
Integrated EEG Signal Fusion for Advanced Epileptic Seizure
Analysis
B. Karthik Raja, K. Nirmal Richard, A. Balachandar, D. Raghu Raman, A. Usharani
and P. Manju Bala
Department of Computer Science and Engineering, IFET College of Engineering, Villupuram, Tamil Nadu, India
Keywords: Epileptic Seizure, Memory Loss, EEG Signals
Abstract: Epileptic seizures can result in substantial harm to the brain, which can lead to cognitive decline and memory
loss. Reducing the severity of seizures is largely dependent on early identification. Currently, the doctors
visually inspect EEG signals in order to diagnose seizure activity, which can be time-consuming and difficult.
In order to automatically monitor and detect seizures through the brain's bio-signals, we propose a new
method: simplistic convolutional neural network-long short-term memory model (1DCNN-LSTM). First, the
unprocessed EEG dataset is pre-treatment and normalized, and we extract the sequence of features by a 1D
CNN, and pass them to the LSTM layer. The temporal features are supplied to a few fully connected layers
for final seizure recognition. Using data from UCI epileptic seizure detection dataset, the suggested model
was assessed. In terms of recognition accuracy, the results are excellent: 82.00% for five-class seizure
recognition and 99.39% for binary seizure recognition. The attribution of accuracy is considerably above that
of classical machine learning methods and outshines other deep learning models widely recognized as
competitors.
1 INTRODUCTION
Millions of individuals worldwide suffer from the
neurological condition known as epilepsy. It develops
as a result of a confluence of acquired and inherited
elements, with the body undergoing abnormal brain
activity that results in disorientation,
unconsciousness, uncontrollable movements, etc. 5
crore individuals throughout the globe to be troubled
by seizure disorder; most of whom are adults, with the
rest being children. Abnormal birth oxygen levels,
brain injuries that occur in pregnancy, intracranial
tumor, and unfamiliar blood sugar are some of the
possible causes, however these are mostly
unexplained. There were two kinds of seizure: focal
seizures and generalized Tonic-Clonic seizures. A
generalized seizure attacks the brain as a whole
whereas a focal seizure attacks only certain regions of
the brain. There are various classes into which
generalized seizures have been divided. These
include myoclonic, Tonic-Clonic, Atonic, Tonic-
Clonic seizures, Absence, and Clonic, among others,
that end in convulsive seizures. The rests differentiate
epilepsy from a significant condition that may have a
devastating impact on the patient's physical as well as
mental well-being-even causing death. These people
would be much better off if they received adequate
and appropriate care at the right time.
Electroencephalography can be used to study the
brain non-invasively. This technique can yield all the
epilepsy-related information that cannot be gathered
through other physiological procedures. EEG signals
are mostly covered on the scalp but may also be
recorded intracranially. EEG signals can be broadly
classified into 4 states; these are Interictal, Postictal,
Ictal, and Preictal. The Preictal stage has further
significance because, minutes before the seizure
occurs, it serves as an information source upon when
the seizure onset is going to happen. By using the
classification of interictal and preictal stages to
predict the ictal state, seizures may be avoided and
their harmful effects mitigated by taking medication
on time. In the past, the primary method used by
medical professionals to diagnose epilepsy or
determine the origin of seizures was the visual
interpretation of EEG signal data. However, new
developments in deep learning techniques have made
Karthik Raja, B., Nirmal Richard, K., Balachandar, A., Raghu Raman, D., Usharani, A. and Manju Bala, P.
Integrated EEG Signal Fusion for Advanced Epileptic Seizure Analysis.
DOI: 10.5220/0013594500004664
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 3rd International Conference on Futuristic Technology (INCOFT 2025) - Volume 2, pages 449-456
ISBN: 978-989-758-763-4
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
449

it possible to create automated algorithms for
identifying seizure activity associated with epilepsy.
Deep learning has grown significantly in the last few
years and is now applied in many domains, most
notably image and natural language processing.
CNNs basically use two characteristics in a different
way to outperform other neural networks in different
tasks. These characteristics include different filters
which are applied over a variety of layers-for
example: Convolution, pooling, normalization, and
fully connected layers. However, the process of
learning relevant and representational features active
from EEG bio-signals presented as a time series is
inherently difficult for CNNs. Hence, CNNs were
unable to apply an accurate modeling upscaling of
raw EEG signals onto seizure detection outcomes.
2 RELATED WORKS
110 features were then created for each seizure in the
time, frequency, and time-frequency domains after
preprocessing of the signals by the traditional
pipeline. The features were ranked added in order to
find the important ones using the method of extreme
Gradient Boosting with statistical tests, Abirami S et
al, (Abirami, Tikaram, et al. , 2024). With the
introduction of machine learning algorithms,
automated diagnosis systems can help doctors make
rapid and accurate diagnoses, inform the patients, and
speed up the classification procedure. It is the
presentation of a new multi-path deep learning
network for seizure-type classification, H. Albaqami
et al, (Albaqami, Hassan, et al. , 2023)
There is a new feature extraction method because
of specific bands common spatial pattern, MSBCSP,
for multi-class. It applies the joint approximation
diagonalization, JAD, to the original CSP algorithm
in the case of a multi-classification problem. Energy
of Intrinsic Mode Functions is extracted through
Complete Ensemble Empirical Mode Decomposition
with Adaptive Noise, D. Wu et al, (Wu, Li, et al. ,
2023). Whereas self-regulating primitive discovery of
seizures from a average EEG has been obtainable,
classification of seizure types has not been attempted.
