
Improving Disease Classification Accuracy with Hybrid CNN-RNN
Architectures for Lung Tumors
Vishal R Patil, Vineet S Hiremani, Adil Mulimani, Shreeniwas R Kolagal and Channabasappa Muttal
School of Computer Science and Engineering (SoCSE), KLE Technological University, Hubballi, India
Keywords:
Lung Nodules, CNN, RNN, CT Scans, Medical Imaging, Hybrid Model.
Abstract:
The detection of lung nodules is essential in medical imaging, playing a critical role in diagnosing lung cancer
at its early stages and supporting timely treatment. This study introduces a hybrid CNN-RNN model designed
to enhance the accuracy and precision of lung nodule identification in computed tomography (CT) scans. The
framework combines the spatial feature extraction capabilities of Convolutional Neural Networks (CNNs)
with the temporal sequence analysis strengths of Recurrent Neural Networks (RNNs), effectively integrating
spatial and temporal information for improved detection performance. Trained on a labeled dataset of CT
images, the model’s performance was assessed using metrics such as precision, recall, F1 score, and area
under the curve (AUC). The proposed method surpassed existing techniques, achieving an accuracy of 96.1%,
an F1 score of 0.8434, an AUC of 0.901, a precision of 76.02%, and a recall of 94.81%. It demonstrated
significant advancements over hybrid CNN-LSTM models previously used in related fields like Parkinson’s
disease detection, agricultural disease analysis, and lung cancer prognosis estimation, which recorded lower
precision, recall, and F1 scores. These findings highlight the potential of CNN-RNN architectures for lung
nodule detection and their promise in advancing early lung cancer diagnosis.
1 INTRODUCTION
Lung cancer continues to be the leading cause
of cancer-related deaths worldwide, accounting for
nearly 18% of all cancer fatalities annually (Society,
2024). Early detection plays a pivotal role in im-
proving survival rates, as identifying lung nodules at
an initial stage provides the best chance for effective
treatment and favorable patient outcomes. Computed
tomography (CT) imaging has emerged as a critical
tool in identifying these nodules. However, interpret-
ing CT scans manually is both time-consuming and
subject to observer variability, often leading to incon-
sistent diagnoses (Patel and Sharma, 2024). This in-
consistency arises from the inherent complexity of an-
alyzing three-dimensional imaging data, coupled with
subtle variations in nodule size, shape, and location
(Verma and Singh, 2023). To mitigate these chal-
lenges, there is growing interest in developing auto-
mated systems that can enhance the efficiency, accu-
racy, and reliability of lung nodule detection while re-
ducing clinicians’ workload.
Advances in artificial intelligence (AI), partic-
ularly in deep learning, offer promising solutions
to these challenges. Convolutional neural networks
(CNNs) have transformed medical imaging by en-
abling the extraction of intricate spatial features from
CT scans, facilitating precise lung nodule identifica-
tion (Lee and Gupta, 2023). These models excel at
handling large datasets and identifying patterns that
may elude human interpretation (P. Mishra and Ku-
mar, 2024). In addition, recurrent neural networks
(RNNs), including long short-term memory (LSTM)
models, have proven effective for processing sequen-
tial data and capturing temporal relationships, further
enhancing diagnostic potential (Verma and Kumar,
2023). Combining CNNs for spatial analysis with
RNNs for temporal modeling has led to significant
progress in lung cancer detection and classification
(Kumar and Sharma, 2024). This hybrid approach is
particularly valuable in scenarios involving serial CT
imaging, where tracking changes in nodule character-
istics over time is crucial for early diagnosis (et al.,
2024).
Despite the potential of hybrid CNN-RNN archi-
tectures, several hurdles must be overcome before
they can be integrated into clinical practice. One of
the most pressing challenges is the limited availabil-
ity of large, annotated datasets, which are essential for
training robust AI models. Generating these datasets
574
Patil, V. R., Hiremani, V. S., Mulimani, A., Kolagal, S. R. and Muttal, C.
