
Automated Alzheimer’s Disease Diagnosis
K. Saraswathi, N. T. Renukadevi, Harita T, Neha Parveen N and Vibinikha E M
Department of Computer Technology-UG, Kongu Engineering College, Perundurai, Erode, India
Keywords: Random Forest, KNN, Decision Tree, Gradient Boosting, Neural Networks, CatBoost.
Abstract: Alzheimer's disease (AD) is a progressive neurodegenerative disorder characterized by cognitive decline and
the accumulation of beta-amyloid plaques and tau protein tangles in the brain, leading to neuronal dysfunction
and eventual cell death. This research aims to develop a computer-aided diagnostic system utilizing machine
learning algorithms to analyze clinical data and identify indicators of Alzheimer’s disease. The project
involves data collection, algorithm development, and rigorous testing to ensure accuracy and reliability.
Algorithms such as K Nearest Neighbor, Neural Network, Gradient Boosting Machine, Decision Tree,
Random Forest, and CatBoost are employed to enhance diagnostic precision. Hyperparameter tuning and an
ensemble model with a Voting Classifier will further improve accuracy. The objective is to deliver a user-
friendly system that provides comprehensive reports, enabling healthcare professionals to make informed
decisions. This system strives to improve diagnostic accuracy, ensuring its practical utility in healthcare
environments by leveraging advanced machine learning techniques.
1 INTRODUCTION
A prompt and precise diagnosis is essential for the
efficient management of Alzheimer's disease is
leading causes of mortality worldwide. However, the
large amount of complex medical data required for an
accurate diagnosis presents a major challenge. The
goal of this research is to enhance the prediction of
Alzheimer's disease by using machine learning
algorithms. The intention is to assist medical
professionals in making more rapid and accurate
diagnoses by developing reliable models that undergo
stringent accuracy and precision testing. Machine
learning algorithms can analyze large amounts of
complex data, identifying patterns that indicate
Alzheimer's disease. This approach improves patient
outcomes by facilitating early intervention and
increasing diagnostic accuracy. By efficiently
evaluating patient data and detecting early warning
signs of intelligent decline, machine intelligence can
have a high impact on Alzheimer's care.
The study climaxes the transformational potential of
machine intelligence in healthcare, emphasizing the
importance of integrating this technology into clinical
practice to achieve better health outcomes. By
leveraging state-of-the-art examining capacities,
machine intelligence can play a pivotal act in the early
discovery and administration of Alzheimer's disease,
eventually reconstructing the status of growth for
patients and alleviating the work on caregivers and
healthcare methods.
2 LITERATURE REVIEW
In older persons, Alzheimer's disease (AD) is ultimate
prevailing cause of dementia. Currently, there is a lot
of interest in using machine learning to discover
metabolic disorders that impact a huge number of
people globally. Diseases that impair memory and
functionality will affect an increasing number of
people, their families, and the healthcare system as
our aging population grows. There will be significant
social, financial, and economic repercussions from
these. Alzheimer's disease is unpredictable when it is
first developing. When AD is treated early on, less
mild damage is caused and the efficacy of the
treatment is higher than when it is treated later. To
determine the optimal parameters for Alzheimer's
disease prediction, a number of methods have been
used, containing Decision Tree, Random Forest,
Support Vector Machine, Gradient Boosting, and
Voting classifiers. The Open Access Series of
Imaging Studies (OASIS) data is the action for
Alzheimer's disease prognoses, and versification to a
degree Precision, Recall, Accuracy, and F1-score for
Saraswathi, K., Renukadevi, N. T., T, H., N, N. P. and E M, V.
Automated Alzheimer’s Disease Diagnosis.
DOI: 10.5220/0013588000004664
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 3rd International Conference on Futuristic Technology (INCOFT 2025) - Volume 2, pages 113-122
ISBN: 978-989-758-763-4
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
113

machine intelligence models are used to assess model
performance. Clinicians can diagnose these disorders
using the proposed classification approach. When
these ML algorithms are used for early diagnosis, the
annual death rates from Alzheimer's disease can be
significantly reduced. With high-quality confirmation
average veracity of 83% on the test dossier of AD, the
suggested work demonstrates superior outcomes.
This test's accuracy score is noticeably greater than
that of previous studies. (Kavitha, Mani, et al. 2022)
The most common neurodegenerative disease is
Alzheimer's disease. Initially innocuous, the
manifestations worsen with time. One type of
dementia that is common is Alzheimer's disease. The
difficulty with this illness is that there isn't a cure. The
disease is diagnosed, but only in its final stages.
Therefore, the disease's progression or symptoms
may be slowed down if the illness is diagnosed early.
In this study, psychological variables such age, visits,
MMSE, and education are used to predict the
likelihood of Alzheimer's disease utilizing machine
learning algorithms. (Neelaveni, Devasana, et al.
