
Enhancing COVID-19 Diagnosis with Deep Learning Models
DenseNet and ResNet on Medical Imaging Data
S. Chowdri
1
, S. Shanthi
1
, R. Dhanaeswaran
1
, P. Giridar Prasad
1
, K. Nirmala Devi
1
and A. Kavitha
2
1
Dept. of Computer Science and Engineering, Kongu Engineering College, Erode, India
2
Dept. of Computer Science and Engineering, Government College of Engineering (IRTT), Erode, India
Keywords: Covid-19 Diagnosis, Deep Learning, DenseNet, ResNet, CT Scan, Chest X-Ray, Image Classification,
Accuracy.
Abstract: The COVID-19 pandemic has put immense pressure on health systems worldwide. Early and accurate
diagnosis is thus an important modality of management of the disease. RT-PCR tests are the gold standard for
diagnosis but suffer from a number of limitations such as high processing time and occasional false negatives.
Diagnostic imaging via chest X rays and CT scans offers a rapid, non-invasive alternative for detecting
COVID-19-induced lung abnormalities. This study evaluates the performance of various configurations of
DenseNet (121, 169, 201) and ResNet-152 for automated COVID-19 detection using chest X-rays and CT
scans. More in particular, DenseNet-201 yielded a good result of approximately 96% accuracy for CT scans
and 99% for X-rays when trained with the Adam optimizer using a batch size of 32. It highlights that the
choice of optimizer and batch size has paramount importance. DenseNet-201’s efficient gradient flow, feature
reuse, and parameter utilization make it especially suitable for medical imaging applications with limited
annotated datasets. Its robust feature extraction capabilities position it as a reliable diagnostic tool, potentially
enhancing clinical workflows and accelerating COVID-19 diagnosis. This study underscores DenseNet-201’s
potential to improve patient outcomes and pandemic management through accurate, automated medical image
analysis.
1 INTRODUCTION
COVID-19 is a disease that appeared suddenly at the
end of 2019, caused by the virus SARS-CoV-2, and
has since caused huge destruction to the health,
economies, and daily life of the world. It is transmitted
by aerosols, droplets, contaminated surfaces, and air.
The symptoms range from very mild to the most
severe and can involve fever, cough, difficulty
breathing, severe problems in the lungs, organ failure,
and, in extreme cases, death. For example, the Delta
and Omicron variants spread so quickly because of
their high contagion rate, sometimes for evading
immunity. This is not mentioning the vaccine
coverage that was reached or the lingering effects that
many people were still battling against COVID-19 in
mid-2024.Conventional medical imaging represents
another field where CNNs revolutionized the way it
presents unparalleled capabilities for the detection,
segmentation, and classification of different image
classes.
It is of great help in diagnosing diseases of the
lungs, such as lung cancer, tuberculosis, and
pneumonia, all at once. During the pandemic period of
COVID-19, CNNs became important for researching
chest X-ray features along with computed
tomography. The specialty in architectures of CNN
comes forth through ResNets and VGG, and then
further Inception, aside from others. Each of them is
unique and has its particular power, like residual
connections and skip connections in ResNet networks,
which enable the training of deeper networks; making
simpler designs boosts feature learning in VGG, while
Inception further improves this with its two most
innovative connectivity ideas: convolutional blocks
on several parallel branches with concatenated feature
maps. It allows every layer to directly feed into all
previous ones, encourages feature reuse, and makes
the flow of gradients better. This architecture will
learn both low-level and high-level features for
finding subtle abnormalities in a medical image.The
efficiency and resistance to overfitting make
506
Chowdri, S., Shanthi, S., Dhanaeswaran, R., Giridar Prasad, P., Devi, K. N. and Kavidha, A.
Enhancing COVID-19 Diagnosis with Deep Learning Models DenseNet and ResNet on Medical Imaging Data.
DOI: 10.5220/0013595700004664
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 3rd International Conference on Futuristic Technology (INCOFT 2025) - Volume 2, pages 506-513
ISBN: 978-989-758-763-4
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

DenseNet very relevant to high-dimensional data
associated with medical applications. Most of the
recent works on COVID-19 diagnostics, tumor
segmentation, skin lesion detection, and diabetic
retinopathy classification have been based on this
approach. Compared to ResNet and VGG models,
DenseNet is more parameter-efficient with high
accuracy and fewer parameters, hence having less risk
of overfitting. This is of extreme value in providing
accurate diagnoses in resource-poor settings. This
would, in turn, enable the radiologists to provide
accurate and reproducible results on well-
preprocessed and well-annotated datasets with
significantly less workload. Its performance can be
improved by performing hyperparameter tuning, with
evaluation metrics such as accuracy and AUC-ROC.
