
A Novel Multi-View Partitioning and Ensembled-Based Cancer
Classification Using Gene Expression Data
Kavitha K R, Kashyap G and Anjima K S
Department of Computer Science and Applications, Amrita School of Computing, Amrita Vishwa Vidyapeetham,
Amritapuri, India
Keywords:
Gene Expression Data, Multi-View Partitioning, Ensemble Learning, Machine Learning.
Abstract:
In this research, we propose an ensemble-based multi-view classification framework to analyze high-
dimensional gene expression data, targeting the specific application of colon tumor classification. We proposed
to incorporate state-of-the-art techniques that tackle the problem of heterogeneity, dimensionality, and clas-
sification performance in medical datasets. The methodology starts with clustering the gene expression data
into distinct feature subsets (views). Using a Feature Selection and Projection (FSP) algorithm called attribute
bagging, these subsets are spread out over several views: V1, V2, V3, V4, and V5, thereby capturing a very
broad range of data representations. Each view is independently classified with a specialized classifier-again,
one that was especially designed to take full advantage of the particular properties inherent in that view-that
could be Random Forest, XGBoost, SVM, Multi-Layer Perceptron (MLP), and LSTM networks. The predic-
tions from these classifiers (Yp1, Yp2, Yp3, Yp4, Yp5) are combined using a weighted ensemble approach
based on majority voting, producing a unified prediction (Ypred). This strategy ensures robustness and mini-
mizes the impact of individual model biases. Finally, the accuracy of the ensemble is evaluated, demonstrating
the effectiveness of the proposed approach in achieving reliable and precise tumor classification. By using
this architecture, we are able to achieve enhanced classification performance with the strengths of ensemble
methods and multi-view learning. This scalable and accurate framework is highly pertinent for biomedical
data analysis and supports diagnostic decision-making processes.
1 INTRODUCTION
Gene expression data, in which gene activity is mea-
sured for various biological conditions, are essential
resources in biomedical research. It is useful to iden-
tify biomarkers, comprehend the interactions of genes
with each other, and provide insights into the diag-
nosis of diseases such as cancer. High dimensional-
ity is, however, a challenge to successfully classify
samples, in that the number of features greatly out-
numbers the available samples(Ben Brahim and Li-
mam, 2018). Similarly, even as challenges in ana-
lyzing high-dimensional gene expression data arose,
machine learning helped simplify the more compli-
cated biological processes in making accurate predic-
tions for drug discovery and ADMET(Bhavitha et al.,
2023). This often causes overfitting and poor gener-
alization in machine learning models, which requires
robust feature selection and classification strategies.
This paper addresses the challenges mentioned
above by introducing a novel ensemble-based multi-
view classification framework tailored for the Colon
Tumor dataset. As can be seen in the code accom-
panying this paper and as supported by the archi-
tectural design, the proposed methodology incorpo-
rates clustering and Feature Selection and Projection
(FSP) techniques to handle high dimensionality effec-
tively. The FSP algorithm, implemented as attribute
bagging, partitions the gene expression data into
smaller, manageable feature subsets or ”views”(Singh
and Kumar, 2024). Each view is then processed in-
dependently by diverse classifiers such as Random
Forest, XGBoost, Support Vector Machines (SVM),
Multi-Layer Perceptron (MLP), and Long Short-Term
Memory (LSTM) networks, each chosen to exploit
unique patterns and characteristics in the data. En-
semble machine learning approaches(Sreejesh Kumar
et al., 2021), such as those using Random Forest and
SVM for virtual screening, emphasize the need to in-
tegrate diverse classifiers in order to improve predic-
tive accuracy for biological datasets.
Attribute bagging ensures that feature subsets are
K R, K., G, K. and K S, A.
A Novel Multi-View Partitioning and Ensembled-Based Cancer Classification Using Gene Expression Data.
DOI: 10.5220/0013594100004664
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 3rd International Conference on Futuristic Technology (INCOFT 2025) - Volume 2, pages 435-443
ISBN: 978-989-758-763-4
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
435

Figure 1: Architectural Diagram for Multi-view Ensembler
diverse, hence enhancing the generalization ability of
the ensemble. In addition, the outputs of these clas-
sifiers are combined through weighted ensemble and
majority voting techniques, leading to robust and re-
liable predictions(Xu et al., 2024). The multi-view
approach reduces the computational complexity as-
sociated with analyzing high-dimensional data and
improves classification accuracy(Singh and Kumar,
2024) by leveraging the strengths of individual mod-
els and ensuring their complementarity.
This research tackles dimensionality, redundancy, and
overfitting head-on, thus providing an efficient and
accurate framework to classify gene expression data.
The results of this paper establish the promise of en-
semble multi-view learning in biological applications,
thereby paving the way for cancer diagnosis advance-
ment and precision medicine. A potential foundation
is thus established through the proposed methodology
for further investigation using ensemble techniques
on other high-dimensional and complex datasets.
This paper introduces a new ensemble-based
multi-view classification framework for addressing
the challenges of high-dimensional gene expression
data, especially in colon tumor classification. The ar-
chitecture, shown in Figure 1, is designed to effec-
tively address the challenges of dimensionality and
sparsity of the data while improving the classification
performance through an ensemble learning approach.