Improving Disease Classification Accuracy with Hybrid CNN-RNN Architectures for Lung Tumors.
DOI: 10.5220/0013597000004664
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 3rd International Conference on Futuristic Technology (INCOFT 2025) - Volume 2, pages 574-580
ISBN: 978-989-758-763-4
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

requires expert annotation of extensive medical image
collections, a resource-intensive and time-consuming
process (Sharma and Lee, 2023). Additionally, the
computational demands of processing high-resolution
volumetric CT scans pose significant challenges for
real-time clinical use, where timely decision-making
is critical (Liu and Zhang, 2022). Model generaliza-
tion across diverse clinical settings is further com-
plicated by variations in imaging protocols, scanner
configurations, and patient demographics (Mehta and
Agarwal, 2024). To address these issues, standardiz-
ing preprocessing methods has become a priority to
enhance the adaptability and reliability of these mod-
els across various medical environments (Rao and Pa-
tel, 2023). Moreover, ensuring that these advanced
AI systems integrate seamlessly into clinical work-
flows is essential for bridging the gap between re-
search innovations and practical application (Wang
and Li, 2023).
In this proposed work, we describe the develop-
ment of a hybrid framework for the accurate and ef-
fective detection of lung nodules in CT scans that
combines Convolutional Neural Networks (CNNs)
and Recurrent Neural Networks (RNNs). The com-
plex intricacies of nodules are captured from indi-
vidual slices by the CNN component, which is ex-
cellent at extracting spatial features. In order to pro-
vide a more comprehensive understanding of nodule
features, the RNN component models temporal rela-
tionships across successive CT slices. These elements
work together to create a pipeline that tackles issues
including nodule size, shape, and location variability.
The goal of the hybrid model is to lessen frequent
challenges in medical imaging, like observer variabil-
ity and the laborious process of manual interpretation.
Our method improves lung nodule detection consis-
tency and reliability by integrating spatial and tem-
poral data processing. Additionally, the framework’s
modular design facilitates adaptability to a range of
clinical needs, including focused diagnostic activities
and extensive screenings.
In situations when early diagnosis is essential to
enhancing patient outcomes, this pipeline is very ben-
eficial. It makes use of sophisticated preprocessing
methods, such as data augmentation, segmentation,
and normalization, to strengthen the system’s resis-
tance to changes in patient demographics and imaging
protocols. By using a hybrid CNN-RNN technique,
the model is guaranteed to be able to process intricate
medical data with computational efficiency appropri-
ate for real-time applications.
In this paper, we discuss our work in the follow-
ing sections. In Section 2, a comprehensive back-
ground study is presented, exploring recent advance-
ments in pulmonary nodule detection using hybrid
CNN-RNN techniques and other machine learning
approaches. Section 3 delves into the methodology,
detailing the dataset preparation, the hybrid CNN-
RNN model architecture, and the training process em-
ployed in our proposed approach. Section 4 highlights
the results and performance metrics of the model, in-
cluding comparisons with contemporary methods. Fi-
nally, Section 5 provides the conclusion, summarizing
the findings and outlining potential future improve-
ments for this work.
2 BACKGROUND STUDY
Recent advancements in machine learning have sig-
nificantly enhanced the detection and classification of
pulmonary nodules, which is crucial for early lung
cancer diagnosis. A variety of studies have explored
different machine learning techniques beyond deep
learning, contributing to this progress.
Marinakis, Karampidis, and Papadourakis (Mari-
nakis et al., 2024) conducted an in-depth review of
the existing literature on pulmonary nodule detection,
segmentation, and classification through the use of
deep learning. Their analysis underscores the crit-
ical role of extracting nodule data from radiologist-
annotated pixel data to effectively train models. They
examined methods for creating 2D and 3D nodule
patches, emphasizing the benefits of multi-view patch
usage in improving model outcomes. The review
also addresses challenges and outlines prospective ad-
vancements in applying deep learning to pulmonary
nodule research.