DenseNet bridges advanced AI technology with
practical healthcare applications; thus, it holds
tremendous potential to revolutionize diagnostics and
support clinicians worldwide in combating COVID-
19 and other diseases.
2 LITERATURE REVIEW
A study designed a CAD system that classified chest
X- rays into COVID-19 pneumonia, other pneumonia,
and normal cases using transfer learning -based CNN
following the use of preprocessing techniques like
removal of the diaphragm region and histogram
equalization(A. T, S. S, N. K, A. K, D. R. B and N.
Rajkumar, Sentiment Analysis on Covid-19 Data
Using BERT Model, 2024 International Conference
on Advances in Modern Age Technologies for Healt,
n.d.) (Nirmala Devi, K., Shanthi, S., Hemanandhini,
K., Haritha, S., Aarthy, S. (2022). Analysis of
COVID-19 Epidemic Disease Dynamics Using Deep
Learning. In Kim, J.H., Deep, K., Geem, Z.W.,
Sadollah, A., Yadav, A. (Eds) Proceed, n.d.).The
model achieved an accuracy of 94. 5% on a dataset of
8,474 images and reported that the performance was
enhanced significantly with the use of preprocessing
techniques and revealed how significant image
enhancement is towards the achievement of better
performance in COVID-19 detection (Heidari et al.,
2020). The analysis of deep learning models to
identify COVID- 19 from chest X-rays on a dataset of
5,000 images showed the four CNNs that were part of
the experiment, including ResNet18, ResNet50,
SqueezeNet, and DenseNet-121, achieving a 98%
sensitivity rate and a 90% specificity rate after transfer
learning training. Precision in high lighting the
infected lung regions of COVID-19 was observed in
the heatmaps generated by the models while matching
with the annotations made by the radiologists. The
results are promising, but the study points out that
larger datasets need to be created for even more
reliable accuracy assessments (Minaee et al., 2020).
ACoS is an abbreviation for Automatic COVID
Screening system in this study which is the
classification of patients into normal, suspected, and
infected with COVID 19 using radiomic texture
descriptors from chest X-rays. The ensemble uses a
majority 3 voting of five supervised classifiers in a
two- phase classification approach. The validation
was performed using 258 images with an accuracy of
98. 06% in the first phase (normal vs. abnormal) and
91. 33% in the second phase (pneumonia vs. COVID-
19). The obtained results manifested a statistical
difference and even surpassed some of the techniques
currently used for COVID-19 detection (Chandra et
al., 2021). A recent study presents a Deep
Convolutional Neural Network (CNN)-based
approach for the detection of COVID-19 from chest
X-ray images. Models used in this solution are
DenseNet201, ResNet50V2, and Inceptionv3, which
are specifically trained and then combined using a
weighted average ensembling. With 538 images
positive for COVID-19 and 468 negative images for
COVID-19, the model was able to achieve a
classification accuracy of 91.62%. In addition, the
study created an intuitive graphical user interface
application to make medical practitioners quickly
detect the existence of COVID-19 in the chest X-ray
images (Das et al., 2021). A study proved that AI can
be used to automate and improve the detection
accuracy for COVID-19 using Chest X-ray (CXR) and
CT images. Besides, AI can be also utilized in DL
techniques such as Convolutional Neural Networks
(CNN). This paper dis cusses research works on this
topic, challenges, and recent breakthroughs on the DL
based classification of COVID-19. The review also
suggests further research that should further improve
the performance and reliability of automated systems
for COVID-19 image classification (Aggarwal et al.,
2022). An article classifies COVID-19 patient
individuals using chest X-ray scans and com pares
various CNN models that base their work on deep
learning. In this, a dataset consisting of 6432 samples
from the Kaggle repository was tested using data
augmentation with Xception, ResNeXt, and Inception
V3. It was seen that among these, Xception is having
the highest accuracy as 97. 97%. The findings of the
analysis are not medical but show that automated deep
learning techniques might be useful for the screening
of COVID-19 patients (Jain et al., 2021). To overcome
the deficiencies of previous networks, a paper
proposed a dual path way, 11-layer deep 3D
Enhancing COVID-19 Diagnosis with Deep Learning Models DenseNet and ResNet on Medical Imaging Data
507

Convolutional Neural Network for the segmentation
of brain lesions. It fused an efficient dense training
scheme with a dual pathway architecture that
computes multi-scale input. The proposed approach
demonstrated superior performance on benchmarking
BRATS 2015 and ISLES 2015 experiments in multi-
channel MRI data of ischemic stroke, brain tumors,
and traumatic brain injuries. The method is found to
be effective and computationally efficient and thus
suitable for research as well as clinical applications
(Kamnitsas et al., 2017). It performed an experiment
using Transfer Learning to evaluate the state-of-the-
art convolutional neural networks on X-ray images
from patients infected with COVID 19, bacterial
pneumonia, and normal conditions. Based on the
study, the Deep Learning models were shown to detect
COVID-19 with as high as 96. 78% accuracy, 98. 66%
4 sensitivity, and 96. 46% specificity. Results indicate
that X- ray imaging could be an important step toward
the diagnosis of COVID-19; thus, more research needs
to be done in the medical field (Apostolopoulos &
Mpesiana, 2020). A research introduced COVID-
CAPS, a model of capsule network for the substitution
of CNN diagnosis in COVID-19 using X-ray images.