2020)
Alzheimer's is a backward senility that starts
accompanying a slight loss of memory and someday
results in the complete deficit of insane and tangible
skills. For the patient benefits, the diagnosis should
be made as soon as possible to begin treatment and
preventive measures. While assessments like the
Mini-Mental State Tests Examination are typically
utilized for preliminary detection, brain analysis
through magnetic resonance imaging (MRI) provides
the basis for diagnosis. Techniques like OASIS (Open
Access Series of Imaging Studies) is individual
public project that makes neuroimaging datasets
freely accessible for scientific inquiry. This study
proposes and compares a novel approach for MRI-
located Alzheimer's disease that is established deep
learning and image processing techniques to earlier
research in the field. Findings: Our approach obtains
a balance veracity (BAC) of 0.88 for the production
of the ailment stage (healthful tissue, very gentle, and
harsh stage) and until 0.93 for concept-located
computerized diagnosis of the illness. Conclusions:
Using the OASIS collection, the results produced
outperformed the state-of-the-art suggestions. This
brings that methods based on deep learning are useful
in developing a strong solution for MRI data-driven
Alzheimer's-assisted diagnosis. (Saratxaga, et al.
2021)
The time of prevention and treatment outcomes of
Alzheimer's disease depend on a precise and early
diagnosis. In order to evaluate the course of
Alzheimer's disease (AD), pinpoint its previous
phases, and exploring this for future fields of study,
this review summarizes the most recent research
studies that use the deep learning methods and
machine learning techniques. Several number of
modern AI techniques, including Support Vector
Machines, Random Forest, Logistic Regression,
Convolutional Neural Networks (CNN), Recurrent
Neural Networks (RNN), and Transfer Learning, are
covered in this review in relation to their use in AD
diagnosis. Their efficacy is also investigated, along
with their advantages and disadvantages. The talk
includes an overview of the key conclusions and
medical imaging preprocessing techniques from the
earlier investigations. Finally, we discuss the
limitations and opportunities going forward. As a
result, we emphasize that further data are required and
that advanced neuroimaging technologies will be
created. (Saeed, 2024)
Alzheimer's disease (AD) is a nerve condition that
progresses irreversibly. The patient's treatment
approach must be adjusted in light of the disease's
close AD monitoring. Clinical score prediction using
neuroimaging data is ideal for AD monitoring
because it can accurately reveal the disease status.
The majority of the earlier research on this task
concentrated on a single time point and ignored the
correlation between clinical scores at various time
periods and neuroimaging data, such as magnetic
resonance imaging (MRI). In contrast to previous
research, we suggest developing a framework for
predicting clinical scores using longitudinal data
collected at several time periods. The three
components of the proposed system are as follows:
feature encoding using a multi-layer or deep
polynomial network, ensemble learning techniques
for regression using the support vector regression
approach, and feature selection utilizing correntropy
regularized in joint learning. There are two scenarios
created for score prediction. To be more precise,
scenario 1 makes use of baseline data to forecast
longitudinal scores, but scenario 2 makes use of all
data from prior time points to forecast scores at the
subsequent at time, potentially increasing the
accuracy of score prediction. To address the
incompleteness of the data, the missing clinical scores
at several longitudinal time periods are imputed. (Lei,
et al. 2020)
Relevance of the pathological process of
Alzheimer's disease (AD) begins with a protracted
phase of amyloid (Aβ) buildup that is symptomless.
The length of this stage varies widely from person to
person. The optimal way to forecast the start of
clinical progression is still unknown, despite the
significant relevance of this disease phase for clinical
INCOFT 2025 - International Conference on Futuristic Technology
114

trial designs. Goal to assess the efficacy of various
plasma biomarker combinations in predicting
cognitive deterioration in cognitively unimpaired
(CU) people who test positive for Aβ.The main result
was a series of longitudinal cognitive tests that
assessed over a median of 6 years (range, 2–10) using
the Mini-Mental State Examination (MMSE) and the
modified Preclinical Alzheimer Cognitive Composite
(mPACC). The development of AD dementia was the
secondary result. Linear regression models were
employed to estimate the rates of longitudinal
cognitive change (determined independently) using
baseline biomarkers. The models were calibrated for
baseline cognition, apolipoprotein E ε4 allele status,
years of schooling, sex, and age. The revised Akaike
information criterion and model R2 coefficients were
used to compare multivariable models. (Mattsson-
Carlgren, et al. 2023)
Over the past few years, there has been a
tremendous advancement in the discovery of plasma
biomarkers for pathologies associated with
Alzheimer's disease. Blood tests that are validated for
neurodegeneration, astrocytic activation, and amyloid
and tau pathology are now available. We evaluated
the prediction of research-diagnosed disease status by
using these biomarkers and examined genetic variants
associated with the biomarkers that may provide a
more accurately reflect the risk of biochemically
defined Alzheimer's disease instead of the risk of
dementia to define Alzheimer's disease using
biomarkers rather than clinical evaluation. The
combination of all biomarkers, APOE, and polygenic
risk score attained an area of using the receiver
operating characteristic curve (AUC) showed a
prediction accuracy of 0.81 for clinical diagnosis of
Alzheimer's disease; the most significant contributors
were ε4, Aβ40 or Aβ42, GFAP, and NfL. (Stevenson-
Hoare, et al. 2023)
Relevance Regarding which biomarkers are most
useful in predicting longitudinal tau buildup at
various clinical stages of Alzheimer disease (AD),
there is currently little agreement. Goal In order to
identify which biomarker combinations demonstrated
the most significant relationships with longitudinal
tau PET and best optimal clinical trial enrichment, as
well as to characterize longitudinal [18F]RO948 tau
positron emission tomography (PET) findings across
the clinical continuum of AD. Principal Results and
Measures Using a data-driven method that combines
clustering and event-based modeling, baseline tau
PET using standardized uptake value ratio (SUVR)
and also annual percent change in tau PET SUVR
across regions of interest were determined. In order
to determine which combinations best predicted
longitudinal tau PET, regression models were utilized
to investigate relationships between certain
biomarkers and longitudinal tau PET. The effects of
using these combinations as an enrichment method on
a number of participants in a simulated clinical trial
were then investigated using a power analysis.