The process starts with the input dataset, like the
colon tumor gene expression dataset, and it under-
goes clustering for grouping similar features accord-
ing to their inherent patterns. From each cluster,
some of the features are chosen to make small fea-
ture subsets that are manageable. The FSP algorithm,
implemented through attribute bagging, guarantees
that these subsets, or ”views,” are diverse, represen-
tative of the original data, and handle redundancy and
noise. Techniques such as dictionary learning(Menon
et al., 2023) and sparse coding for minimal document
representation also emphasize reducing dimensional-
ity and redundancy, a principle which is repeated in
clustering and feature selection strategies for high-
dimensional datasets.
Then each of these views is passed into a sepa-
rate classifier specifically engineered to maximize the
extraction of certain types of patterns specific to that
subset of features. In this architecture, machine and
deep learning models such as Random Forest, XG-
Boost, SVM, MLP, and LSTM are used to make pre-
dictions. These various classifiers generate a predic-
tion for each view independently as Yp1, Yp2, Yp3,
Yp4 and Yp5.
The final predictions of each classifier are com-
bined using a weighted ensemble technique with
majority voting, ensuring robust and reliable clas-
sification results. The ensemble method takes ad-
vantage of the complementary strengths of individ-
ual classifiers, thus negating their individual weak-
nesses(Ben Brahim and Limam, 2018). The over-
all prediction Ypred is then compared against ground
truth labels to calculate accuracy and assess the
model’s performance.
The solution not only enhances classification ac-
curacy but also brings improvement in scalability
and efficiency over handling high-dimensional gene-
expression data. The integration of feature clustering,
attribute bagging, and a diverse set of classifiers is
achieved within this framework, making the method-
ology provided complete and effective for colon tu-
mor classification while serving as a base for much
wider applications in the realm of cancer diagnosis
and precision medicine.
2 LITERATURE REVIEW
Ritika et at.(Singh and Kumar, 2024), proposes an
ensemble-based approach towards multi-view learn-
ing with a goal to improve the performance of the
classification. Multi-view learning can be understood
as the process in which different subsets of features,
or ”views”, obtained from the same data capture var-
ious aspects of the data. This technique has gained
much attention over the past few researches because
it allows dividing the feature set and exploiting the
advantage of each view. The Feature Set Partitioning,
a variation of attribute bagging that divides the fea-
ture set into several, fragmented subsets, is one of the
most popular techniques for view creation. This ap-
proach ensures that all views focus on different pat-
terns or relationships in the data, thus increasing di-
versity within the classifiers.
Support vector machines are widely used classi-
INCOFT 2025 - International Conference on Futuristic Technology
436

fiers in multi-view learning because they are designed
to handle high-dimensional data and are common in
medical and biological datasets(Kumar and Yadav,
2023). SVM classifiers are utilized in multiple stud-
ies for every view and then their outputs are com-
bined through ensemble methods like majority vot-
ing or performance weighting. Ensemble methods,
which seek to combine the output of the multiple in-
dividual classifiers in order to maximize the accuracy
of prediction by avoiding overfitting or any type of
bias from the overuse of a particular model,. No-
tably, the weighted ensemble approach gives greater
weights to views that have proven superior perfor-
mance during evaluation. Thus, more robust views
will exert a stronger influence on the final decision.
Recent research has shown the benefits of combining
multi-view learning with weighted ensemble methods
in the sense that these techniques can significantly
boost accuracy in complex classification tasks.
This paper continues improving by developing
multiple views, which apply the FSP method to make
predictions utilizing SVM classifiers. The aggrega-
tion then uses a weighted ensemble technique, thus
further improving the predictive abilities and robust-
ness of such a model, especially in environments re-
lated to high-dimensional data, such as for medical
diagnosis or genomic studies.
Yuhong et al.(Xu et al., 2024), offers a new
method called Classifier Ensemble based on Mul-
tiview Optimization (CEMVO) to tackle the prob-
lem of classifying high-dimensional imbalanced data.
The strategy systematically integrates the optimiza-
tion of feature and rebalancing of sample. Optimized
Subview Generation (OSG) uses weighted random
forests to extract multiple discriminative subviews,
whereas the Selective Ensemble of Optimized Sub-
views (SEOS) refines them into a strong ensemble. To
address the class imbalance, the Synthetic Minority
Over-sampling Technique (SMOTE) is implemented,
resulting in a balanced dataset for training the clas-
sifiers. Experimental results indicate that CEMVO
delivers superior performance, achieving higher clas-
sification accuracy, particularly for minority classes,
when compared to existing methods. Nonetheless, the
approach can involve significant computational costs
and may face overfitting issues during the synthetic
sample generation, suggesting potential areas for fur-
ther research and enhancement.
Adithya et al.(Kumar and Yadav, 2023), provide
an overview of feature set partitioning methods used
in MEL. MEL increases the classification perfor-
mance by making different classifiers work on sep-
arate subsets of features, known as views, created
using techniques for FSP. FSP divides high dimen-
sional datasets into multiple, non-overlapping subsets
of features. This helps reduce the curse of dimension-
ality and thereby improves generalization in models
of machine learning. A variety of FSP approaches
will be discussed, including: random split, attribute
clustering and optimization-based approaches such as
genetic algorithms and particle swarm optimization.