Compared to the previously designed CNN model,
COVID-CAPS reported to have an accuracy of 95.
7%, sensitivity of 90%, and specificity of 95. 8%,
which solved most drawbacks. These included small
amounts of sample data and loss of spatial information
in CNNs. It is promising as a diagnostic tool for
COVID-19, as further improvement to 98.3%
accuracy and 98.6% specificity was achieved by pre-
training with an X-ray image dataset (Afshar et al.,
2020). A study designed clinical predictive models for
the identification of COVID-19 cases based on
laboratory data and deep learning. It tested these
models on data from 600 patients, with an impressive
accuracy of 86. 66%, F1-score of 91. 89%, and a recall
of 99. 42%. The implications are that these clinical
predictive models may help the medical professional
validate the reliability of results from laboratories and
efficiently use resources during the pandemic (Alakus
& Turkoglu, 2020)
3 EXPERIMENTAL SETUP
3.1 Dataset
3.1.1 CT Scan
This dataset was downloaded from Kaggle, "COVID-
19 CT Scan Dataset" by Dr. Surabhi Thorat, and it
consists of COVID and Non-COVID images, which
are well-labeled, hence perfect for training diagnostic
machine learning models. Images were then split into
two categories: COVID-19 and Non-COVID
combined into a single DataFrame for easy
manipulation, then stratified into an 80:20 training-
validation split of 6095 training and 1525 validation.
Stratification has ensured that both categories are well
represented, hence the integrity of the data is
maintained without bias during model training and
validation.
3.1.2 X Ray
The dataset used in this research has been curated by
Prashant from Kaggle and is titled "Chest X-ray
(COVID-19 & Pneumonia)". The dataset comprises a
collection of X-ray images of cases related to
COVID-19, pneumonia, and healthy ones. In this
work, cases of pneumonia are not taken into
consideration, hence, the classes considered for
analysis are COVID-19 and Non- COVID. In all, the
dataset holds 2,159 images. It is split into an 80:20
stratified split for 1,726 training samples and 433
validation samples that maintain a balanced class
representation. Label them, categorize the images as
COVID-19 and Non-COVID, and merge them into
one DataFrame to accelerate the processing that can
later be easily split with the assurance that this would
maintain the integrity of the data and representativity
of the sets for a proper training and validation
process.
3.2 Data Augmentation
To enhance the generalization ability of our models
and compensate for the relatively limited size of the
dataset, various data augmentation techniques were
employed. These included:
Table 1: Data Augmentation Techniques.
Augmentation
Technique
Description
Rotation Range Rotates images randomly within the
s
p
ecified de
g
ree ran
g
e.
Width Shift
Range
Randomly shifts images horizontally by
a specified fraction.
Height Shift
Ran
g
e
Randomly shifts images vertically by a
s
p
ecified fraction.
Shear Range Applies random shearing
transformations to the ima
g
es.
Zoom Ran
g
e Randoml
y
zooms in or out on ima
g
es.
Horizontal Fli
p
Randoml
y
fli
p
s ima
g
es horizontall
y
.
Rescale Normalizes pixel values by
scaling them by specified factor.
INCOFT 2025 - International Conference on Futuristic Technology
508

3.3 Model Architecture
Traditional CNNs have sequential information flow,
from one layer to the next. However, in DenseNet
every layer receives input from all preceding layers
and passes its own feature maps to all subsequent
layers. So, the i-th layer receives feature maps of all
the previous layers,
𝑥0, 𝑥1, 𝑥2, … 𝑥𝑙 − 1, 𝑎𝑠 𝑖𝑛𝑝𝑢𝑡:
𝑥1 =𝐻1([𝑥0,𝑥1,𝑥2,…..,𝑥𝑙−1]) (1)
where H1 is a composite function of batch
normalization, ReLU and convolution. Dense
connectivity leads to the +following benefits: Direct
connections between layers would allow gradients to
flow more easily in the backwards propagation pass
so as to mitigate the problem of vanishing gradients.