Conclusions and Pertinence’s Plasma p-tau217
combined with tau PET may work best for
improvement in preclinical and prodromal AD in
studies where tau PET is the endpoint. Nonetheless,
tau PET was more significant in prodromal AD, but
Plasma p-tau217 was more significant in preclinical
AD. (Leuzy, et al. 2022)
Lately, Alzheimer's disease has emerged as a big
worry. Approximately 45 million individuals are
afflicted with this illness. Alzheimer's is a declining
brain disease that mostly affects the elderly and has
an unclear etiology and pathophysiology. Dementia is
the primary cause of Alzheimer's disease, as it
gradually affects brain cells. This disease caused
people to lose their capability to read, think, and many
other skills. By estimating the illness, a machine
learning system can lessen this issue. The primary
goal is to identify dementia in a range of people. The
investigation and findings of the detection of
dementia using several machine learning models are
presented in this research. The method has been
developed using the Open Access Series of Imaging
Studies (OASIS) dataset. Many machine learning
models have been applied and the dataset examined.
For prediction, decision trees, random forests, logistic
regression, and support vector machines have all been
employed. The system has been used both with and
without fine-tuning. After comparing the outcomes, it
is discovered that the support vector machine
outperforms the other models. Among a large number
of patients, it had the highest accuracy in identifying
dementia. The technique is easy to use and can
identify individuals who may be suffering from
dementia. (Bari et al. 2021)
Background: The health of the elderly is at risk
due to Alzheimer's Disease (AD), a nerve condition
that progresses over time. It is believed that mild
cognitive impairment (MCI) is through to be
prodromal stage of AD. As of right now, the diagnosis
of AD or MCI is made following permanent changes
to the structure of the brain. Thus, the creation of
novel biomarkers is essential to the early diagnosis
and management of this illness. Currently, a few
studies have demonstrated that radiomics analysis can
be a useful diagnostic and classification technique for
AD and MCI. Goal: To look into how the use of
radiomics analysis can be used diagnosis and
categorize of AD patients, MCI patients, and Normal
Automated Alzheimer’s Disease Diagnosis
115

Controls (NCs), a thorough evaluation of the
literature was conducted. Results: In the end, thirty
finished MRI radiomics investigations were chosen
for inclusion. The acquisition of picture data, Region
of Interest (ROI) segmentation, feature extraction,
feature selection, and classification or prediction are
typically steps in the radiomics analysis process. The
majority of the radiomics techniques were devoted to
texture analysis. The histogram, shape-based, texture-
based, wavelet, Gray Level Co-Occurrence Matrix
(GLCM), and Run-Length Matrix (RLM) are
additional characteristics that were retrieved. In
conclusion, even though randomics analysis is now
employes for the diagnosis and classification of AD
and MCI, there is still a long way to go before these
computer-aided diagnosis approaches are applied in
clinical settings. (Feng, and Ding, 2020)
Alzheimer's disease permanently damages brain
cells related to cognition and memory. Given that it
results in death, it has a lethal outcome. In previous
identification of Alzheimer's disease is so crucial.
Accurately diagnosing this illness in its early stages
is essential for clinical research as well as patient
care. Alzheimer's disease (AD) is among the most
costly illnesses to cure, hence many researchers are
focusing on developing an automated algorithm with
great accuracy. Early detection and estimation of
Alzheimer's disease may provide difficulties. An ML
system that can predict the sickness can solve this
problem. The potential of machine learning (ML) to
address issues in a variety of domains, including the
interpretation of medical imaging, has recently led to
ML's significant rise in popularity. Current research
uses machine learning algorithms and 3D magnetic
resonance imaging (MRI) images to predict and
classify Alzheimer's disease. Using 3D MRI
technology, this study integrates the white and grey
matter found in MRI images to produce 2D slices in
the axial, sagittal, and coronal orientations. In order
to forecast and categorize Alzheimer's disease, Multi-
Layer Perceptron (MLP) and SVM algorithms are
used for feature extraction after the most pertinent
slices have been chosen. The precision, recall,
accuracy, and F1-score are among the criteria the
researchers use to evaluate the system's effectiveness.
(Rao, Gandhi, et al. 2023)
This section discusses experimental results and
presents an actual MRI image using the suggested
methods. The trials are conducted using several
grayscale MRI image standards that vary in size. As
seen in Fig. 5(a), the MRI pictures are distorted by
speckle noise, random noise, and salt and pepper
noise generated by MRI scanning equipment. These
three noise characteristics serve as the basis for the
de-noising procedure. In summary is using a variety
of algorithms, the Computer Aided Diagnosis (CAD)
method is suggested as a means of identifying and
categorizing Alzheimer disease on authentic MRI
scans. An extremely expensive diagnostic tool for
Alzheimer's is the picture of the disease, which is
quite dangerous. The biomedical field has gained
popularity recently as a result of computer-aided
diagnosis (CAD), which uses digital image
processing to diagnose clinical patients accurately
and quickly. For people with Alzheimer's disease
(AD), early and appropriate diagnosis and treatment
planning lead to increased life expectancy and quality
of life. Modern methods that consider multimodal
analysis to be accurate and efficient have been
demonstrated to be superior to manual analysis.