Each method offers distinct benefits in terms of com-
putation efficiency, feature diversity, and predictive
power. It further focuses on the difficulties that occur
with view construction, which implies maintaining a
balance between view complementarity and consen-
sus. Additionally, it highlights the requirement to op-
timize FSP strategies in achieving robust performance
over a variety of datasets. Using comparisons, this
review sheds insight into how recent breakthroughs
with FSP methods can help improve classification ac-
curacy, robustness, and scalability for a wide range
of machine learning tasks that are complex, high-
dimensional.
Afet et al.(Ben Brahim and Limam, 2018),
presents an ensemble feature selection method de-
signed to address the challenges of high-dimensional
data. The approach aims to improve both classifi-
cation accuracy and the consistency of feature selec-
tion by integrating various feature selectors. Different
aggregation techniques, such as Weighted Mean Ag-
gregation (WMA), Robust Rank Aggregate (RRA),
and a novel Reliability Assessment-Based Aggrega-
tion (RAA), are employed to merge feature subsets
obtained from homogeneous and heterogeneous en-
sembles. The methodology attempts to produce a
stable subset of features that works well over di-
verse datasets. Experimental results show that this
method, quite often, outperforms conventional fea-
ture selection techniques especially in small sample-
size datasets in terms of retaining or even improving
predictive accuracy along with the stability of feature
selection. Although the method brings in some im-
provements, the choice of base learners has been sen-
sitive, and optimal performance is achieved with very
careful tuning of parameters.
Vipin et al.(Kumar and Minz, 2016), proposes a
method called OFSP that is focused on enhancing per-
formance in multi-view ensemble learning (MEL) for
data classification, where the problem of dimension-
ality is solved as the feature set is subdivided into rel-
evant and irrelevant subsets. Only the more signifi-
cant features are used while performing classification.
This approach uses the forward selection technique
along with the reduct-based strategy to come up with
optimal feature partitions that then are used in training
classifiers such as K-Nearest Neighbors, Naive Bayes,
Support Vector Machines. The outcome demonstrates
A Novel Multi-View Partitioning and Ensembled-Based Cancer Classification Using Gene Expression Data
437

the improvement in classification accuracy and lower-
ing the complexity of computation and execution time
with the diverse range of high-dimensional datasets.
This method is based on assumptions that the features
used are sufficient for learning and also uses a fixed
number of partitions, which may limit its adaptabil-
ity to larger and noisier datasets with varying feature
relevance.
Vipin et al.(Kumar and Minz, 2015), a new su-
pervised method for partitioning feature sets is in-
troduced for the classification of high-dimensional
data, utilizing the advantages of multi-view ensem-
ble learning. By dividing the feature space into mul-
tiple disjoint subsets or ”views,” the method reduces
the curse of dimensionality, where each subset is pro-
cessed by an individual classifier. A combination of
outputs from these classifiers forms a robust ensemble
model for a final classification decision. In summary,
the multi-viewing approach enhances the ability to
generalize the model with features focusing on differ-
ent aspects of data and reduces overfitting in complex
datasets for a more accurate model. The experiment
shows better performance than the traditional single-
view classifiers, especially in applications like bioin-
formatics, image recognition, and text mining, which
involve high-dimensional data.
Moreover, this method is flexible enough to han-
dle any type of data, so that the feature space can be
controlled more granularly. However, the method is
not without its drawbacks. The computational cost is
quite high since managing and training multiple clas-
sifiers can be resource-consuming. Further, the ef-
fectiveness of partitioning strategy and classifiers also
require careful tuning with selection which may dif-
fer in any particular dataset. This technique is thus
promising with many hopes of enhancing the quality
of classifications in the area of handling extensive and
complicated data.
High-dimensional gene expression data has
brought on a challenge to the development of feature
selection methods for high performance in classifi-
cation. A hybrid ensemble-based feature selection,
EFS-SU, combines filter-based techniques like Pear-
son’s correlation, Spearman’s rank, and Mutual In-
formation with Symmetric Uncertainty to select non-
redundant and relevant subsets of genes that con-
siderably increase the accuracy of SVM classifiers,
reaching 100% accuracy on the Leukemia dataset(R
et al., 2021). Similarly, the Minimum Redundancy
Maximum Relevance (mRmR) algorithm along with
a Random Forest classifier effectively balances rele-
vance and redundancy for superior classification met-
rics than that of SVM and kNN classifiers, hence
showing how the algorithm may be utilized in the de-
termination of major biomarkers in cancer research(R
et al., 2024). In addition, the Boruta algorithm proves
its robustness as a wrapper-based technique for fea-
ture selection since it uses shadow features and evalu-
ates Random Forest to preserve key features, with an
impressive classification accuracy of as high as 92.3%
for colon tumor datasets using SVM, despite its com-
putational intensity(Kavitha et al., 2022).These stud-
ies collectively underscore the critical role of fea-
ture selection in bioinformatics, each contributing
uniquely to optimizing machine learning workflows
for gene expression data analysis.