Layers could read the feature maps of all preceding
layers, so reuse of features is encouraged and
redundancy is reduced. This architecture uses fewer
parameters than its traditional counterparts at the
same depth due to the fact that it does not have to
learn redundant feature maps in the first place.
A Dense Net consists of several dense blocks. The
dense block itself comprises multiple convolutional
layers, which are connected densely. Between the
dense blocks, transition layers are provided to carry
out the down sampling operations and decrease the
spatial dimensions of the feature maps. Dense Block:
It is a sequence of layers wherein each layer is fed
forward to every other layer. Each layer feature maps
are concatenated with all inputs from the following
layers. Transition Layer: It lies between the dense
blocks. It utilizes a 1x1 convolution to compress
features, followed by feature map reducing a 2x2
average pooling operation along with batch
normalization. Dense Net exists in many
configurations that are primarily different in depth,
concerning the number of layers. Currently, three
variants are widely used, such as DenseNet121,
DenseNet169, and DenseNet201.
Here, each number corresponds to the total
quantity of the layers inside the network, counting
both convolutional, pooling, and fully connected
layers.
Table 2: Comparison between Traditional CNN and
DenseNet.
Feature Traditional
CNN
DenseNet
Connectivity Sequential Dense (each
layer connected
to all previous
layers)
Gradient Flow Can be
hindered by
depth
(vanishing
g
radient
)
Improved due
to direct
connections
Feature Reuse Limite
d
Extensive
Parameter
Efficiency
Higher due to
redundant
feature ma
p
s
Lower, more
efficient
3.3.1 DenseNet 121
It has 121 layers. It offers an excellent balance
between depth and computational efficiency and best
applicable in places where computation is much
needed.
3.3.2 DenseNet 169
It adds to the architecture through adding more layers,
therefore giving depth for feature extraction when the
complexity goes up a notch. This variation is very
good for tasks which need the recognition of fine
details and is computationally costly.
3.3.3 DenseNet 201
Being one of the deepest variants, with a count of 201
layers, it provides the finest feature extraction
capability. In particular, it is well-suited for more
complex applications but with huge computational
and memory resource requirement
3.3.4 ResNet 152
ResNet-152 deep learning model was designed to
solve problems with the vanishing gradient in deep
neural networks. The gradients can flow through
shortcut connections that bypass layers. It is built
from residual blocks with two or three convolutional
layers, with batch normalization and ReLU for
activation. These shortcut connections add the input
of the block directly to the output, hence enabling the
network to learn residual functions. ResNet-152 is a
152-layer network with about 60.2 million
parameters. An architecture so deep, trained
efficiently for very deep networks, improves the
Enhancing COVID-19 Diagnosis with Deep Learning Models DenseNet and ResNet on Medical Imaging Data
509

accuracy and performance on many tasks like image
classification and medical image analysis
Figure 1: Differentiation of layers between DenseNet
Architectures.
3.4 Methodology
This paper uses the complete dataset of COVID-
19 X-ray and CT-scan images downloaded from
Kaggle for training and testing machine learning
models for diagnosis. A variety of DenseNet
architecture variants, including DenseNet-101,
DenseNet-121, and DenseNet-201, are considered
along with ResNet for the analysis using transfer
learning techniques. Transfer learning was applied by
initializing the weights of DenseNet pre-trained on
ImageNet. This ensured that the starting point for our
models was considerably robust and had leveraged
some of the rich feature representations learned from
a diverse set of images. The dataset was well-
structured, split roughly 80-20 between training and
validation sets; this helps prevent bias and maintains
integrity in the dataset by ensuring that the
distribution of COVID-19 and non-COVID-19
images remains constant across both sets.
Accordingly, early stopping during training kept a
constant eye on the model's performance on the
validation set and stopped it when it saw it did not
improve anymore. In that way, this technique avoids
overfitting; by doing so, it keeps the model
generalizing even to new data fed into it. Each of
these trained models was then comprehensively
evaluated against different metrics, namely AUC-
ROC, accuracy, specificity, recall, and precision.
AUC-ROC expresses the measure of how well the
model is capable of distinguishing between classes,
while accuracy is the general correctness of the
predictions made by the model. Specificity will
explain how well the model provides the true
negatives, and recall will provide the performance of
identifying true positives. Precision provided a view
into how well that model identified true positives
among all that were predicted as positive.