Although numerous technologies have been
developed to diagnose Alzheimer's disease, the
diagnosis system is still very expensive and provides
low-accuracy and inefficient disease detection
because of the limitations of Magnetic Resonance
Imaging (MRI) scanning machines. This study
suggests a fresh approach for CAD procedure that
predicts AD utilizing a variety of algorithms.
(Sathiyamoorthi, Ilavarasi, et al. 2021)
Predicting the long-term course of Alzheimer's
disease (AD), a chronic neurological illness, is
undoubtedly crucial. When describing the cortical
atrophy that is strongly linked with AD prodromal
stages and clinical symptoms, structural magnetic
resonance imaging, or sMRI, might be utilized. A
large number of current techniques have concentrated
on employing a set of morphological traits obtained
from sMRI to predict the cognitive scores at future
time-points. More extensive information can be
obtained from the 3D sMRI than from the cognitive
scores. Nevertheless, relatively few studies attempt to
forecast a single brain MRI scan at a later period. In
order to forecast the overall appearance of a person's
brain over time, we present a disease progression
prediction framework in this paper that includes a 3D
multi-information generative adversarial network
(mi-GAN). and a multi-class classification network
tuned with a focal loss based on 3D DenseNet that
determines the estimated brain's clinical stage. With
respect to the individual of Multi-information and 3D
brain sMRI at the baseline time point, the mi-GAN
may provide individual 3D brain MRI images of
superior quality. On the use Alzheimer's Disease
Neuroimaging Initiative (ADNI), experiments are
conducted. With a structural similarity index (SSIM)
of 0.943 between the produced and real fourth-year
MRI images, our mi-GAN demonstrates advanced
performance. When mi-GAN and focused loss are
INCOFT 2025 - International Conference on Futuristic Technology
116

used in place of conditional GAN and cross entropy
loss, the pMCI vs. sMCI accuracy improves by
6.04%. (Zhao, Ma, et al. 2021)
In order to predict the likelihood that someone
with mild cognitive impairment (MCI) will develop
Alzheimer's disease (AD), this study confirms the
generalizability of the MRI-based classification of
AD patients and controls (CN) to an external data
collection. A deep convolutional neural network
(CNN) and a traditional support vector machine
(SVM) method based on structural MRI data that
were either minimally or heavily pre-processed into
maps of the modulated gray matter (GM). The
Alzheimer's Disease Neuroimaging Initiative (ADNI;
334 AD, 520 CN)employed cross-validation. After
that, trained classifiers were used in the independent
Health-RI Parelsnoer Neurodegenerative Diseases
Biobank data set as well as in ADNI MCI patients
(231 converters, 628 non-converters) to predict
conversion to AD. We enrolled 199 AD patients, 139
participants with subjective cognitive impairment, 48
MCI patients who converted to dementia, and 91 MCI
patients who did not convert to dementia from this
multi-center trial, which represented the population
of a tertiary memory clinic. For AD classification,
deep and conventional classifiers performed similarly
well, with just a minor drop in performance when
applied to the external cohort. We anticipate that this
external validation study will help translate machine
learning into clinical settings. (Bron, et al. 2021)
The main causes of dementia, Alzheimer's disease
(AD) is characterized by a gradual course that takes
years to complete with no known cure or medication.
In this sense, attempts have been made to determine
the likelihood of acquiring AD at an early age. More
recent research has concentrated on the disease and
prognosis of AD utilizing long or period series data
in a manner of disease progression modeling, whereas
many earlier works used cross-sectional analysis. In
this study, we provide a unique computational
framework that can predict, under the same problem
settings, cognitive scores at various future time
points, coupled with the trajectories of clinical status
and phenotypic measures of MRI biomarkers.
However, it typically encounters a large number of
unexpected missing observations when handling time
series data. Given such an adverse scenario, we plan
a subordinate question of estimating those missing
principles and address it accurately by accounting for
the multivariate and temporal linkages present in
period succession data. In particular, we plan a deep
repeating network to jointly address four issues: (i)
phenotypic calculations predicting; (ii) course guess
of a cognitive score; (iv) dispassionate rank guess of
a subject established longitudinal image biomarkers;
and (iii) missing value imputation. Interestingly, our
cautiously constructed loss function is used to train
the learnable parameters of each module in our
prediction models end-to-end using the
morphological features and cognitive scores as input.
We tested our approach using The Alzheimer’s
Disease Prediction Of Longitudinal Evolution
(TADPOLE) challenge cohort, comparing it to rival
approaches in the literature and measuring
performance for a number of measures. Furthermore,
ablation tests and thorough analysis were carried out
to further verify the efficacy of our approach. (Jung,
Jun, et al. 2021)
The extreme predominance of Alzheimer's
disease (AD) and the extreme cost of usual
demonstrative patterns create research into the
mechanical discovery of AD critical. Since AD
materially impacts the meaning and sound character
of spoken conversation, machine intelligence and
robotics offer hopeful methods for dependably
detecting AD. Recently, skilled has happened a
conception of models for AD categorization; still,
these change in agreements of the types of models,
datasets used, and training and testing paradigms. In
this work, we analyze the efficiency of two prevalent
methods to mechanical recognition of AD from
speech on the same, appropriate dataset, in order to
ascertain the benefits of using expertise in the field
vs. had trained transfer models. In order to identify
the best predictive model, it is important to assess its
effectiveness on carefully crafted datasets using
compatible same variables for training and self-
sufficient test datasets. This approach supports the
usefulness of productive machine learning and
linguistically-focused machine learning methods that
identify AD from speech. (Balagopalan Eyre, et al.