Thus, P. Swarubini's and et al, study caters to classify
seven types of seizures using non-seizure EEG
(Abirami, Swarubini, et al., 2023). From every
preictal data segment in 17 EEG channels and 1 ECG
channel, the time-domain characteristics were
extracted. Various classifiers like k-nearest
neighbour, decision tree, random forest, naive Bayes,
support vector machines were utilized to access the
classification accuracy. Wenjuan Xiong's and et al,
research by using random forest on 15-0 min preictal
period of EEG and ECG data achieved the best
classification accuracy results at 87.83% (Xiong,
Nurse, et al. , 2023).
In the case of epileptic patients, accurate
identification of the seizure type is of great
importance to help design a treatment plan and
administer medications. Diagnosis of epileptic
seizures is most commonly carried out using the
electroencephalography technique, commonly
abbreviated as EEG. Signals from the EEG are most
often used in epilepsy research, and the signals carry
vital information regarding electrical activity in the
brain. Among the various deep network architectures
that have been broadly applied in learning
representations for EEG signals in epilepsy research,
CNNs are just one of them. M. Hussain et al,
(Alshaya, and, Hussain, 2023). A Nicolet EEG
machine samples the EEG data set at 125 Hz. It has
been feasible to obtain IEDs, for example spikes,
sharps, slow waves, and spike-wave discharges
(SWD), by robust preprocessing, feature extraction,
and optimal classifiers. Results The results of the
developed classifier are tested against clinical
impressions provided by experienced epileptologists.
R. K. Joshi et al, (Joshi, Kumar, et al. , 2022). Hence,
development procedures automatically would support
medical professionals with the early identification
and diagnosis of epileptic seizures as well as
classification. Intelligent diagnostic techniques
depend on development that needs for the physiology
and pathophysiology of seizure, by using machine
learning in classification and identification of
symptoms. Adetunji C. O et al, (Adetunji, Olaniyan,
et al. , 2023). Design, procedure and strategy Multiple
illness patients encounter many problems especially
in situations where they have been diagnosed with
more than one dysfunction, especially when they use
wheelchairs and are sighted. Neelappa R. U. N et al,
(Neelappa, and, Harish, 2023).
About thirty percent of epilepsy patients remain
unmedicated or unaspirantedly operated upon.The
preictal area is the area of the brain showing abnormal
activity just before a seizure occurs, often sometime
in the minutes leading up to it. Poorani S et al,
(Poorani, and, Balasubramanie, 2023).In this respect,
this research work presented a novel deep learning
methodology for the prediction of successful seizure
in iEEG accurately. It used channel increment
strategy in conjunction with 1D-CNN. As an initial
step, we segmented the iEEG signals using 4-sec
sliding windows non-overlap. Wang X et al, (Wang,
Zhang, et al. , 2023).
INCOFT 2025 - International Conference on Futuristic Technology
450
This paper introduces an epilepsy detection
algorithm which could reduce the memory
requirements of the system by using few
characteristics only. This study also introduces a new
entropy estimation technique for features extraction
so that computation requirement of the algorithm will
be reduced using bitwise operations instead of
logarithmic ones. Yan X et al, (Yan, Yang, et al. ,
2022).This has led to aggressive application of
algorithms of machine learning to classify seizure
diseases from big data, and thus present neurologists
with shortlisted results. According to P.
Boonyakitanont et al, (Boonyakitanont, Lek-Uthai, et
al. , 2020) many features, data transformations, and
classifiers have been researched in order to classify
and assess seizures using EEG signals. Raw EEG
signals, directly obtained without any preprocessing
as input into the system, reduce the amount of
computation. Secondly, BNLSTM and CASA
retained the time and spatial information of the raw
EEG data respectively, M. Ma et al, (Ma, Cheng, et
al. , 2021).
This paper introduces a new CNN algorithm along
with the common spatial pattern (CSP) algorithm for
seizure prediction. According to real signals, Y.
Zhang et al, (Zhang, , et al. , 2020)first divide the pre-
ictal signals and combine them together to form
artificial preictal EEG signals as an approach to the
trial imbalance situation between two states.