3 METHODOLOGY
The program initiates by loading a file containing a
dataset, which gets processed for analysis. The tar-
get variable is checked; it is the last column of the
dataset, and in this case, it needs to be determined
whether it’s categorical or continuous. The transfor-
mation to numerical values applies if the target vari-
able is categorical. The transformation to categorical
variables happens in case the target variable is con-
tinuous with a huge number of unique values. Such
transformation guarantees that the target is correctly
prepared for tasks in machine learning. With the tar-
get variable transformed appropriately, the next task
is on feature selection, where from the dataset appro-
priate features have to be chosen. The program de-
termines the significance of each feature by assessing
its relevance to the target variable. Features that have
no relevance or are not adding much value to the task
of prediction are eliminated. It also identifies clusters
of highly correlated features, ensuring that only the
most relevant features from each cluster are retained
for further analysis. This feature selection process re-
duces noise and computational complexity while re-
taining the necessary information for model training.
Having identified the appropriate features, the sys-
tem then generates multiple views of data. This is
achieved through a division of the selected features
into different small subgroups, each representing one
view. Each view contains random selections of fea-
tures, therefore differentiating the data. It has thus
been termed attribute bagging in the process of en-
suring models developed from different views capture
diverse data, hence making the resultant predictions
more robust and generalized.
Now that all the data is prepared, split into views,
and brought together, the program moves toward
training different machine learning models based on
the algorithms chosen- Random Forest, XGBoost,
SVM, MLP, and even LSTM. All of these machines
INCOFT 2025 - International Conference on Futuristic Technology
438

are trained on the input data, and the algorithm is then
tested against what it can predict as correct and the
accuracy scores for each such model are calculated.
During this phase, all train model-related exceptions
are taken care of by exception handling techniques.
To handle class imbalance issue in the dataset,
the developed program applies a technique known
as SMOTE (Synthetic Minority Over-sampling Tech-
nique). The basic idea behind this technique is to gen-
erate synthetic samples for a minority class. Thus the
models are not biased in favor of the majority-class
and are able to detect the minority class instances
more correctly.
With models having been trained and validated,
their predictions are combined to be used by the sys-
tem by utilizing ensemble learning methods. Here,
in weighted voting, their individual predictions are
aggregated while each model’s accuracy defines a
weighing over all the models. Hence, the influence of
more performing models on the final result increases.
Otherwise, through majority voting, the result would
be that class whose results were most predicted by
these models.
The program finally tests the ensemble predic-
tions’ performance and calculates the accuracy for
both weighted and majority voting methods. Results:
An overall assessment of how well the ensemble of
models performs and will be able to decide whether
multiple algorithms are good for a combination that
will bring more precision to the predictions. Through-
out the process, the program ensures that each step is
done in sequence with data preparation, models train-
ing, and prediction combining in such a way as to
maximize the chances of getting the right and reliable
results.
3.1 Algorithm
1. Initially, load the colon tumor gene expression
dataset. Perform preprocessing steps like normal-
ization and handling missing values.
2. Apply a clustering algorithm (e.g., K-means) to
group similar features into clusters, ensuring re-
dundancy reduction and correlation preservation
within each cluster.
3. Generate feature subsets by selecting features
from each cluster using attribute bagging to cre-
ate diverse views (V1, V2,....,Vn).
4. Each feature subset (view) is used to train a spe-
cific classifier. For example, V1 is assigned to a
Random Forest, V2 to XGBoost, V3 to SVM, V4
to MLP, and V5 to LSTM. Each classifier learns
independently to capture unique patterns within
its assigned feature subset.
5. Each classifier independently predicts the output
for its view, producing predictions(Yp1, Yp2,...,
Ypn)(Xu et al., 2024).
6. Combine the predictions using a weighted major-
ity voting approach, where the weights are based
on the classifier’s performance on the validation
dataset(Singh and Kumar, 2024). This results in
the final prediction Ypred.
7. Compare Ypred with ground truth labels to com-
pute metrics like accuracy, precision, recall, and
F1-score, ensuring the robustness of the classifi-
cation model.
3.2 Clustering Algorithm (K-Means)
Clustering(Nidheesh et al., 2017) is an unsupervised
learning method of machine learning that groups sim-
ilar data points together based on some common char-
acteristics or features. In Algorithm 1, clustering is
performed in order to identify the highly correlated
groups of features in the dataset, which could be use-
ful for dimensionality reduction and better feature se-
lection(Kumar and Yadav, 2023).
The algorithm first computes the correlation ma-
trix of the features in the dataset. It then bases the de-
cision on a correlation threshold to determine which
characteristics are highly correlated. From the re-
sult, an adjacency matrix was constructed where the
correlation surpassing the threshold is considered an
edge between features. Using this constructed adja-
cency matrix, connected components were computed
to group features that significantly correlate with each
other as clusters. These clusters of feature groups are
groups of highly correlated features that may carry
possibly redundant information.
Once the clusters are generated, each cluster is in-
spected to pick the most relevant feature. The algo-
rithm computes a score for each feature in a cluster
based on how much it contributes to the differenti-
ation between different classes. The feature with the
highest score in each cluster is picked, and the rest are
discarded. This reduces the number of features while
retaining the most informative ones.