Liu et al. (Liu et al., 2023) introduced a data
augmentation framework coupled with an embedding
mechanism to enhance pulmonary nodule detection
and classification, especially in limited-data scenar-
ios. Their methodology includes a 3D pixel-based sta-
tistical algorithm to create synthetic nodules, which
are merged with healthy lung samples to generate ex-
panded training datasets. The embedding approach
they proposed improves feature representation, lead-
ing to better accuracy and reliability across both de-
tection and classification tasks, with potential appli-
cability to other imaging domains.
Wang et al. (Wang et al., 2022) introduced a
deep learning model specifically designed for diag-
nosing solid pulmonary nodules. This multi-task
framework not only determines lesion malignancy
but also highlights critical features, enabling inter-
pretability by visually identifying these manifesta-
tions. The model achieved an impressive test AUC
of 0.992 on the LIDC dataset and 0.923 on an inter-
Improving Disease Classification Accuracy with Hybrid CNN-RNN Architectures for Lung Tumors
575

nal dataset. By incorporating manifestation-specific
tasks, the model enhanced malignancy classification
accuracy, improving its utility in clinical settings and
facilitating better collaboration with radiologists.
Hesse et al. (Hesse et al., 2020) explored transfer
learning techniques to determine the origins of pri-
mary tumors in lung nodules using spectral CT im-
ages. They implemented a 3D convolutional neural
network (CNN) for nodule detection and leveraged
a pre-trained model as a feature extractor to classify
nodules as benign, primary lung cancer, or metas-
tases. This approach achieved a classification accu-
racy of 78% in a three-class setting, demonstrating
the potential of pre-trained models to deliver robust
results with minimal fine-tuning.
Chen and Xie (Chen and Xie, 2024) proposed a
novel detection network designed to handle hard sam-
ples in nodule detection. Their method integrates
deformable convolution with self-paced learning and
achieved competitive results on the LUNA16 dataset.
This approach underscores the importance of priori-
tizing difficult cases to improve overall detection ac-
curacy.
Hosseini et al. (Hosseini et al., 2022) provided
a systematic review of deep learning applications for
early-stage lung cancer diagnosis, examining a vari-
ety of models and their performance. Their study
highlights ongoing challenges and proposes strategies
to refine diagnostic tools, offering valuable insights
for clinicians and researchers.
Aslani et al. (Aslani et al., 2022) proposed a time-
series deep learning architecture combining multi-
modal data, such as nodule-specific, lung-specific,
and demographic details. Their approach demon-
strated superior performance in malignancy predic-
tion, showcasing the value of integrating longitudinal
data for lung cancer screening.
Al Ewaidat and El Brag (Ewaidat and El Brag,
2022) utilized a convolutional neural network-based
YOLOv5 model for localizing nodules in CT scans.
Their method achieved an accuracy of 92.27% for
nodule identification, illustrating the effectiveness of
CNN-based solutions in medical imaging.
These studies collectively highlight the broad
scope of machine learning in advancing pulmonary
nodule detection and classification. Techniques in-
volving clinical data integration, handling complex
samples, and applying multiscale analysis have driven
improvements in the early diagnosis of lung cancer.
3 METHODOLOGY
This section details the proposed hybrid CNN-RNN
framework for lung nodule detection, incorporating
both spatial feature extraction and temporal sequence
modeling using the LUNA 16 (Grand Challenge,
2016) dataset.
3.1 Dataset Description
The dataset used for our proposed work is LUNA16
derived from LIDC-IDRI dataset (Grand Challenge,
2016), which includes low-dose lung CT images,
which is divided into 10 subsets to provide tenfold
cross-validation. The dataset is a collection of 888
CT scans with about 1,186 lung nodules annotated by
doctors. All the CT scans are stored in .mhd format
for medical imaging with dimensions around 512 x
512 pixels with minimum voxel spacing of 1.00mm
and have a slice thickness of less than 2.5mm, which
provides higher-resolution imaging suitable for analy-
sis. The collection of lung nodules in diameter range
from 3.0 mm to 28.3 mm, with an average diameter
of 8.3 mm and position co-ordinates mentioned in a
.csv file for set of candidate nodules.