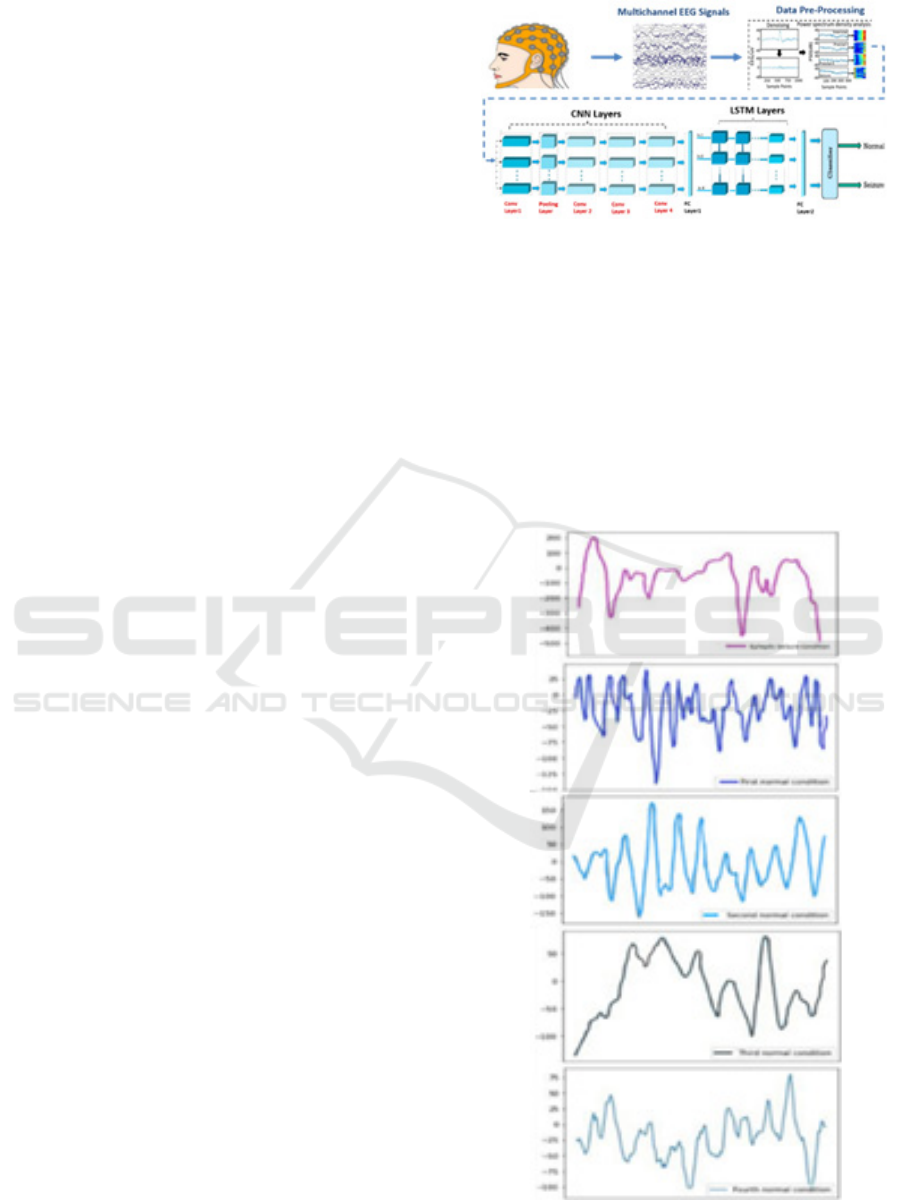
3 PROPOSED SYSTEM
It includes real-time monitoring, feature extraction,
preprocessing, classification, and data acquisition.
This research describes a novel method for 1D CNN-
LSTM-based epileptic seizure identification .
First, the raw EEG signal data was preprocessed.
Next the LSTM and 1D CNN were used in turn. Then
1D CNN combined with the LSTM model identifies
epileptic seizures for data processing and getting an
accurate outcome as shown below as the result graph.
Now let us see discuss the Dataset Description,
1D CNN, LSTM Structure, and CNN Combined with
LSTM Model in 1D which has to be implemented to
detect the Seizure after the multichannel signals have
been gathered to provide a key improvement in
accuracy.
Figure 1 : Block Diagram
3.1 Dataset Description :
This study uses the publicly accessible 500-subject
UCI Epileptic Seizure Recognition Data Set. Each of
five folders included in the dataset had 100 recordings
in total, and every recording sample held 4097 data
points that were gathered over a period of 23.5
seconds. UCI preprocesses that dataset and then splits
each sample into 23 1-second segments, randomly
shuffles the data, and produces 11,500 timeseries
EEG’s signal datas and samples. There are 5 types of
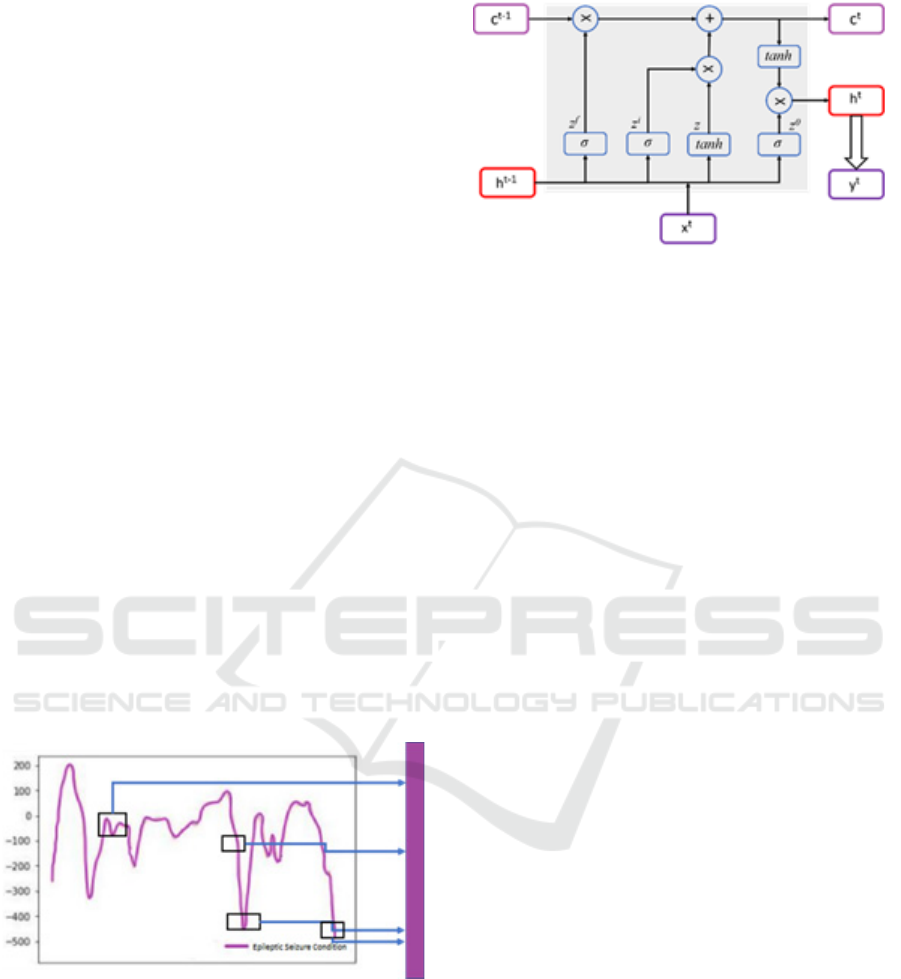
Figure 2: Quartet normal circumstances and the EEG raw
signa which is in undulation of one tonic seizure state
Integrated EEG Signal Fusion for Advanced Epileptic Seizure Analysis
451
medical conditions in the UCI dataset. These include
four normal conditions where no seizure takes place
and one associated with epileptic seizures. Such
conditions include records in which patients undergo
seizures, records in which patients are open-eyed
during an EEG, records of patients who are closed-
eyed during an EEG, healthy brain regions of
subjects, and the brain tumor region of the subjects.
Despite the fact that the raw EEG signal waveform
for the epileptic seizure condition is significantly
different compared with normal conditions, many
normal situations cannot be differentiated. Hence,
both tasks of binary and five-class epileptic seizure
recognition are dealt with in this model to estimate
the efficiency of the advanced approach
appropriately. The dataset is openly accessible to all
users.
3.2 1D CNN :
To extract relevant and comprehensive features from
1D time-series data, 1D convolution operations along
with multiple filters are applied within the 1D CNN.
For this experiment, one dimensional feature maps