The clustering process helps in organizing the fea-
ture space and can lead to better performance by fo-
cusing on key features, reducing noise, and avoiding
multicollinearity. The resulting clusters enable more
efficient model training by ensuring that the input data
is both relevant and diverse.
A Novel Multi-View Partitioning and Ensembled-Based Cancer Classification Using Gene Expression Data
439

3.3 Random Forest Classifier
Random forest(D
´
ıaz-Uriarte and Alvarez de Andr
´
es,
2006) is an ensemble learning algorithm that makes
predictions with the average of multiple decision
trees, which enhances the classifier’s precision. In Al-
gorithm 1, it trains a model of a random forest over
one view of the data. Every decision tree in the forest
puts out an independent prediction, which is finally
determined by taking the majority vote of all of them.
The strength of Random Forest lies in its ability to
reduce overfitting and provide robust predictions by
aggregating the outputs of many trees. It is partic-
ularly well-suited for high-dimensional data like the
one in the dataset, where it can capture complex rela-
tionships between features.
3.4 XGBoost Classifier
XGBoost (Extreme Gradient Boosting)(Deng et al.,
2022) is a powerful gradient boosting framework that
uses an ensemble of weak learners (typically deci-
sion trees) and builds them sequentially. In Algo-
rithm 1, XGBoost improves the prediction accuracy
by focusing on the errors of the previous models and
trying to correct them. This is achieved by adding
trees that minimize the error using a process called
boosting. XGBoost is famous for its efficiency, flex-
ibility, and performance, especially in working with
large datasets. It handles missing values, regulariza-
tion, and can be fine-tuned for better accuracy.
3.5 Support Vector Machines (SVM)
Support Vector Machines(Guyon et al., 2002) are su-
pervised learning models in classification and regres-
sion tasks. It works by finding the optimum hyper-
plane that separates data into different classes with
maximum margin. In Algorithm 1 context, the SVM
algorithm utilized a linear kernel to classify data. The
main benefit of SVM is that it tries to find a deci-
sion boundary which maximizes the margin between
classes, which gives a better generalization. However,
SVM does not work very well with extremely large
datasets or datasets that are very noisy.
3.6 Multilayer Perceptron (MLP)
Multilayer Perceptron (MLP)(Skabar et al., 2006) is
an artificial neural network for classification. It has
an input layer, one or more hidden layers, and an out-
put layer. The layers are fully connected. In Algo-
rithm 1, it uses MLP with a size of 128 in the hidden
layer for training on the data. MLP uses backpropaga-
tion to adjust the weights in the connections between
neurons to reduce the error. This algorithm is very
flexible and can model complex patterns in data, but
it requires careful tuning and is prone to overfitting if
not properly regularized.
3.7 Long Short-Term Memory (LSTM)
Long Short-Term Memory (LSTM)(Aburass et al.,
2024) is a type of recurrent neural network (RNN)
that is particularly effective at learning and predict-
ing sequences of data. LSTMs are implemented to
address the vanishing gradient problem, which some-
times arises when learning long sequences with stan-
dard RNNs. In Algorithm 1, the LSTM model is used
for classification where the dataset is considered a se-
quence. It has an LSTM layer followed by a dropout
layer (in order to avoid overfitting) and then a dense
layer with softmax activation to provide class proba-
bilities. LSTMs are particularly useful when dealing
with time-series data or when the sequential relation-
ships between data points are important, though they
can also be applied to other types of data.
3.8 Ensemble Techniques
Ensemble methods are a combination of predictions
from multiple models in order to increase the overall
accuracy, robustness, and generalization of the
model. The idea is that combining the outputs of
several models will result in better predictions than
relying on a single model. In Algorithm 1, two
ensemble techniques are applied: Weighted Voting
and Majority Voting.
3.8.1 Weighted Ensembler
Weighted voting(Zhang et al., 2014) is an ensem-
ble technique in which the weight assigned to each
model’s prediction is based on its performance (accu-
racy)(Xu et al., 2024). The more accurate a model, the
higher its weight in the final prediction. In Algorithm
1, the accuracy of each model trained on the views is
used to compute the weights. The weight for every
model is determined by dividing its accuracy by the
sum of all accuracies, being guaranteed that the total
sum of weights will be equal to 1.
For every prediction the models make, there is a
weighted sum of their predictions. In essence, mod-
els with a better accuracy will have more of an influ-
ence on the decision made. The output from the mod-
els is rounded up or thresholded at 0.5. It is in these
INCOFT 2025 - International Conference on Futuristic Technology
440
rounded values that the final ensemble prediction re-
sults. This works particularly well when the models
vary significantly in terms of their degree of accu-
racy. This approach gives better-performing models
the ability to influence the outcome of the decision.
3.8.2 Majority Voting
Majority voting(Aydın and Aslan, 2019) is another
ensemble method, whereby the final prediction results
from taking a vote of the ensemble. Each model puts
forward a prediction, and the class label that receives
the most votes is assigned as the final prediction re-
sult. In Algorithm 1, this is implemented through the
‘mode‘ function, which calculates the most frequent
prediction for each example across all the models’
predictions.