3.5 Training And Optimisation
In this work, transfer learning is adopted by
initializing the DenseNet models with pre-trained
weights from ImageNet. In that way, the DNNs will
leverage the rich feature representations they have
learned on such a large and diverse dataset for fast
convergence and improvement in performance
toward the COVID-19 diagnosis task. In training, the
base layers in DenseNet were frozen in order to
maintain all the valuable pre-trained features while
training the head for classification, for adapting it to
our binary classification task. The custom head
consists of a global average pooling layer followed by
fully connected layers, culminating in a sigmoid
activation that outputs class probabilities using the
loss function as Binary cross-entropy.
The DenseNet is densely connected; this aids in
better gradient flow and feature propagation, which
can be of great help for such complex tasks as medical
image classification. An optimization of the model
performance was basically carried out with the Adam
optimizer because it has an adaptive learning rate and
it is efficient in handling sparse gradients, which
helps speed up the convergence.
However, in order to really assess model
performance, further exploration of the models with
different optimizers is intended, including RMSprop,
SGD, and Adam, along with various batch sizes. Each
of these optimizers has its merits: Adam is adaptive
with its learning rates but sometimes converges to
suboptimal solutions; SGD offers better
generalization but needs careful tuning and may result
in slow convergence; and RMSprop acts best on non-
stationary problems, though it can converge more
slowly than Adam on deeper networks.
Table 3: Model Architecture.
Component Description
Global Average
Pooling
Reduces spatial dimensions of
feature maps
Dense Layer (ReLu) Adds non-linearity and
enhances learnin
g
Output Layer
(
Si
g
moid
)
Binary output for
classification
Batch size does play an important role while
training a model. Larger batch size provides smoother
estimates of gradient, faster convergence, requires a
lot of memory, though carries the risk of trapping into
a local minimum; smaller batch size introduces extra
noise into the gradient estimation. This might help the