and convolution filters are utilized that suit the raw
EEG properties. The more layers added to the CNN
through the incorporation of more convolutional
layers, the more progressive the disclosing of
sophisticated traits that are reliable and unique in
diagnosing epileptic seizures.
Figure 3: One-dimensional Filtering Process
3.3 LSTM Structure
The LST block consists of four gates: an InputGate-
zi controls the information that flows into the cell; a
ForgetGate-zf controls the amount of information
retained within the cell, the cell state gate z that saves
information over time, and the output gate zo, which
chooses how much information from the cell will be
used for output computation.
Figure 4 : Layout of the LSTM cell
Every gate consists of an activation function along
with a fully connected layer. In addition to this, the
LSTM block also contains three outputs: the Current
CellState (ct), the Current HiddenState (ht), and the
Current Output (yt), whereas there are also three
inputs involved, including the PreviousCellState (ct-
1), the PreviousHidden State (ht-1), and the
CurrentInput (xt). It is the hidden state which
produces the current output. The statistical expression
is given by
𝑧
= 𝜎𝑊
𝑥
,ℎ
{
}
𝑧^𝑖 = \𝑠𝑖𝑔𝑚𝑎(𝑊^𝑖 [𝑥_𝑡,ℎ_{𝑡− 1}])
𝑧 = \𝑡𝑎𝑛ℎ(𝑊[𝑥_𝑡,ℎ_{𝑡− 1}])
𝑧
= 𝜎𝑊
𝑥
,ℎ
{
}
𝑐^𝑡 = 𝑧^𝑓 \𝑡𝑖𝑚𝑒𝑠 𝑐^{𝑡− 1} + 𝑧^𝑖 \𝑡𝑖𝑚𝑒𝑠 𝑧
ℎ^𝑡 = 𝑧^0 \𝑡𝑖𝑚𝑒𝑠 \𝑡𝑎𝑛ℎ(𝑐^𝑡)
𝑦^𝑡 = \𝑠𝑖𝑔𝑚𝑎(𝑊^ℎ ℎ_𝑡)
3.4 CNN Combined with LSTM Model
in 1D
The advanced approach toward CNN combined with
LSTM 1-Dimensional model architecture consists of
an InputLayer, followed by four convolutional layers,
a PoolingLayer, two LSTM layers, four fully
connected layers, and a Softmax OutputLayer. Since
1-Dimensional EEG’s indication is in the form of
178x1, it can be supplied directly as the input data to
the model. The first convolutional layer, responsible
for extraction of features from the raw data, applies
64 1D convolutional kernels sized at 3x1 and strides
at 1Convolutional layer followed by a ReLU
ActivationLayer helps to begin the Non-Linearity
within the reprensentation. Definitions: numerical
precision of 1-Dimensional convolution and
mathematical definition of ReLU activation:
INCOFT 2025 - International Conference on Futuristic Technology
452
𝑦
=𝜎 𝑐𝑜𝑛𝑣
1𝐷𝑤
,
,𝑥
+𝑏
As a result of the 1-Dimensional convolution and
ReLU Activation, 64 176 x 1 feature maps are
generated. Then the output is passed to a layer called
1D max-pooling. This represents the whole
mathematical formula of the process of 1D max-
pooling.