3.2 Dataset Preparation
The LUNA 16 (Grand Challenge, 2016) dataset, con-
sisting of annotated CT scans, was used to train and
evaluate the model. A preprocessing pipeline was ap-
plied to normalize, segment, and augment the data.
In normalization pixel values were scaled to the
range [0, 1] for uniformity. In segmentation Lung
regions were isolated using thresholding techniques
based on Hounsfield Unit (HU) values to exclude ir-
relevant background. In resizing each CT slice was
resized to 32 ×32 ×32 voxels to optimize the compu-
tational efficiency of the CNN model. In augmenta-
tion random rotations, flipping, and intensity shifting
were applied to simulate real-world variations, pre-
venting overfitting.
3.3 Model Architecture
The model consists of a CNN for feature extraction
followed by an RNN for temporal modeling 1(figure
1). The proposed architecture leverages a U-Net-
based segmentation model to extract regions of inter-
est from medical images, followed by a classification
model to determine whether the regions are cancer-
ous. If cancerous, the system performs type classifi-
cation to identify malignancy or benignity, enabling
precise diagnostic outcomes.
INCOFT 2025 - International Conference on Futuristic Technology
576
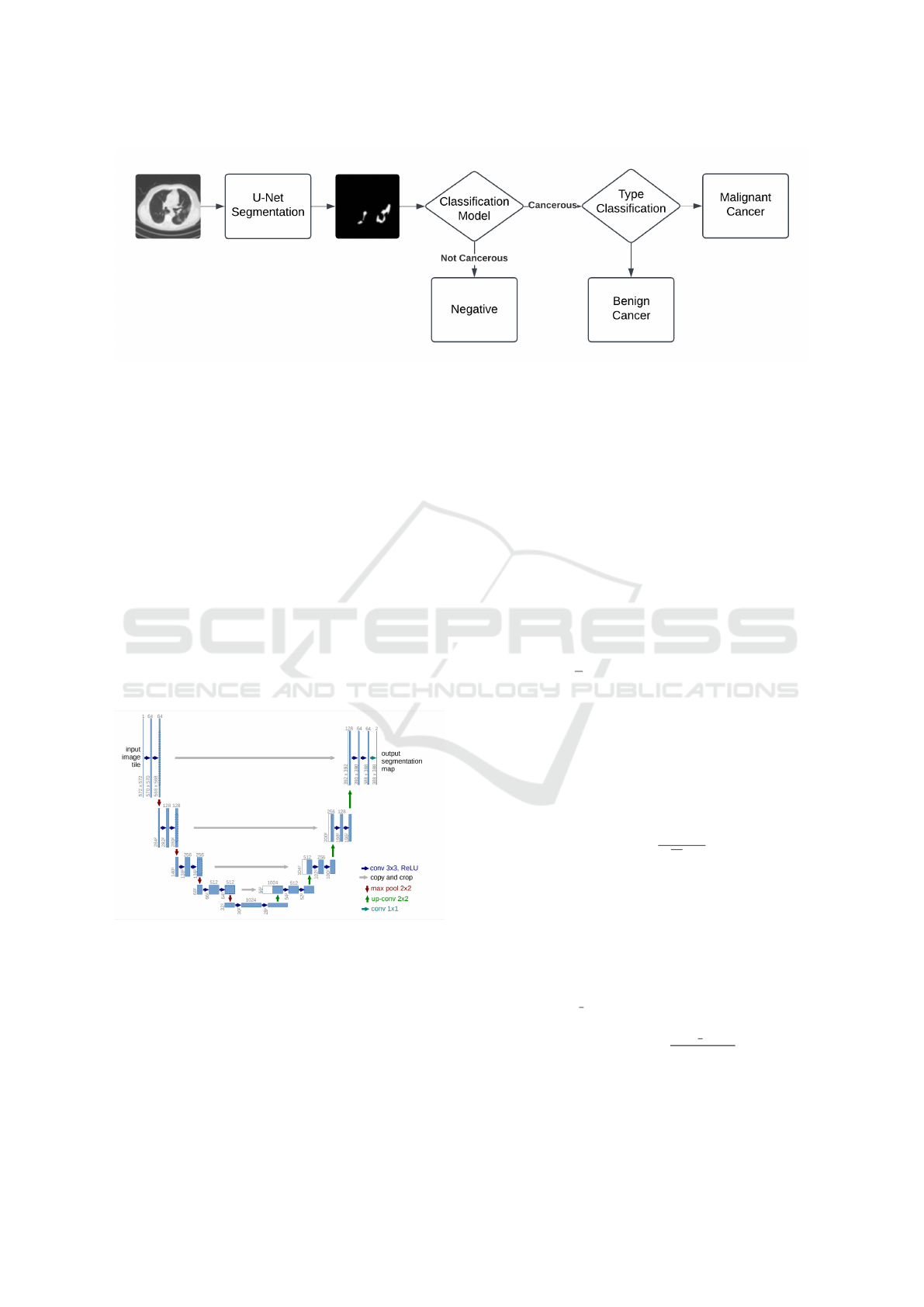
Figure 1: Pipeline of the proposed implementation showing the integration of U-Net for image segmentation and Hybrid
CNN-RNN for tumor classification
3.3.1 CNN Component
The CNN is based on a modified U-Net architecture
designed to capture spatial features. The convolu-
tional operation is defined as follows:
y = σ(W ·x +b) (1)
where x is the input, W is the convolutional kernel,
b is the bias term, and σ is the ReLU activation func-
tion. Batch normalization and dropout are applied af-
ter each convolutional layer to stabilize training and
mitigate overfitting. The U-Net Architecture 2 (figure
2) is used for CT image segmentation of the lungs, to
extract the nodule features present and is then passed