models from escaping local minima with somewhat
slow convergence. We systematically try these
INCOFT 2025 - International Conference on Futuristic Technology
510
various optimizers and batch sizes in order to see
what the best combination of the two might be that
yields better performance for our particular
classification problem.
4 RESULT AND DISCUSSION
Table 4: Accuracy for DenseNet121 for CT SCAN
Dataset.
Table 5: Accuracy for DenseNet169 for CT SCAN Dataset
Optimizer Batch
Size
(
16
)
Batch
Size
(
32
)
Batch
Size
(
64
)
Adam 95. 20 94. 50 92. 80
RMS
p
ro
p
94. 00 93. 50 91. 50
SGD 89. 80 88. 50 86. 70
Table 6: Accuracy for DenseNet201 for CT SCAN
Dataset.
Optimizer Batch
Size(16)
Batch
Size(32)
Batch
Size(64)
Adam 94. 00 96. 90 95. 70
RMS
p
ro
p
94. 50 96. 10 95. 10
SGD 93. 30 95. 20 94. 30
Table 7: Accuracy for ResNet152 for CT SCAN Dataset.
Optimizer Batch
Size(16)
Batch
Size(32)
Batch
Size(64)
Adam 94. 50 95. 70 94. 00
RMSprop 92. 80 93. 50 92. 10
SGD 91. 80 92. 20 90. 50
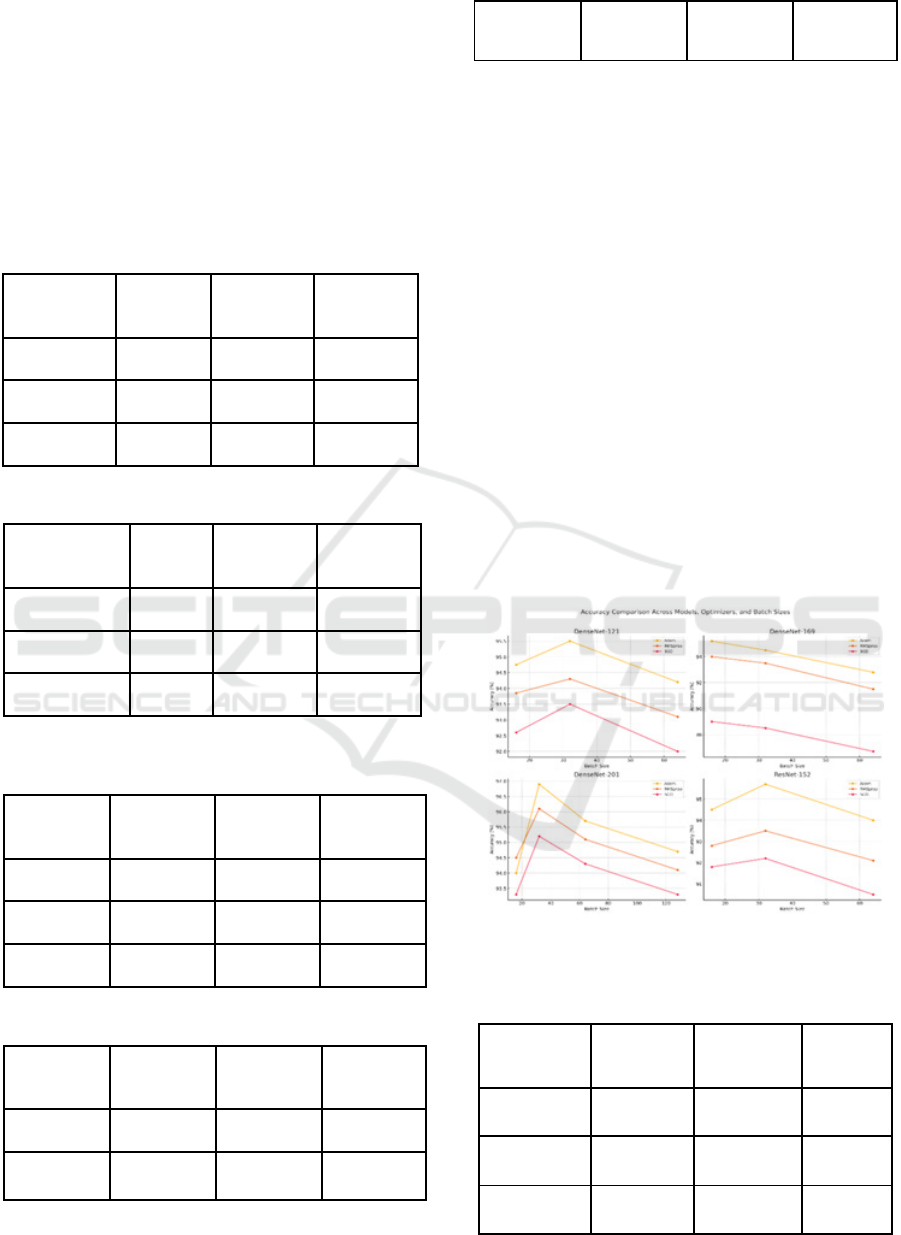
Comparing all different models, namely
DenseNet-121, DenseNet-169, DenseNet-201, and
ResNet-152, with a set of different optimizers and
batch sizes, it can be found that the best results come
out to be from DenseNet-201. With the Adam
optimizer and batch size of 32, this has achieved an
accuracy of 96.9%. Relatively, one more working
combination for the DenseNet-201 model was with
RMSprop as the optimizer and a batch size of 32,
which yielded the same result of 96.1% accuracy. The
best after that is DenseNet-121, which reaches a peak
of 95.5% with the Adam optimizer and batch size 32.
Next comes DenseNet-169, which, compared to its
other variants, reached a peak of 95.2% with the
Adam optimizer and batch size of 16. ResNet-152
was competitive but reached a peak of 95.7% only at
the Adam optimizer with a batch size of 32.
Therefore, in all models, the best performance was
obtained when, for the Adam optimizer, the batch size
was set to 32. Thus, it is the best configuration. On
the other hand, DenseNet-201 yielded the best
performance on the Covid-19 CT-scan dataset when
using Adam with a batch size of 32.
Figure 2: Comparison of various DenseNet and ResNet
Architectures for CT scan Dataset.
Table 8: Accuracy for DenseNet121 for X Ray Dataset.