4 EXPERIMENTAL RESULTS
Ninety percent of the data available in this experiment
was used to train the 1D convolutional LSTM, 1D
CNN, and DNN models. Ten percent remained as test
data. Dropout prevented overfitting during training
for 100 epochs. At random, scrambling happened
before feeding these models the data. The accuracy
for the final training and test data sets of every epoch
was calculated while evaluating the model's
generalization capacity and looking out for
overfitting. Finally, after 10 training cycles, if
generalization no longer increased, checkpoints were
created and the learning rate was changed. Major
Tasks for the Study This paper considered the
development of tasks concerning recognition of
seizure, first, in binary and then, as 5-class. While
five-class task required the identification of seizures
and normal situations, such as both opened eyes and
closed eyes, EEG activity from wholesome mental
state areas, and EEG’s motion from the malignancy
affected region, the binary work just required the
identification of seizures and normal conditions.
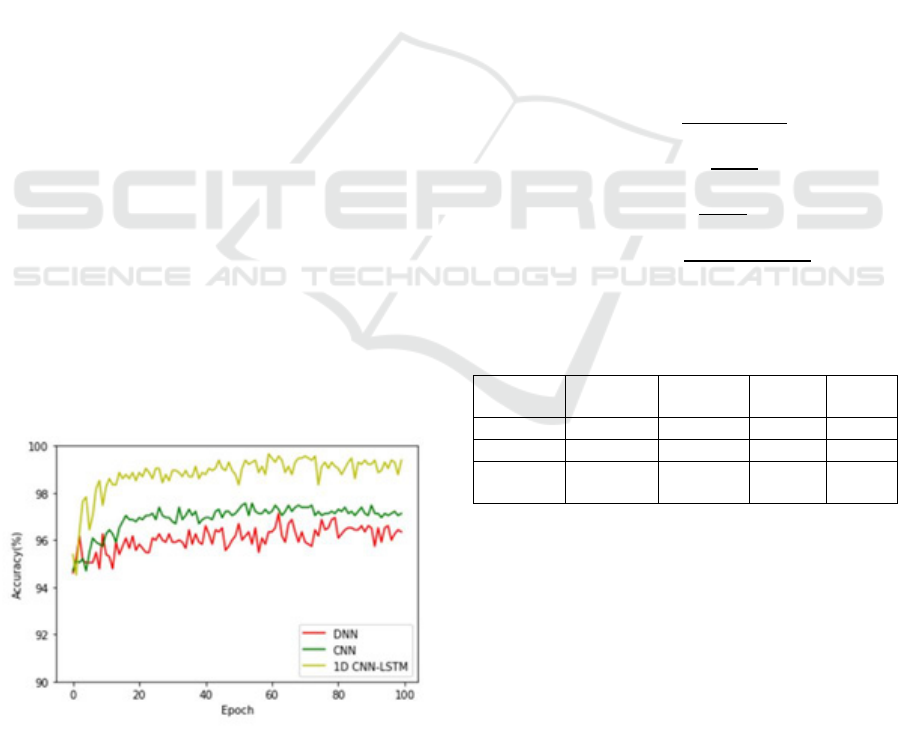
Figure 5 : Assessing each of these output of the models on
the Binary Recognition test
4.1 Binary Recognition Task:
Figure 5 of this chapter depicts the 1-Dimensional
CNN combined LSTM model's performance on the
task of Binary Recognition. This compared the results
produced by the proposed model to the yields of two
other deep learning models applied in the space of
utilization of epileptic seizure, namely DNN and
standard CNN. The figure clearly states that though
the DNN model converged the fastest, loss
parameters for training and testing the suggested
model were found to be less, hence increasing the
accuracy. The typical CNN performed similarly in
training compared with the proposed model, however
it performed much worse in testing. Since this model
achieved the best accuracy while testing to be done
across most of the training period, Figure 6 further
shows that the advanced 1-Dimensional CNN
combined LSTM Model outperforms the CNN and
then DNN Models. However, all three models are
compared in Table 1 and it is shown how the
suggested model is better in terms of seizure activity
validation.