2021)
Alzheimer's disease (AD) is a step-by-step
affecting animate nerve organs illness that frequently
influences middle-old and older persons, gradually
impairing their cognitive function. There is currently
no treatment for AD. In addition, it takes too long to
diagnose AD clinically today. In order to predict AD
clinical scores, we have designed a combined and
deep learning system in this research. To be more
precise, features of brain regions linked to AD are
screened and dimensions are reduced using a process
of feature selection that combines group LASSO and
correntropy. In order to investigate the temporal
association between longitudinal data and the internal
connections between various brain regions, we
interrogate the multi-layer alone repeating brain
network reversion. The clinical score is concluded
Automated Alzheimer’s Disease Diagnosis
117

apiece jointly submitted deep learning network, that
likewise examines the equivalence betwixt the
clinical score and drawing resonance depict. The
expected clinical score principles enable physicians
to treat patients' illnesses promptly and with an early
diagnosis. (Lei, et al. 2022)
A crucial but unmet clinical issue is creating
multi-biomarker models that are cross-validated to
predict the rate of cognitive deterioration in
Alzheimer's disease (AD). Global understanding (R2
= 24%) and thought (R2 = 25%) decline rates in rare
AD were predicted by a model integrating all
diagnostic categories and tested in ADAD over a 4-
year period. By utilizing model-based risk-
enrichment, the sample size needed to identify
simulated intervention effects was decreased by 50%
to 75%. Our alone confirmed machine-knowledge
approach concede possibility significantly lower the
sample amount necessary in AD clinical troubles by
forecasting cognitive degeneration in scattered
prodromal AD. In order to think rates of intelligent
decline, we applied support heading reversion to AD
biomarkers obtained from fundamental attractive
resonance depict (MRI), amyloid-PET,
fluorodeoxyglucose positron-diffusion tomography
(FDG-PET), and cerebrospinal fluid. Prediction
models were checked in sporadic premature AD (n =
216), after being trained in autosomal-dominant AD
(ADAD, n = 121). When promoting model-located
risk enrichment, the sample content necessary to
identify situation belongings was premeditated.
(Franzmeier, et al. 2020)
The most prevailing type of senility, Alzheimer's
disease (AD), can influence a affecting animate nerve
organs condition that damages brain cells and impairs
function, ultimately leading to gradual memory loss
and difficulty carrying out daily tasks. We can
identify AD patients based on whether they currently
have the lethal disease or may not in future by using
MRI (Magnetic Resonance Imaging) scan brain
images to aid in the identification and prediction of
this disease. The primary goal of all of our work is to
create the greatest tools for detection and prediction
that radiologists, physicians, and other caregivers can
use to treat patients with this illness and save time and
money. Deep Learning (DL) algorithms have shown
great promise in recent years for the diagnosis of AD
due to their ability to operate on enormous datasets.
In this study, we have used MRI images from the
ADNI 3 class, which has a total 2480 records, 2633
normal, 1512 moderate cases, to cultivate
Convolutional Neural Networks (CNNs) for earlier
diagnosis and classification of AD. When compared
to numerous other relevant papers, the model
performed well, with a noteworthy accuracy of
99%.Additionally, we contrasted the outcome with
our earlier research, which used the OASIS dataset to
apply machine learning algorithms. This revealed that
methods that use deep learning can be a better choice
than standard methods for machine learning when
handling large amounts of data, such as medical data.
(Salehi, Baglat, et al. 2020)
It is difficult to anticipate when healthy people or
people with modest cognitive impairment will
progress to the stage of active Alzheimer's disease.
Recently, a deep learning-based survival analysis was
created to make predictions about when an event
would occur in a dataset that contains censored data.
Here, we studied either an comprehensive study of
addition survive forecast the happening of
Alzheimer's disease in a matching style. We
employed the white matter dimensions of various
brain regions in patients who were cognitively normal
and those who had mild cognitive impairment as
predictive variables. The prediction results of our
deep survival model, which is based on a Weibull
distribution, the DeepHit model, and the conventional
standard Cox proportional-hazard model were then
compared. Our model produced the highest
correlation index of 0.835, which was similar to the
DeepHit model's and greater than the Cox model's. As
far as we are aware, this is the sole research that
discusses using brain-MRI data to apply a deep
survival model. Our findings show that this kind of
study could accurately forecast when a person would
develop Alzheimer's disease. (Nakagawa, et al.
2020)
3 METHODOLOGY
3.1 Random Forest:
• Ensemble Learning: Random Forest
builds multiple decision trees and
merges them together to get a more
accurate and stable prediction.
• Bagging Technique: It uses the bagging
method, where each model is trained on
a random subset of the data. This helps
in reducing the variance and avoids
overfitting.