Optimizer Batch
Size
(
16
)
Batch
Size
(
32
)
Batch
Size
(
64
)
Adam 97. 85 98. 50 98. 20
RMSprop 97. 70 98. 00 97. 85
SGD 96. 50 97. 00 96. 70
Optimizer Batch
Size(16)
Batch
Size(32)
Batch
Size(64)
Adam 94. 75 95. 50 94. 20
RMS
p
ro
p
93. 85 94. 30 93. 10
SGD 92. 60 93. 50 92. 00
Enhancing COVID-19 Diagnosis with Deep Learning Models DenseNet and ResNet on Medical Imaging Data
511
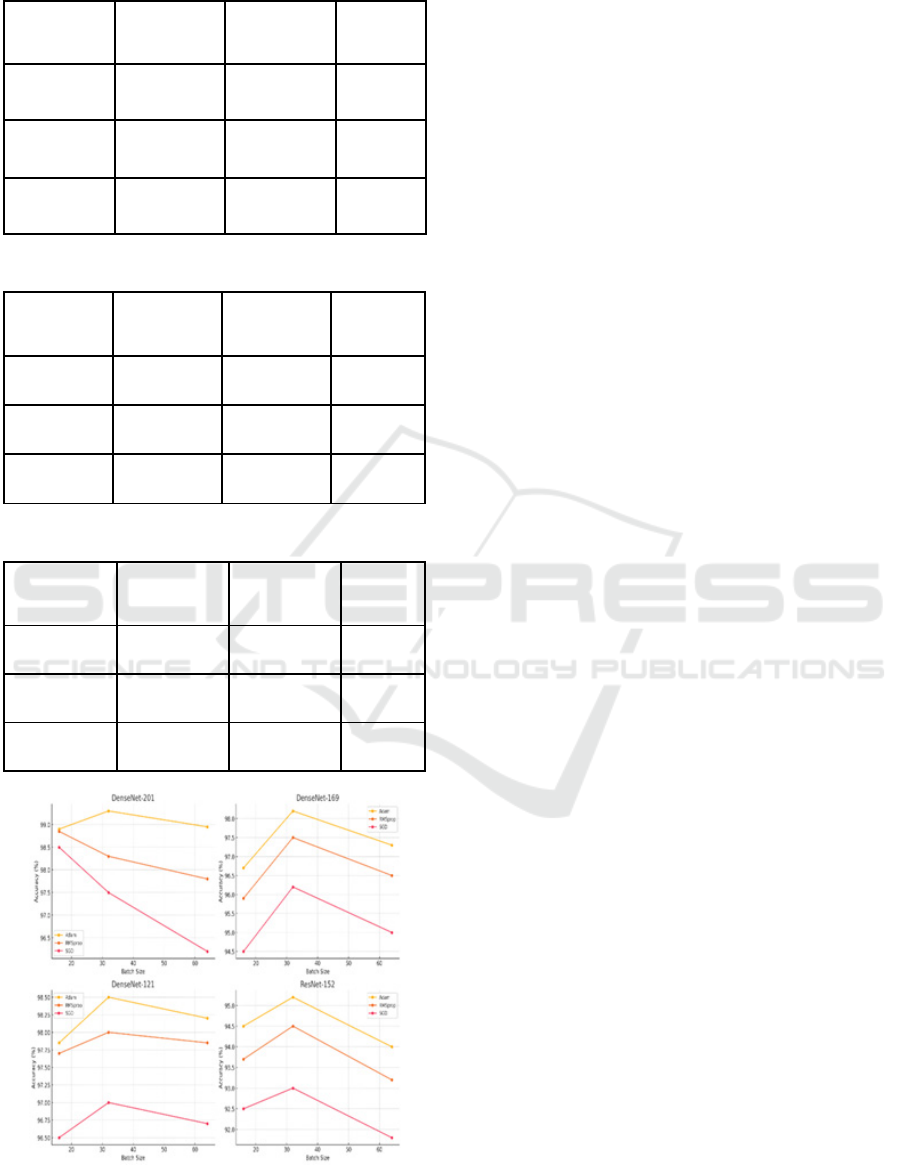
Table 9: Accuracy for DenseNet169 for X Ray Dataset.
Optimizer Batch
Size(16)
Batch
Size(32)
Batch
Size(64)
Adam 96. 70 98. 20 97. 30
RMSprop 95. 90 97. 50 96. 50
SGD 94. 50 96. 20 95. 00
Table 10: Accuracy for DenseNet201 for X Ray Dataset.
Optimizer Batch
Size
(
16
)
Batch
Size
(
32
)
Batch
Size
(
64
)
Adam 98. 90 99. 30 98. 95
RMSprop 98. 30 98. 85 98. 50
SGD 97. 50 98. 20 97. 80
Table 11: Accuracy for ResNet152 for X Ray Dataset.
Optimizer Batch
Size(16)
Batch
Size(32)
Batch
Size(64)
Adam 94. 50 95. 20 94. 00
RMSprop 93. 70 94. 50 93. 20
SGD 92. 50 93. 00 91. 80
Figure 3: Comparison of various DenseNet and ResNet
Architectures for X-ray Dataset.
A comparison of the accuracies attained for the
various models using the X-ray dataset shows that the
highest, from DenseNet-201 with the Adam
optimizer and batch size of 32, was 99.30%. This is
closely followed by DenseNet-121, which had
attained an accuracy of 98.50% under the same
conditions and consistently showed quite good
performance under other batch sizes and optimizers.
Among these, DenseNet-169 had the highest
accuracy of 98.20% with the Adam optimizer and
batch size 32, ranking slightly after the top two.