Accuracy =
Precision =
Recall =
F1 Score = 2 ×
×
Table 1: The DNN, CNN, and the suggested 1-
Dimensional CNN combined with LSTM Model's relative
efficiency in handling binary classification problems
Methods Accuracy Precision Recall F1-
score
CNN 94.17% 93.34% 91.25% 0.9319
DNN 97.34% 92.17% 84.70% 0.9227
Proposed
Model
99.40% 98.40% 98.37% 0.9754
TN and FN state the number of seizures that arose
accurately classed and inaccurately classified,
respectively; and non-seizure activities that were not
classified as seizure activities are indicated by TN.
The count of any other sort of different kind of a
seizure task which is mistakenly labeled is given by
FP. The proposed LSTM and 1D CNN model
outperforms the normal CNN and DNN models since
it presents high F1-score, precision, recall, accuracy
values at 0.9754, 98.40%, 98.37%, and 99.40%,
respectively. Values outperform the regular CNN and
DNN models with increases in F1-score as 0.0435
Integrated EEG Signal Fusion for Advanced Epileptic Seizure Analysis
453
and 0.0527; precision: 5.06% and 6.23%; recall:
7.12% and 13.67%; as well as increases in accuracy:
5.23% and 2.06%
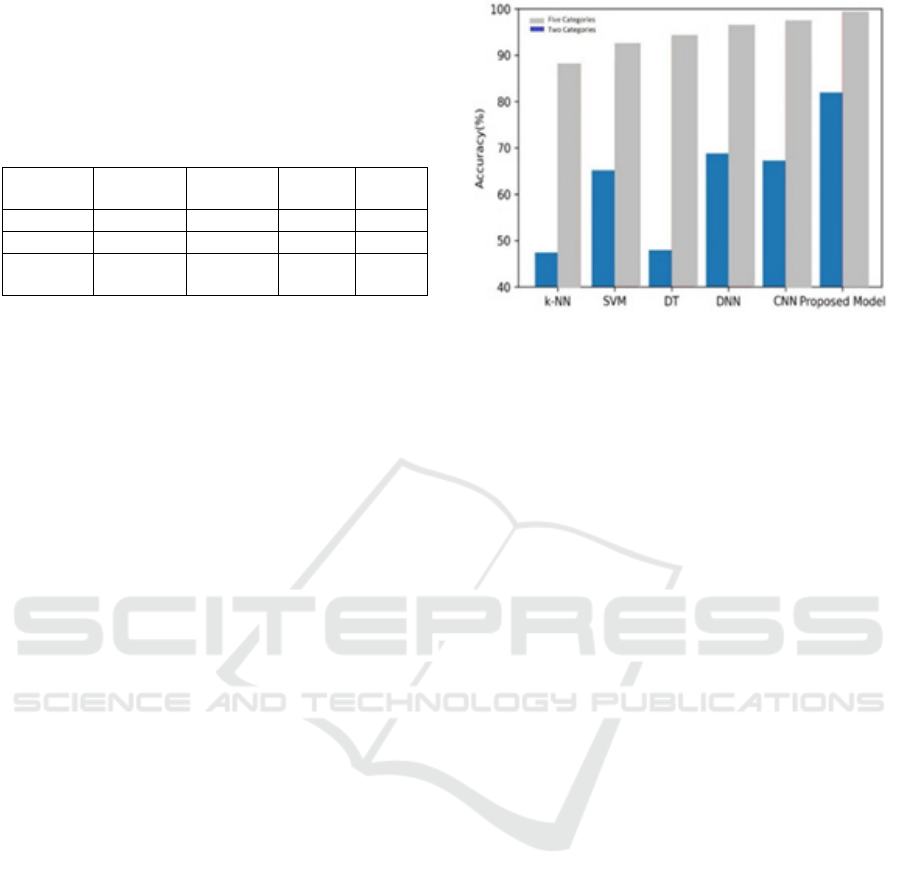
Table 2: The Five-Class classification task variation of
CNN, DNN, and the suggested 1-Dimensional CNN
combined with LSTM model.
Methods Accuracy Precision Recall F1-
score
CNN 64.40% 65.74% 66.77% 0.6775
DNN 67.74% 69.43% 67.57% 0.6641
Proposed
Model
81.00% 81.77% 82.70% 0.8174
4.2 FiveClass Recognition Task:
Figure 7 presents the results of the three models
applied to the FiveClass Identification operation, and
it is evident that the 1-Dimensional CNN combined
LSTM model exhibits the efficient identification of
variation compared to the DNN and CNN models.
This conclusion is further supported by Table 2,
which shows that the advanced approach outshines
the several two models in Accuracy, Precision,
Recall, and F1-score.
5 RESULT
Capturing the 1D Convolution Operation from the
raw EEG data tends to identify the seizure and non-
seizure circumstances by considering their temporal
characteristics. Normal conditions are represented by
four classes, where the waveform pattern of the EEG
is smoother and more periodic. The case of the
epileptic seizure condition is introduced as containing
strong spikes and anomalies. Long-term
dependencies in the time series data are followed by
making use of the LSTM block structure. Models
such as CNN, LSTM, and CNN-LSTM showed high
testing accuracies for the binary recognition
challenge of seizure versus non-seizure. CNN-LSTM
generally outperforms the others. On the other hand,
model performances were varied for the five-class
recognition challenge. In this case, 1D CNN-LSTM
seems to offer superior cross-class generalization.