3.2 K-Nearest Neighbor:
• K-NN stores all the data and classifies
INCOFT 2025 - International Conference on Futuristic Technology
118
the new data point according to the
similarity. Therefore, when new data
appears, it can easily be classified into
the well suite category by K-NN
algorithm.
• At the training phase, KNN only stores
the datasets, when it receives new data,
it classifies according to the similarity
of the new data.
3.3 Decision Tree:
• The decision tree is built by recursively
dividing the training data into sub-data
sets based on the attributes' values until
a threshold is reached, such as a
maximum depth or minimum number
of samples to split a node.
• The aim is to find an attribute that gets
the most information or reduces the
amount of impurity after splitting the
data.
3.4 Gradient Boosting:
•
It builds an ensemble of models
sequentially, where each model
attempts to correct the errors of its
predecessor.
• This method is particularly known for
its effectiveness in improving the
accuracy of predictions.
3.5 Neural Networks:
•
Neural networks comprise layers of
neurons, accompanying each layer tr
ansforming the input
data before passing it to the next lay
er. The layers contain an input layer,
hidden layers and output layer.
•
They use activation functions to
introduce non-linearity, enabling the
network to learn from complex
patterns and Neural networks are
trained using backpropagation.
3.6 CatBoost:
• It builds an ensemble of trees
sequentially, each one correcting errors
from the previous one.
• Automatically handles categorical
features without the need for extensive
preprocessing and Uses ordered
boosting to reduce overfitting and
improve accuracy.
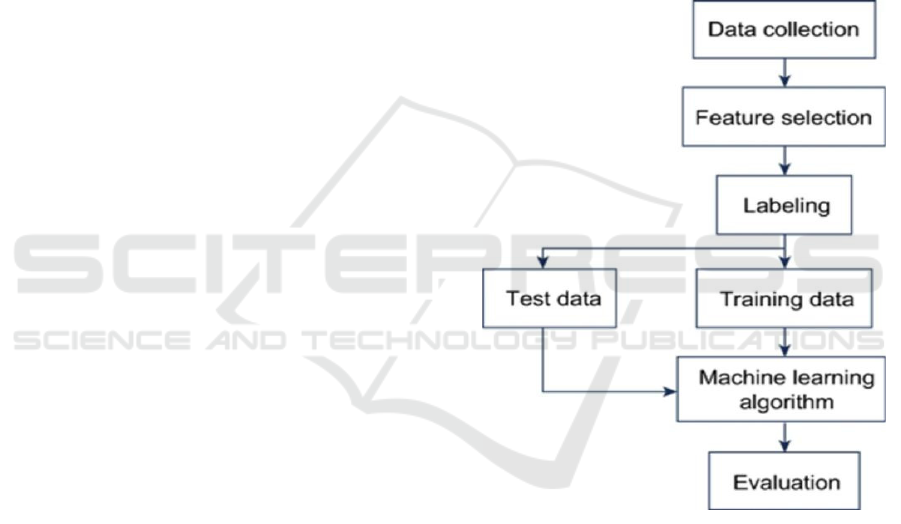
4 WORK FLOW
Figure 1: Work Flow
4.1 Explanation for work flow
4.1.1 Data Collection:
•
Collect raw data from various sources.
Ensure data is relevant to the problem.
Organize it for processing.
4.1.2 Feature Selection:
•
Identify key attributes or features.
Eliminate irrelevant or redundant data.
Prepare data for labeling and training.
Automated Alzheimer’s Disease Diagnosis
119
4.1.3 Labeling:
•
Assign labels to the dataset if it's
supervised learning. Categorize data based
on classes or outputs. Prepare it for training
and testing.
4.1.4 Data Split (test, train):
•
Split the data into training and test sets.
Training data is used to model learning.
Test data will validate model.
4.1.5 ML Algorithm:
•
Choose a machine learning model and Use
training data to train the model. Understand
patterns and relationships in the data.
4.1.6 Evaluation:
•
Apply the model to test set. Measure the
performance using metrics (accuracy,
precision, recall, etc.). Optimize the model
based on the results if necessary.