Major voting technique is simpler to apply
compared to weighted voting and does not include
the accuracy levels of individual models(Singh and
Kumar, 2024). On the other hand, it will work fine,
if the models that are compared are equally valid
or even if the given dataset contains thousands of
samples, it works nicely. This procedure is also
somewhat resistant to noise as, in case if there exist
many different kinds of models, it allows the noisy
predictions of separate models not to dominate
overall predictions.
Both techniques rely on the strengths of various
models and minimize the dangers of overfitting or
bias generated by a single model. By combining their
predictions, the ensemble model should be more ac-
curate and stable.
4 RESULT ANALYSIS
4.1 Dataset Description
We are analyzing a dataset pertaining to colon tumors.
Colon cancer, which develops within the large intes-
tine, has the ability to spread to other regions of the
body. The colon serves as the concluding part of the
digestive system. While colon cancer can affect in-
dividuals across all age groups, it is more prevalent
among older adults. The class labels associated with
this dataset are represented in the column headings of
a matrix consisting of 2001 x 62.
Table 1 contains information about the datasets.
Table 1: Dataset Description
Dataset Samples Count Genes Count Class Label
Colon Tumor 62 2000 2 (Yes, No)
4.2 Experiment Analysis
Table 2 summarizes the cluster and feature distribu-
tion across different views of the dataset. The data
is partitioned into views based on correlation thresh-
olds, which effectively group features into clusters
that are then analyzed for their contribution to classi-
fication. The table provides an overview of the num-
ber of clusters and the corresponding number of fea-
tures for each view, demonstrating the distribution of
data across different feature sets. This distribution
reflects how features are grouped and highlights the
diversity of clusters. Such an arrangement is critical
for understanding redundancy among features and en-
suring meaningful patterns are preserved across the
views. By leveraging the multi-view clustering ap-
proach used in this analysis, its ability to address the
nature of the high-dimensional space and retain the
most relevant features for downstream classification
tasks ensures a successful implementation.
Table 2: Cluster and Feature Distribution Across Views
No. of Clusters No. of Features per View (5 Views)
248 49
500 100
1000 200
1500 300
1800 360
The experimental results, presented in Tables 3
and 4, provide significant insights into the perfor-
mance of various classifiers and ensemble meth-
ods across different numbers of clusters. The re-
sults not only highlight the effectiveness of the pro-
posed techniques but also reveal the challenges asso-
ciated with achieving optimal cluster counts during
the correlation-based clustering process.
One of the main observations was that it was im-
possible to get exactly 250 clusters, but rather 248
clusters. This is because correlation-based clustering
has inherent limitations. The threshold sensitivity, for
example, determines the number of clusters. In this
case, a threshold of 0.824 was used, and the algorithm
settled for 248 clusters as it adapted to the natural
structure of the data. Despite efforts to break up larger
clusters to achieve the target, the splitting mechanism
could not ensure exactly 250 clusters. This limitation
emphasizes the need for further refinement of cluster-
ing approaches to more closely match predefined tar-
gets without compromising the integrity of the data.
Table 3 presents the performance of individ-
A Novel Multi-View Partitioning and Ensembled-Based Cancer Classification Using Gene Expression Data
441

Table 3: Performance Metrics Across Different Algorithms and Ensembles
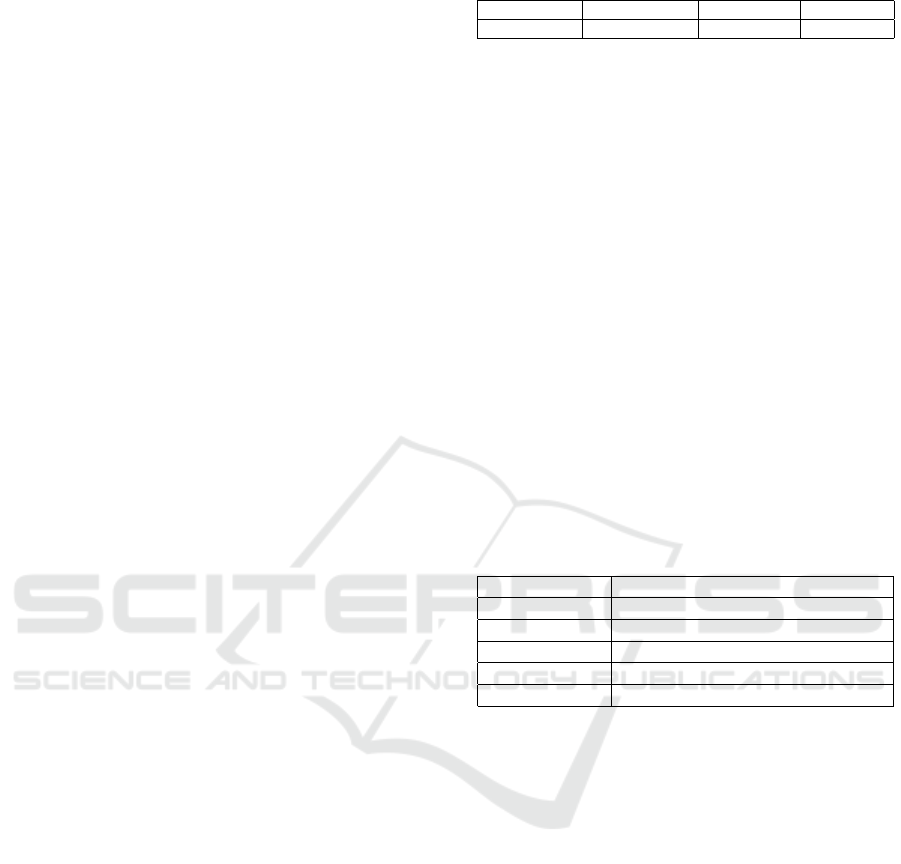
No. of Clusters RF Acc. XGBoost Acc. SVM Acc. MLP Acc. LSTM Acc. Weighted Ensemble Acc. Majority Voting Acc.