ResNet-152 is a little lagging and achieved a
maximum of 95.70% accuracy under similar settings.
Hence, DenseNet-201 and DenseNet-121 are the best
models for this dataset, especially with the Adam
optimizer and batch size 32, predestining these
models for X-ray image classification tasks.
ACKNOWLEDGEMENTS
The author acknowledges AICTE for providing the
fund for conducting this research. AICTE, the
reference no. F.No.8-80/FDC/RPS/POLICY-1/2021-
22/ Dated: 18.02.2022.
REFERENCES
A. T, S. S, N. K, A. K, D. R. B and N. Rajkumar, Sentiment
Analysis on Covid-19 data Using BERT Model, 2024
International Conference on Advances in Modern Age
Technologies for Healt. (n.d.).
Afshar, P., Heidarian, S., Naderkhani, F., Oikonomou, A.,
Plataniotis, K. N., & Mohammadi, A. (2020). COVID-
CAPS: A capsule network-based framework for
identification of COVID-19 cases from X-ray images.
Pattern Recognition Letters, 138, 638–643.
https://doi.org/10.1016/J.PATREC.2020.09.010
Aggarwal, P., Mishra, N. K., Fatimah, B., Singh, P., Gupta,
A., & Joshi, S. D. (2022). COVID-19 image
classification using deep learning: Advances,
challenges and opportunities. Computers in Biology
and Medicine, 144, 105350.
https://doi.org/10.1016/J.COMPBIOMED.2022.10535
0
Alakus, T. B., & Turkoglu, I. (2020). Comparison of deep
learning approaches to predict COVID-19 infection.
Chaos, Solitons & Fractals, 140, 110120.
https://doi.org/10.1016/J.CHAOS.2020.110120
Apostolopoulos, I. D., & Mpesiana, T. A. (2020). Covid-
19: automatic detection from X-ray images utilizing
transfer learning with convolutional neural networks.
Physical and Engineering Sciences in Medicine, 43(2),
635–640. https://doi.org/10.1007/s13246-020-00865-4
INCOFT 2025 - International Conference on Futuristic Technology
512

Chandra, T. B., Verma, K., Singh, B. K., Jain, D., & Netam,
S. S. (2021). Coronavirus disease (COVID-19)
detection in Chest X-Ray images using majority voting
based classifier ensemble. Expert Systems with
Applications, 165, 113909.
https://doi.org/10.1016/J.ESWA.2020.113909
Das, A. K., Ghosh, S., Thunder, S., Dutta, R., Agarwal, S.,
& Chakrabarti, A. (2021). Automatic COVID-19
detection from X-ray images using ensemble learning
with convolutional neural network. Pattern Analysis
and Applications, 24(3), 1111–1124.
https://doi.org/10.1007/s10044-021-00970-4
Heidari, M., Mirniaharikandehei, S., Khuzani, A. Z.,
Danala, G., Qiu, Y., & Zheng, B. (2020). Improving the
performance of CNN to predict the likelihood of
COVID-19 using chest X-ray images with
preprocessing algorithms. International Journal of
Medical Informatics, 144, 104284.
https://doi.org/10.1016/J.IJMEDINF.2020.104284
Jain, R., Gupta, M., Taneja, S., & Hemanth, D. J. (2021).
Deep learning based detection and analysis of COVID-
19 on chest X-ray images. Applied Intelligence, 51(3),
1690–1700. https://doi.org/10.1007/s10489-020-
01902-1
Kamnitsas, K., Ledig, C., Newcombe, V. F. J., Simpson, J.
P., Kane, A. D., Menon, D. K., Rueckert, D., &
Glocker, B. (2017). Efficient multi-scale 3D CNN with
fully connected CRF for accurate brain lesion
segmentation. Medical Image Analysis, 36, 61–78.
https://doi.org/10.1016/J.MEDIA.2016.10.004
Minaee, S., Kafieh, R., Sonka, M., Yazdani, S., &
Jamalipour Soufi, G. (2020). Deep-COVID: Predicting
COVID-19 from chest X-ray images using deep
transfer learning. Medical Image Analysis, 65, 101794.
https://doi.org/10.1016/J.MEDIA.2020.101794
Nirmala Devi, K., Shanthi, S., Hemanandhini, K., Haritha,
S., Aarthy, S. (2022). Analysis of COVID-19 Epidemic
Disease Dynamics Using Deep Learning. In Kim, J.H.,
Deep, K., Geem, Z.W., Sadollah, A., Yadav, A. (eds)
Proceed. (n.d.).
Enhancing COVID-19 Diagnosis with Deep Learning Models DenseNet and ResNet on Medical Imaging Data
513