The average accuracies for DT, DNN, CNN, CNN-
LSTM, and SVM, k-NN, and SVM, among others,
indicated that deep learning models—especially
CNN and CNN-LSTM—were drastically better than
a more traditional model such as k-NN and SVM.
Figure 6: The mean precisions of 1-Dimensional CNN
combined with LSTM, k-NN and DNN approaches
6 CONCLUSION
From this, the suggested model made use of an LSTM
network combined with a CNN for the analysis of
Epileptic Seizures by using the EEG’s indications.
The LSTM approach would classify the sequential
EEG signals that were recognized after the 1D CNN
had gotten the features out from the EEG data, thus
completing the whole end-to-end network. So, the
model was tested on the two different recognition
tasks, which include binary and five-class recognition
along with the UCI epileptic seizure recognition
dataset. It depicted excellent performances for both
five-class and binary recognition, where the five-class
recognition was showing an accuracy of 82.00%,
whereas binary recognition resulted in 99.39%. The
proposed model showed a key improvement in
accuracy compared to some other techniques
including DT, CNN, SVM, DNN and k-NN with the
help of 3.04%, 2.26%, 7.09%, 5.43%, and 5.35%
above the accuracy of the binary recognition
challenge, respectively.
REFERENCES
S. Abirami , Tikaram , M. Kathiravan , Rajamanickam
Yuvaraj , Ramshekhar N. Menon, John thomas , P. A.
karthick, A. amalin prince , and Jac Fredo Agastinose
Ronickom, “Automated Multi-Class Seizure-Type
Classification System Using EEG Signals and Machine
Learning Algorithms”, IEEE Access (Vol.12), Sept
2024, DOI: 10.1109/ACCESS.2024.3462772.
H. Albaqami, G. M. Hassan, and A. Datta, ‘‘MP-SeizNet:
A multipath CNN bi-LSTM network for seizure-type
classification using EEG,’’ Biomed. Signal Process.
INCOFT 2025 - International Conference on Futuristic Technology
454

Control, vol. 84, Jul. 2023, Art. no. 104780, DOI:
10.1016/j.bspc.2023.104780.
D. Wu, J. Li, F. Dong, J. Liu, L. Jiang, J. Cao, X. Wu, and
X. Zhang, ‘‘Classification of seizure types based on
multi-class specific bands common spatial pattern and
penalized ensemble model,’’ Biomed. Signal Process.
Control, vol. 79, Jan. 2023, Art. no. 104118, DOI:
10.1016/j.bspc.2022.104118.
S. Abirami, P. Swarubini, J. Thomas, R. Yuvraj, R. N.
Menon, and A. R. J. Fredo, ‘‘Multi-class seizure type
classification using features extracted from the EEG,’’
Healthcare Transformation Inform. Artif. Intell., vol.
305, pp. 68–71, Jun. 2023, DOI: 10.3233/SHTI230426.
Wenjuan Xiong; Ewan S. Nurse; Elisabeth Lambert, Mark
J. Cook, Tatiana Kameneva, “Classification of
Epileptic and Psychogenic Non-Epileptic Seizures
Using Electroencephalography and
Electrocardiography”, IEEE Transactions on Neural
Systems and Rehabilitation Engineering, Vol .31, June
2023, DOI: 10.1109/TNSRE.2023.3288138.
H. Alshaya and M. Hussain, ‘‘EEG-based classification of
epileptic seizure types using deep network model,’’
Mathematics, vol. 11, no. 10, p. 2286, May 2023, DOI:
10.3390/math11102286.
R. K. Joshi, V. M. Kumar, M. Agrawal, A. Rao, L. Mohan,
M. Jayachandra, and H. J. Pandya, ‘‘Spatiotemporal
analysis of interictal EEG for automated seizure
detection and classification,’’ Biomed. Signal Process.
Control, vol. 79, Jan. 2023, Art. no. 104086, DOI:
10.1016/j.bspc.2022.104086.
C. O. Adetunji, O. T. Olaniyan, O. Adeyomoye, A. Dare,
M. J. Adeniyi, and A. Enoch, ‘‘An intelligent diagnostic
approach for epileptic seizure detection and
classification using machine learning,’’ in Artificial
Intelligence for Neurological Disorders. New York,
NY, USA: Academic Press, 2023, pp. 225–243
DOI:10.1016/B978-0-323-90277-9.00011-0.
R. U. N. Neelappa and H. M. Harish, ‘‘Automatic diseases
detection and classification of EEG signal with
pervasive computing using machine learning,’’ Int. J.
Pervasive Comput. Commun., vol. 19, no. 3, pp. 432–
450, May 2023, DOI: 10.1108/IJPCC-09-2021-0216.
S. Poorani and P. Balasubramanie, ‘‘Deep learning based
epileptic seizure detection with EEG data,’’ Int. J. Syst.
Assurance Eng. Manage., vol. 2023, pp. 1–10, Jan.
2023.
X. Wang, C. Zhang, T. Kärkkäinen, Z. Chang, and F. Cong,
‘‘Channel increment strategy-based 1D. convolutional
neural networks for seizure prediction using
intracranial EEG,’’ IEEE Trans. Neural Syst. Rehabil.