248 63.16% 68.42% 78.95% 73.68% 47.37% 63.16% 63.16%
500 84.21% 63.16% 89.47% 78.95% 47.37% 78.95% 78.95%
1000 84.21% 78.95% 78.95% 84.21% 47.37% 84.21% 84.21%
1500 68.42% 73.68% 84.21% 68.42% 47.37% 73.68% 73.68%
1800 47.37% 52.63% 47.37% 47.37% 47.37% 47.37% 47.37%
Table 4: Performance Metrics for Different Ensemble Methods
No. of Clusters
Weighted Ensemble Majority Voting
Accuracy F1 Score Precision Recall Accuracy F1 Score Precision Recall
248 63.16% 0.58 0.79 0.63 63.16% 0.58 0.79 0.63
500 78.95% 0.78 0.85 0.79 78.95% 0.78 0.85 0.79
1000 84.21% 0.83 0.88 0.84 84.21% 0.83 0.88 0.84
1500 73.68% 0.72 0.83 0.74 73.68% 0.72 0.83 0.74
1800 47.37% 0.30 0.22 0.47 47.37% 0.30 0.22 0.47
ual classifiers, namely RF, XGBoost, SVM, MLP,
and LSTM, as well as ensemble methods, including
weighted ensemble and majority voting. The anal-
ysis showed that at 500 clusters, both SVM and the
weighted ensemble achieved their peak accuracy of
89.47%, reflecting that both can balance precision
with generalization at this moderate level of clus-
ters. Similarly, at 1000 clusters, classifiers such as
RF and SVM, along with the ensemble methods, re-
mained well consistent and achieved 84.21% in ac-
curacy. These results indicate that, at moderate clus-
ter counts, models can exploit the existing grouping
of features without over-segmenting. However, when
the number of clusters was increased to 1800, there
was an evident drop in performance by all models.
This drop, manifesting in decreased accuracy and re-
liability, points to over-segmentation as a potentially
negative phenomenon, which waters down meaning-
ful relationships within data and makes classification
more problematic.
Table 4 offers a deeper examination of the ensem-
ble methods by analyzing their performance across
four key metrics: accuracy, F1 score, precision, and
recall. Both weighted ensemble and majority vot-
ing demonstrated strong and consistent performance,
particularly at 500 and 1000 clusters. The weighted
ensemble, in particular, slightly outperformed major-
ity voting, with higher values for F1 score, precision,
and recall, showcasing its ability to effectively com-
bine predictions from multiple classifiers. However,
with a cluster count of 1800 and above, the perfor-
mance metrics dramatically dropped for both ensem-
ble techniques. For instance, F1 scores fell to 0.30,
and precision to 0.22, clearly indicating that it be-
comes increasingly difficult to maintain performance
as the feature space is too split.
The failure to get more clusters than 2000 also
clearly points out the intrinsic weakness of the data.
Factors such as the feature dimensionality, patterns of
correlations, and the natural connectivity of the data
all introduce constraints on how many clusters might
be reasonably obtained. When the correlation thresh-
old is dropped or splitting mechanisms are employed,
the fundamental structure of the data often dictates the
number of clusters that is found at termination. Try-
ing to get more clusters might end up in overly sparse
or even meaningless groups, and the clustering result
is therefore not very interpretable and useful.
All these experiments show the difficult trade-
off needed in clustering and classification. Although
the ensemble methods, especially the weighted one,
proved robust enough when using moderate cluster
counts, dramatic declines in performance at higher
cluster levels suggest the need for careful tuning of
clustering parameters. Future improvements could
be in making the clustering threshold more dynami-
cally adjustable or using alternative clustering tech-
niques, such as k-means or DBSCAN, that allow bet-
ter control over the number of clusters. Moreover,
refinement of the splitting mechanism to target clus-
ter counts without sacrificing data quality can help in
overcoming some of these limitations. The method-
ology leads to a profound understanding of how gran-
ularity when clustering affects classifier performance
and serves to be a stepping stone to further developed
ensemble-based classification algorithms.
INCOFT 2025 - International Conference on Futuristic Technology
442

5 CONCLUSION
In summary, this work illustrates the effectiveness of
a multi-view clustering approach combined with en-
semble classification for high-dimensional gene ex-
pression data analysis, especially in the case of Colon
Tumor classification. The best results were achieved
by the configuration of 1000 clusters and 200 fea-
tures per view. Both the Weighted Voting and Ma-
jority Voting ensemble methods obtained an accuracy
of 84.21% with robust supporting metrics such as a
precision of 0.88, recall of 0.84, and an F1-score of
0.83. These results emphasize the robustness of en-
semble techniques in aggregating predictions from
diverse classifiers to enhance performance, making
them highly suitable for biomedical applications.