to the classification model.
Figure 2: Unet architecture (Ronneberger et al., 2015)
3.3.2 RNN Component
The temporal modeling component uses an LSTM
network, which processes sequential data extracted
from adjacent CT slices.
3.3.3 Output Layer
Sigmoid function is used as activation function in our
final output layer, producing a probability p for the
presence of a nodule:
3.4 Loss Function and Optimization
The loss function used was the binary cross-entropy
(BCE) loss function used to evaluate model perfor-
mance. The BCE loss is defined as:
BCE Loss = −
1
S
S
∑
i=1
h
x
i
log(p
i
)+(1 −x
i
)log(1 −p
i
)
i
(2)
where S is the total number of samples, x
i
is the
true label, and p
i
is the predicted probability for each
sample.
Optimizer: The Adam optimizer was applied
with an initial learning rate of 10
−4
. The parameter
update rule can be expressed as:
θ
t
= θ
t−1
−
η ·m
t
√
v
t
+ ε
(3)
where θ
t
represents the model parameters, m
t
and
v
t
are the first and second moment estimates, η is the
learning rate, and ε is a small constant to avoid divi-
sion by zero.
Gradient Clipping: To prevent the exploding
gradients problem, gradient clipping was applied,
where gradients are rescaled if their L2 norm exceeds
a threshold max norm:
g
clipped
= g ·min
1,
max norm
∥g∥
2
(4)
Improving Disease Classification Accuracy with Hybrid CNN-RNN Architectures for Lung Tumors
577

3.5 Training and Evaluation
The model utilized the Binary Cross-Entropy loss
function and was optimized using the Adam opti-
mizer. A dynamic learning rate adjustment strategy
was implemented through the ReduceLROnPlateau
scheduler, which halved the learning rate whenever
the validation loss showed no significant improve-
ment.
To evaluate the performance of the model, met-
rics such as precision, recall, F1 score and the area
under the curve of the Receiver Operating Character-
istics (ROC) curve were used. Additionally, a 5-fold
cross-validation approach was employed to evaluate
the model’s ability to generalize effectively.