Eng., vol. 31, pp. 316–325, 2023, DOI:
10.1109/TNSRE.2022.3222095.
X. Yan, D. Yang, Z. Lin, and B. Vucetic, “Significant low-
dimensional spectral-temporal features for seizure
detection,” IEEE Trans. Neural Syst. Rehabil. Eng.,
vol. 30, pp. 668–677, 2022, DOI:
10.1109/TNSRE.2022.3156931.
P. Boonyakitanont, A. Lek-Uthai, K. Chomtho, and J.
Songsiri, “A review of feature extraction and
performance evaluation in epileptic seizure detection
using EEG,” Biomed. Signal Process. Control, vol. 57,
pp. 1–28, 2020, DOI :10.1016/j.bspc.2019.101702.
M. Ma, Y. Cheng, Y. Wang, X. Li, Q. Mao, Z. Zhang, Z.
Chen, and Y. Zhou, ‘‘Early prediction of epileptic
seizure based on the BNLSTMCASA model,’’ IEEE
Access, vol. 9, pp. 79600–79610, 2021, DOI:
10.1109/ACCESS.2021.3084635.
Y. Zhang et al., “Epilepsy seizure prediction on EEG using
common spatial pattern and convolutional neural
network,” IEEE Journal of Biomedical and Health
Informatics, vol. 24, no. 2, pp. 465–474, Feb 2020,
DOI: 10.1109/JBHI.2019.2933046.
N. Ilakiyaselvan, A. N. Khan, and A. Shahina, “Deep
learning approach to detect seizure using reconstructed
phase space images,” J. Biomed. Res., vol. 34, no. 3,
pp. 240–250, 2020.
R. D. Thijs, R. Surges, T. J. O’Brien, and J. W. Sander,
‘‘Epilepsy in adults,’’ Lancet, vol. 393, no. 10172, pp.
689–701, Feb. 2019, DOI: 10.1016/s0140-
6736(18)32596-0.
I. R. D. Saputro, N. D. Maryati, S. R. Solihati, I. Wijayanto,
S. Hadiyoso, and R. Patmasari, ‘‘Seizure type
classification on EEG signal using support vector
machine,’’ J. Phys., Conf. Ser., vol. 1201, no. 1, May
2019, Art. no. 012065, DOI: 10.1088/1742-
6596/1201/1/012065.
Z. Jiang, F.-L. Chung, and S. Wang, ‘‘Recognition of
multiclass epileptic EEG signals based on knowledge
and label space inductive transfer,’’ IEEE Trans. Neural
Syst. Rehabil. Eng., vol. 27, no. 4, pp. 630–642, Apr.
2019, DOI: 10.1109/TNSRE.2019.2904708.
L. Wang, W. Xue, Y. Li, M. Luo, J. Huang, W. Cui, and C.
Huang, “Automatic epileptic seizure detection in EEG
signals using multidomain feature extraction and
nonlinear analysis,” Entropy, vol. 19, no. 6, p. 222,
2017.
S. Ryu and I. Joe, ‘‘A hybrid DenseNet-LSTM model for
epileptic seizure prediction,’’ Appl. Sci., vol. 11, no.
16, p. 7661, Aug. 2021, DOI: 10.3390/e19060222.
S. N. Baldassano et al., “Crowdsourcing seizure detection:
algorithm development and validation on human
implanted device recordings,” Brain, vol. 140, no. 6, pp.
1680–1691, 2017, DOI: 10.1093/brain/awx098.
A. K. Jaiswal and H. Banka, “Local pattern transformation
based feature extraction techniques for classification of
epileptic EEG signals,” Biomedical Signal Processing
and Control, vol. 34, pp. 81–92, 2017, DOI:
10.1016/j.bspc.2017.01.005.
Y. Zhang et al., “Epilepsy seizure prediction on EEG using
common spatial pattern and convolutional neural
network,” IEEE Journal of Biomedical and Health
Informatics, vol. 24, no. 2, pp. 465–474, Feb 2020,
DOI: 10.1109/JBHI.2019.2933046.
M. Sharma, R. B. Pachori, and U. R. Acharya, ‘‘A new
approach to characterize epileptic seizures using
analytic time-frequency flexible wavelet transform and
fractal dimension,’’ Pattern Recognit. Lett., vol. 94, pp.
172–179, Jul. 2017, DOI:
10.1016/j.patrec.2017.03.023.
Integrated EEG Signal Fusion for Advanced Epileptic Seizure Analysis
455

A. Theodorakopoulou, ‘‘Machine learning data preparation
for epileptic seizures prediction,’’ T.E.I. Crete, School
Eng. (STEF), PPS Inform. Multimedia, Crete, Greek,
Tech. Rep. 8374, 2017.
K. M. Tsiouris, V. C. Pezoulas, D. D. Koutsouris, M.
Zervakis, and D. I. Fotiadis, “Discrimination of preictal
and interictal brain states from long-term EEG data,” in
Proc. IEEE 30th Int. Symp. Comput.-Based Med. Syst.
(CBMS), Jun. 2017, pp. 318–323.
INCOFT 2025 - International Conference on Futuristic Technology
456
