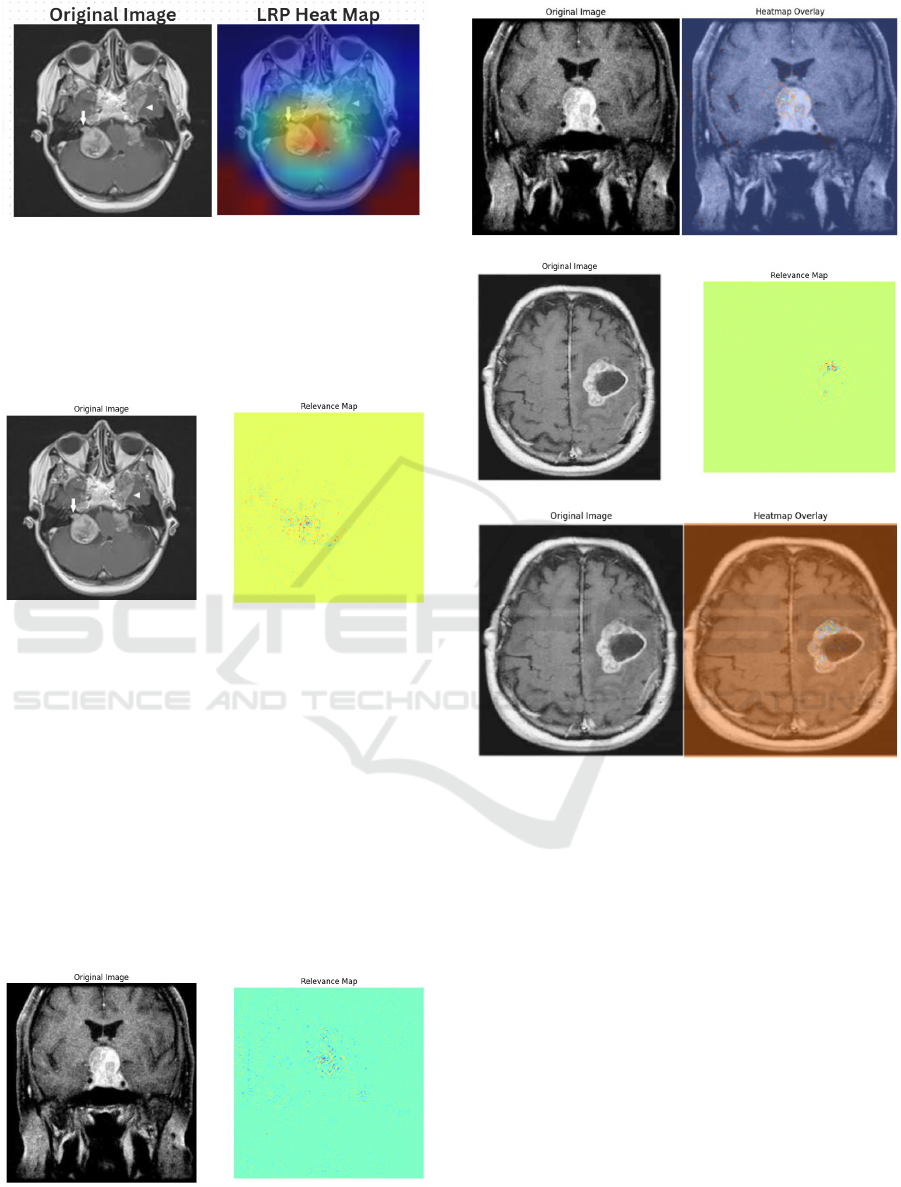
Figure 4: Layer-wise Relevance Propagation Using Over-
layed Heatmaps.
provides a spatial context for the model’s focus, en-
suring that the relevance aligns with clinical expec-
tations and helping users understand the relationship
between the tumor and surrounding tissues.
Figure 5: Pixel-wise Relevance of Presence of Tumor.
Figure 5 presents LRP without the heatmap
overlay, offering a simpler depiction of the relevant
areas. This visualization provides a straightforward
representation of relevance, using binary masks to
identify tumor regions, as well as showing non-
contributing areas and tumor contours with distinct
color coding: blue for tumor regions, red for tumor
borders, and green for non-relevant areas.
Similarly the relevance maps for pituitary ( Figure
6, Figure 7) and glioma ( Figure 8, Figure 9) type of
tumors are also depicted. The model does not gener-
ate any type of map if tumor is not detected.
Figure 6: Pixel-wise Relevance of Pituitary Tumor
Figure 7: Overlayed heatmap of Pituitary Tumor
Figure 8: Pixel-wise Relevance of Glioma Tumor
Figure 9: Overlayed heatmap of Glioma Tumor
4.2 Detailed Analysis of Model
Performance
The model demonstrated high accuracy in identifying
and classifying brain tumors. Performance metrics,
including precision and F1-score, validate its effec-
tiveness. The use of LRP heatmaps further enhances
the confidence in the model’s decision-making pro-
cess by visually confirming that it focuses on the cor-
rect areas, such as the tumor regions.
The heatmaps provide an intuitive understanding
of the model’s attention to specific areas, while the
overlayed heatmaps give a more contextual view, al-
lowing clinicians to assess the spatial relationship be-
tween the tumor and its surrounding tissue. These vi-
sualizations not only validate the model’s predictions
but also serve as a reliable interpretability tool that
can support clinical decision-making.