4 RESULTS
The hybrid CNN-RNN model was evaluated using a
validation dataset of 51,429 negative and 154 positive
samples. The model achieved the following perfor-
mance metrics: 94.8% recall, precision of 0.7602,
F1-score of 0.8434, and AUC of 0.901. These re-
sults, achieved with a validation accuracy of 96.1%,
demonstrate robust performance in detecting lung
nodules. Specifically, in the validation set, of the 154
cancerous nodules, our model has detected 148 cor-
rectly.
Comparison with Contemporary Models:
We compared the performance of our model with
several contemporary approaches from recent studies.
Table 1 highlights the performance metrics of these
models. The comparison underscores the superior re-
call and AUC of our hybrid CNN-RNN model rela-
tive to contemporary approaches. Recall, often con-
sidered critical in medical diagnostics, indicates the
model’s ability to correctly identify positive cases.
Our model’s recall of 94.8% surpasses all listed ap-
proaches, including the hybrid CNN-LSTM model
for Parkinson’s Disease (91%) and the Hybrid CNN-
RNN model for lung cancer survival (92%). This high
recall rate ensures minimal false negatives, a crucial
attribute when detecting potentially life-threatening
conditions like lung nodules.
Precision, which reflects the proportion of true
positive predictions among all positive predictions, is
another vital metric. Our model achieves a precision
of 0.7602, slightly lower than some other methods,
such as the Tomato Leaf Disease Detection model
(0.75) but consistent with other high-performing di-
agnostic systems. While precision could be further
optimized, the trade-off for higher recall is acceptable
in the context of critical health applications, where
missing positive cases is far more detrimental.
The F1-score provides a balanced view of the
trade-off between precision and recall. Our model’s
F1-score of 0.8434 is competitive, exceeding that
of the Hybrid CNN-LSTM for Parkinson’s Disease
(0.79) and the Respiratory Disease Prediction model
(0.79). This robust F1-score signifies an effective bal-
ance in the model’s performance, making it suitable
for real-world clinical environments.
The AUC (Area Under the Curve) metric quanti-
fies the model’s ability to distinguish between positive
and negative samples. Our model achieves an AUC of
0.901, which, while slightly lower than some other
methods such as the Hybrid CNN-RNN for Lung
Cancer Survival (0.97) and the Tomato Leaf Disease
Detection model (0.96), still demonstrates strong dis-
criminatory power. This solid AUC, combined with
the model’s high recall, underscores its reliability and
suitability for clinical diagnostics.
Table 1: Comparison of performance metrics with contem-
porary models
Model Precision Recall F1-Score AUC
Proposed Hybrid CNN-RNN Model 0.7602 0.9481 0.8434 0.901
Hybrid CNN-LSTM for Parkinson’s Disease (El-Sayed, 2024) 0.72 0.91 0.79 0.95
Tomato Leaf Disease Detection (Davida et al., 2022) 0.75 0.88 0.81 0.96
Hybrid CNN-RNN for Lung Cancer Survival (Lu et al., 2024) 0.68 0.92 0.78 0.97
Respiratory Disease Prediction (Li et al., 2024) 0.73 0.87 0.79 0.94
As shown in Table 1, our model demonstrates su-
perior recall and AUC, achieving a recall of 94.8%
and an AUC of 0.901. The balance between high re-
call and robust AUC highlights its potential for clini-
cal applications.
In medical diagnostics, recall is critical as it mea-
sures the model’s capacity to accurately identify pos-
itive cases. With a recall of 94.8%, our model out-
performs other approaches, such as the hybrid CNN-
LSTM model for Parkinson’s Disease (91%) and the
Hybrid CNN-RNN for lung cancer survival (92%).
This high recall minimizes false negatives, which is
essential in detecting serious conditions like lung nod-
ules.
Precision, an important indicator of how many
identified positive cases are genuinely positive, is an-
other key metric. Our model achieves a precision of
0.7602, comparable to similar systems, including the
Tomato Leaf Disease Detection model (0.75). While
there is room to enhance precision, the higher recall
justifies this trade-off, particularly in health-related
applications where failing to identify true positives
could have severe consequences.