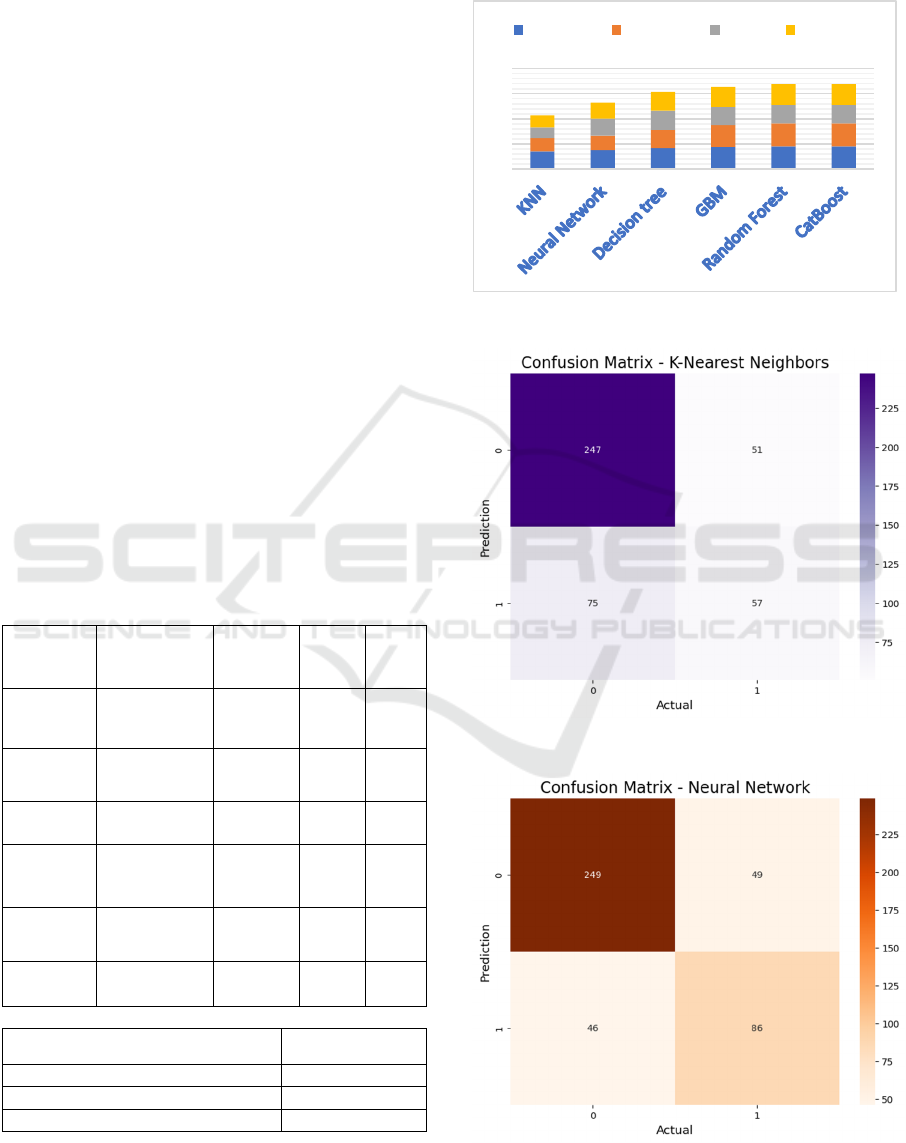
5 RESULTS
Table 1: Output values.
Algorith
m Used
Classificatio
n On
Accurac
y
Precisio
n
Recal
l
F1-
Scor
e
KNN 0.73 0.54 0.44 0.50
Neural
Network
0.81 0.71 0.70 0.70
Decision
Tree
0.83 0.73 0.77 0.75
GBM 0.88 0.87 0.73 0.79
Random
Forest
0.90 0.92 0.74 0.82
CatBoost 0.90 0.92 0.74 0.82
Ensemble Methods
Accuracy
Random forest with KNN
0.86
Random forest with CatBoost
0.89
Random forest with GBM
0.94
5.1 Diagrammatic representation of
outputs
Figure 2: Performance Metrics.
Figure 3: Confusion Matrix of KNN.
Figure 4: Confusion matrix of NN.
0.7
0.75
0.83
0.88
0.9 0.9
0.53
0.58
0.73
0.87
0.92 0.92
0.43
0.68
0.77
0.73
0.74 0.74
0.48
0.63
0.75
0.79
0.82 0.82
0
1
2
3
4
Accuracy Precision Recall F1-Score
INCOFT 2025 - International Conference on Futuristic Technology
120
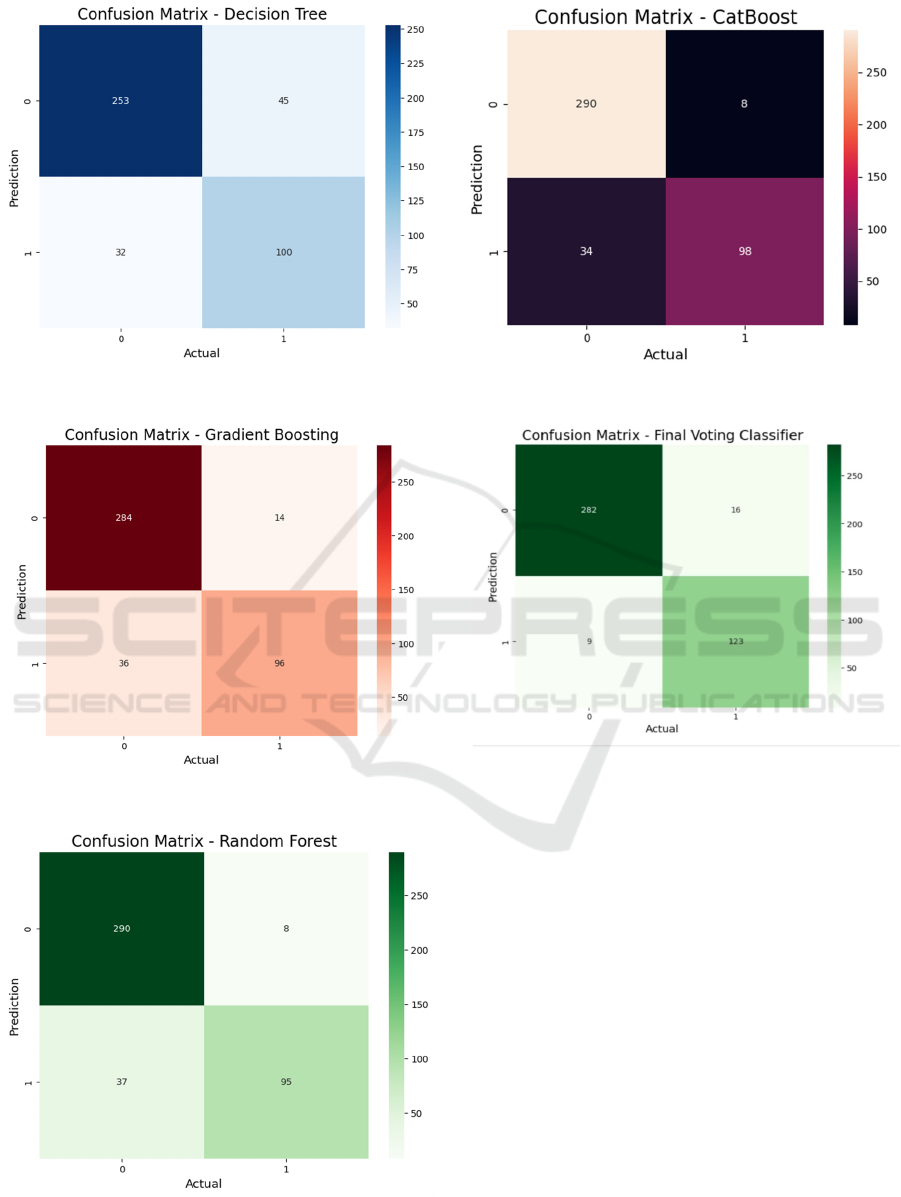
Figure 5: Confusion matrix of DT.