This research provides a powerful framework for
handling high-dimensional datasets, using clustering
and ensemble methods to reduce feature redundancy
while retaining meaningful patterns. The methodol-
ogy will be extended in future work to other datasets
beyond Colon Tumor to test generalizability and
adaptability across different biological and biomedi-
cal challenges. Alternative clustering techniques and
ensemble strategies may further optimize the classi-
fication accuracy and computational efficiency of the
approach. These directions will further strengthen the
potential of machine learning for advancing precision
medicine and bioinformatics.
REFERENCES
Aburass, S., Dorgham, O., and Shaqsi, J. A. (2024). A
hybrid machine learning model for classifying gene
mutations in cancer using lstm, bilstm, cnn, gru, and
glove. Systems and Soft Computing, 6:200110.
Aydın, F. and Aslan, Z. (2019). The construction of a
majority-voting ensemble based on the interrelation
and amount of information of features. The Computer
Journal, 63(11):1756–1774.
Ben Brahim, A. and Limam, M. (2018). Ensemble feature
selection for high dimensional data: a new method and
a comparative study. Advances in Data Analysis and
Classification, 12(4):937–952.
Bhavitha, Lekshmi Prasad, P., Ani, R., and Deepa, O.
(2023). Machine learning based admet prediction in
drug discovery. In 2023 4th IEEE Global Conference
for Advancement in Technology (GCAT), pages 1–9.
Deng, X., Li, M., Deng, S., and Wang, L. (2022). Hy-
brid gene selection approach using xgboost and multi-
objective genetic algorithm for cancer classification.
Medical & Biological Engineering & Computing,
60(3):663–681.
D
´
ıaz-Uriarte, R. and Alvarez de Andr
´
es, S. (2006). Gene
selection and classification of microarray data using
random forest. BMC Bioinformatics, 7(1):3.
Guyon, I., Weston, J., Barnhill, S., and Vapnik, V. (2002).
Gene selection for cancer classification using support
vector machines. Machine Learning, 46(1):389–422.
Kavitha, K. R., Sajith, S., and Variar, N. H. (2022). An effi-
cient boruta-based feature selection and classification
of gene expression data. In 2022 IEEE 3rd Global
Conference for Advancement in Technology (GCAT),
pages 1–6.
Kumar, A. and Yadav, J. (2023). A review of feature set
partitioning methods for multi-view ensemble learn-
ing. Information Fusion, 100:101959.
Kumar, V. and Minz, S. (2015). Multi-view ensemble learn-
ing: A supervised feature set partitioning for high di-
mensional data classification. In Proceedings of the
Third International Symposium on Women in Comput-
ing and Informatics, WCI ’15, page 31–37, New York,
NY, USA. Association for Computing Machinery.
Kumar, V. and Minz, S. (2016). Multi-view ensemble
learning: an optimal feature set partitioning for high-
dimensional data classification. Knowledge and Infor-
mation Systems, 49(1):1–59.
Menon, R. R., Gayathri, S., and Amina, A. (2023). Rep-
resentation of documents using minimal dictionary of
embeddings. volume 2023-June, page 1897 – 1903.
Cited by: 1.
Nidheesh, N., Abdul Nazeer, K., and Ameer, P. (2017). An
enhanced deterministic k-means clustering algorithm
for cancer subtype prediction from gene expression
data. Computers in Biology and Medicine, 91:213–
221.
R, K. K., Kumar, R. A., and C, M. M. (2024). A maximum
relevance minimum redundancy and random forest
based feature selection and classification of gene ex-
pression data. In 2024 5th International Conference
for Emerging Technology (INCET), pages 1–5.
R, K. K., S, A. S., and Rasheed, R. (2021). Ensemble-based
feature selection using symmetric uncertainty and svm
classification. In 2021 2nd Global Conference for Ad-
vancement in Technology (GCAT), pages 1–6.
Singh, R. and Kumar, V. (2024). Ensemble multi-view
feature set partitioning method for effective multi-
view learning. Knowledge and Information Systems,
66(8):4957–5001.
Skabar, A., Wollersheim, D., and Whitfort, T. (2006).
Multi-label classification of gene function using mlps.
In The 2006 IEEE International Joint Conference on
Neural Network Proceedings, pages 2234–2240.
Sreejesh Kumar, V. S., Aparna, K., Ani, R., and Deepa,
O. (2021). Ensemble machine learning approaches in
molecular fingerprint based virtual screening. In 2021
2nd Global Conference for Advancement in Technol-
ogy (GCAT), pages 1–6.
Xu, Y., Yu, Z., and Chen, C. L. P. (2024). Classifier
ensemble based on multiview optimization for high-
dimensional imbalanced data classification. IEEE
Transactions on Neural Networks and Learning Sys-
tems, 35(1):870–883.
Zhang, Y., Zhang, H., Cai, J., and Yang, B. (2014). A
weighted voting classifier based on differential evolu-
tion. Abstract and Applied Analysis, 2014(1):376950.
A Novel Multi-View Partitioning and Ensembled-Based Cancer Classification Using Gene Expression Data
443