The F1-score, which balances precision and re-
call, further emphasizes our model’s effectiveness.
With a score of 0.8434, it surpasses other approaches
such as the Hybrid CNN-LSTM for Parkinson’s Dis-
INCOFT 2025 - International Conference on Futuristic Technology
578

ease (0.79) and the Respiratory Disease Prediction
model (0.79). This balanced performance demon-
strates the model’s suitability for deployment in clin-
ical settings, where both precision and recall are piv-
otal. Our model’s AUC of 0.901, while slightly be-
low the Hybrid CNN-RNN for Lung Cancer Survival
(0.97) and the Tomato Leaf Disease Detection model
(0.96), indicates a high level of reliability. The com-
bination of this solid AUC and exceptional recall un-
derscores the model’s strength and its potential for ap-
plication in real-world clinical diagnostics.
5 CONCLUSIONS
This proposed work presents a hybrid CNN-RNN
model for lung nodule detection, demonstrating
strong performance, particularly in recall and AUC,
which are crucial for identifying malignant nodules.
The model achieved an accuracy of 96.1% recall
of 94%, ensuring that most malignant cases are de-
tected, and an AUC of 0.901, indicating strong over-
all classification performance. While the precision of
0.76 and F1-score of 0.84 are promising, there re-
mains room for improvement in reducing false pos-
itives, which can be achieved through further model
refinement, threshold adjustments, and class balanc-
ing techniques.
The results underscore the importance of balanc-
ing sensitivity and specificity in medical imaging
models, especially when dealing with class imbal-
ances and small sample sizes, which are common in
lung cancer detection tasks. Future work could fo-
cus on integrating advanced data augmentation, semi-
supervised learning techniques, and more efficient
preprocessing pipelines to further enhance precision
while maintaining high recall.
REFERENCES
Aslani, R. et al. (2022). Time-series deep learning model
for malignancy risk prediction in pulmonary nodules.
Computers in Biology and Medicine.
Chen, J. and Xie, R. (2024). Improved detection network
for lung nodule localization using deformable convo-
lution and self-paced learning. IEEE Transactions on
Medical Imaging.
Davida, H. E., Ramalakshmi, K., Venkatesan, R., and
Hemalatha, G. (2022). Tomato leaf disease detection
using hybrid cnn-rnn model. Journal of Applied Biol-
ogy, 65:134–145.
El-Sayed, R. S. (2024). A hybrid cnn-lstm deep learn-
ing model for classification of the parkinson disease.
IEEE Access, 12:12345–12355.
et al., R. P. (2024). Ai-enhanced lung cancer detection
using the resnext50 architecture. In Proc. IEEE Int.
Conf. Imaging Systems (ICIS), pages 33–39. Avail-
able: https://ieeexplore.ieee.org/document/10627413.
Ewaidat, A. and El Brag, A. (2022). A convolutional neural
network-based approach using yolov5 for lung nodule
localization in ct scans. arXiv preprint.
Grand Challenge (2016). Luna16: Lung nodule analysis
2016.
Hesse, L. S. et al. (2020). Primary tumor origin classifica-
tion of lung nodules in spectral ct using transfer learn-
ing. arXiv preprint.
Hosseini, M. et al. (2022). Deep learning applications for
lung cancer diagnosis: A systematic review. Journal
of Medical Imaging and Health Informatics.
Kumar, A. and Sharma, P. (2024). Deep learning based
lung cancer prediction using cnn. In Proc. IEEE
Int. Workshop on Machine Learning and Applications
(IWMLA), pages 14–20. Available: https://ieeexplore.
ieee.org/document/10627846.
Lee, J. and Gupta, H. (2023). Lung cancer diagnosis
and classification using hybrid neural network tech-
niques. In Proc. IEEE Conf. Bioinformatics and Com-
putational Biology (BICB), pages 89–95. Available:
https://ieeexplore.ieee.org/document/10370424.