Layer-Wise Relevance Propagation for Classifying Brain MRI Images
9

4.3 Comparison with Previous
Approaches
When compared with previous methods in brain
tumor classification, the proposed model provides
both high performance and excellent interpretabil-
ity through LRP visualizations. Traditional meth-
ods may lack interpretability or offer limited visual
insights into model predictions. The proposed ap-
proach, by contrast, offers a comprehensive under-
standing of how the model arrives at its predictions,
which is crucial in medical applications where trust
in the model’s decision-making is essential.
4.4 Interpretation of Results and
Implications
The results underscore the ability of the proposed
model to focus on clinically relevant regions, offer-
ing interpretability through heatmaps and overlayed
heatmaps. This helps clinicians to trust the model’s
predictions and provides assurance that the areas
identified by the model correspond to the tumor re-
gions in the MRI images. Additionally, the simplified
visualization without heatmaps ensures that clinicians
can rely on accurate, quantitative data when making
decisions. The report generated as shown in Figure 6
at the end of the process also facilitates patient care
by summarizing important tumor-related information
and offering treatment recommendations based on the
tumor type.
Figure 10: PDF Report of Meningioma Tumor
Figure 11: PDF Report of Pituitary Tumor
Figure 12: PDF Report of Glioma Tumor
4.5 Limitations of the Current
Approach
While the proposed model performs well in classify-
ing brain tumors and providing interpretability, there
are some limitations. One challenge arises when the
relevance is distributed across multiple areas, which
can reduce the clarity of heatmap visualizations. In
such cases, the model’s focus may become ambigu-
ous, making it harder to interpret the results clearly.
Additionally, although the heatmaps provide valuable
insights, they may not always offer the level of preci-
sion required in all medical applications, particularly
when multiple tumors or complex cases are involved.
INCOFT 2025 - International Conference on Futuristic Technology
10

Figure 13: PDF Report of No Tumor
5 CONCLUSION AND FUTURE
WORK
The proposed approach bridges the gap by applying
Layer-wise Relevance Propagation (LRP) to gener-
ate heatmaps that explain model predictions, enabling
medical practitioners to verify the rationale behind AI
outputs. This is crucial for understanding tumor prop-
erties, which vary in size, location, and type. LRP en-
hances the clarity and reliability of the system, help-
ing clinicians make better decisions, develop person-
alized treatment plans, and increase trust in AI-driven
diagnostic tools. Using ResNet-18 and LRP, the sys-
tem classifies brain tumors in MRI scans into four cat-
egories: glioma, meningioma, pituitary, and no tumor.
The LRP results are intuitive and easy to understand,
making the system suitable for medical use. Fu-
ture work could improve performance by expanding
the dataset and exploring more complex architectures,
such as EfficientNet (Litjens et al., 2017b). Incorpo-
rating multi-modal data and refining LRP for clearer
visual explanations could further enhance model ac-
curacy. An intuitive interface with real-time predic-
tions could help healthcare providers quickly and ac-
curately identify patients in clinical settings.
REFERENCES
Babu Vimala, B., Srinivasan, S., Mathivanan, S., et al.
Detection and classification of brain tumor using
hybrid deep learning models. sci rep 13, 23029
(2023).
Bach, S., Binder, A., Montavon, G., Klauschen, F.,
M
¨
uller, K.-R., and Samek, W. (2015a). On pixel-
wise explanations for non-linear classifier deci-
sions by layer-wise relevance propagation. PloS
one, 10(7):e0130140.
Bach, S., Binder, A., Montavon, G., Klauschen, F.,
M
¨
uller, K.-R., and Samek, W. (2015b). On pixel-
wise explanations for non-linear classifier deci-
sions by layer-wise relevance propagation. PloS
one, 10(7):e0130140.
He, K., Zhang, X., Ren, S., and Sun, J. (2016). Deep
residual learning for image recognition. In Pro-
ceedings of the IEEE conference on computer vi-
sion and pattern recognition, pages 770–778.
Isensee, F., Jaeger, P. F., Kohl, S. A., Petersen, J.,
and Maier-Hein, K. H. (2021). nnu-net: a
self-configuring method for deep learning-based
biomedical image segmentation. Nature meth-
ods, 18(2):203–211.
Kulkarni, S. M. and Sundari, G. (2020). A frame-
work for brain tumor segmentation and classifi-
cation using deep learning algorithm. Interna-
tional Journal of Advanced Computer Science
and Applications, 11(8).
Litjens, G., Kooi, T., Bejnordi, B. E., Setio, A. A. A.,
Ciompi, F., Ghafoorian, M., Van Der Laak, J. A.,
Van Ginneken, B., and S
´
anchez, C. I. (2017a). A
survey on deep learning in medical image analy-
sis. Medical image analysis, 42:60–88.
Litjens, G., Kooi, T., Bejnordi, B. E., Setio, A. A. A.,
Ciompi, F., Ghafoorian, M., Van Der Laak, J. A.,
Van Ginneken, B., and S
´
anchez, C. I. (2017b). A
survey on deep learning in medical image analy-
sis. Medical image analysis, 42:60–88.
Pang, T., Li, P., and Zhao, L. (2023). A survey on
automatic generation of medical imaging reports
based on deep learning. BioMedical Engineering
OnLine, 22(1):48.
Ronneberger, O., Fischer, P., and Brox, T. (2015).
U-net: Convolutional networks for biomedi-
cal image segmentation. In Medical image
computing and computer-assisted intervention–
MICCAI 2015: 18th international conference,
Munich, Germany, October 5-9, 2015, proceed-
ings, part III 18, pages 234–241. Springer.
Vankdothu, R. and Hameed, M. A. (2022). Brain tu-
mor mri images identification and classification
based on the recurrent convolutional neural net-
work. Measurement: Sensors, 24:100412.
Layer-Wise Relevance Propagation for Classifying Brain MRI Images
11