Li, L., Ayiguli, A., Luan, Q., Yang, B., and Subinuer, Y.
e. a. (2024). Prediction and diagnosis of respiratory
disease by combining cnn and bilstm methods. Jour-
nal of Healthcare Informatics, 12:112–120.
Liu, D. and Zhang, S. (2022). Lung cancer detection
using ct images and cnn algorithm. IEEE Access,
10:87766–87774. Available: https://ieeexplore.ieee.
org/document/9697158.
Liu, Y., Hou, Y.-J., Qin, C.-X., et al. (2023). A data aug-
mentation method and the embedding mechanism for
detection and classification of pulmonary nodules on
small samples. arXiv preprint.
Lu, Y., Aslani, S., Zhao, A., Shahin, A., and Barber, D.
e. a. (2024). A hybrid cnn-rnn approach for survival
analysis in a lung cancer screening study. Journal of
Medical Imaging and Health Informatics, 14:35–44.
Marinakis, I., Karampidis, K., and Papadourakis, G. (2024).
Pulmonary nodule detection, segmentation and classi-
fication using deep learning: A comprehensive litera-
ture review. BioMedInformatics, 4(3):2043–2106.
Mehta, S. G. A. and Agarwal, V. (2024). Detection and ro-
bust classification of lung cancer disease using hybrid
deep learning approach. In Proc. IEEE Int. Conf. Arti-
ficial Neural Networks (ICANN), pages 27–35. Avail-
able: https://ieeexplore.ieee.org/document/10452545.
P. Mishra, T. S. and Kumar, R. (2024). Early lung cancer
detection using cnn. In Proc. IEEE Int. Symp. Compu-
tational Intelligence (ISCI), pages 98–104. Available:
https://ieeexplore.ieee.org/document/10627340.
Patel, A. G. R. and Sharma, K. (2024). A hybrid deep learn-
ing approach for early detection and classification of
lung cancer using the pelican optimization algorithm.
In Proc. IEEE Int. Conf. Artificial Intelligence (ICAI),
pages 45–52. Available: https://ieeexplore.ieee.org/
document/10515355.
Improving Disease Classification Accuracy with Hybrid CNN-RNN Architectures for Lung Tumors
579

Rao, M. and Patel, P. (2023). Lung cancer detection using
vgg16 and cnn. In Proc. IEEE Conf. Imaging and AI
(ICIA), pages 11–18.
Ronneberger, O., Fischer, P., and Brox, T. (2015). U-net:
Convolutional networks for biomedical image seg-
mentation.
Sharma, K. and Lee, J. (2023). Detection of lung can-
cer from pathological images using cnn model. In
Proc. IEEE Conf. Computational Pathology (CPath),
pages 91–98. Available: https://ieeexplore.ieee.org/
document/9574590.
Society, A. C. (2024). Lung cancer statistics. Ameri-
can Cancer Society. Online. Available: https://www.
cancer.org/research/cancer-facts-statistics.html.
Verma, R. A. S. and Singh, A. (2023). Ai-enhanced
lung cancer prediction: A hybrid model’s pre-
cision triumph. IEEE Trans. Med. Imaging,
43(6):1234–1240. Available: https://ieeexplore.ieee.
org/document/10643642.
Verma, S. and Kumar, A. (2023). Enhancing lung cancer
classification: Leveraging existing cnns within a 1d
framework. In Proc. IEEE Conf. Data Science and
Engineering (ICDSE), pages 67–73. Available: https:
//ieeexplore.ieee.org/document/10695984.
Wang, C. et al. (2022). Towards reliable and explainable
ai model for solid pulmonary nodule diagnosis. arXiv
preprint.
Wang, N. and Li, Y. (2023). Revolutionizing lung cancer
prognosis through cnn-based predictive models. IEEE
J. Biomedical Engineering, 67(4):455–462.
INCOFT 2025 - International Conference on Futuristic Technology
580