Figure 6: Confusion matrix of GBM.
Figure 7: Confusion matrix of KNN.
Figure 8: Confusion matrix of CatBoost.
Figure 9: Confusion matrix of Ensemble Method.
6 CONCLUSIONS
In this proposed work, the prediction of the target
variable was performed using classification
techniques, including K-Nearest Neighbors (KNN),
Neural Network (NN), Decision Tree (DT), Gradient
Boosting, Random Forest and CatBoost. While
comparing these algorithm results, Random Forest
and CatBoost emerged as the best-performing
algorithm with an accuracy of 90%, outperforming
the other classification algorithms. The model's
robustness to noise and ability to handle overfitting
contributed to its superior performance. However, by
applying hyperparameter tuning and creating an
ensemble model with Voting Classifier (combining
Random Forest and Gradient Boosting), the accuracy
was significantly improved to 94%.
Automated Alzheimer’s Disease Diagnosis
121

7 FUTURE SCOPES
In future work, exploring hybridized algorithms can
help enhance both accuracy and robustness.
Additionally, advanced techniques such as deep
learning may be explored, especially when working
with more complex data, offering improved feature
extraction and predictive capabilities. These
approaches hold excellent potential for further
improving model performance and could contribute
significantly to more accurate and reliable
forecastings in Alzheimer's disease classification and
additional healthcare uses.
REFERENCES
Kavitha, C., Mani, V., Srividhya, S. R., Khalaf, O. I.,
Tavera Romero, C. A., 2022. Early-Stage Alzheimer’s
Disease Prediction Using Machine Learning Models.
Front. Public Health, 10, 853294.
Neelaveni, J., Devasana, M. S. G., 2020. Alzheimer Disease
Prediction Using Machine Learning Algorithms. In
2020 6th International Conference on Advanced
Computing and Communication Systems (ICACCS),
Coimbatore, India. IEEE, pp. 101–104.
Saratxaga, C. L., et al., 2021. MRI Deep Learning-Based
Solution for Alzheimer’s Disease Prediction. JPM,
11(9), 902.
Saeed, F., 2024. Applications of ML and DL Algorithms in
the Prediction, Diagnosis, and Prognosis of
Alzheimer’s Disease. AJBSR, 22(6), 779–786.
Lei, B., et al., 2020. Deep and Joint Learning of
Longitudinal Data for Alzheimer’s Disease Prediction.
Pattern Recognition, 102, 107247.
Mattsson-Carlgren, N., et al., 2023. Prediction of
Longitudinal Cognitive Decline in Preclinical
Alzheimer Disease Using Plasma Biomarkers. JAMA
Neurol, 80(4), 360.
Stevenson-Hoare, J., et al., 2023. Plasma Biomarkers and
Genetics in the Diagnosis and Prediction of
Alzheimer’s Disease. Brain, 146(2), 690–699.
Leuzy, A., et al., 2022. Biomarker-Based Prediction of
Longitudinal Tau Positron Emission Tomography in
Alzheimer Disease. JAMA Neurol, 79(2), 149.
Bari Antor, M., et al., 2021. A Comparative Analysis of
Machine Learning Algorithms to Predict Alzheimer’s
Disease. Journal of Healthcare Engineering, 2021, pp.
1–12.
Feng, Q., Ding, Z., 2020. MRI Radiomics Classification
and Prediction in Alzheimer’s Disease and Mild
Cognitive Impairment: A Review. CAR, 17(3), 297–
309.
Rao, K. N., Gandhi, B. R., Rao, M. V., Javvadi, S., Vellela,
S. S., Khader Basha, S., 2023. Prediction and
Classification of Alzheimer’s Disease using Machine
Learning Techniques in 3D MR Images. In 2023
International Conference on Sustainable Computing
and Smart Systems (ICSCSS), Coimbatore, India.
IEEE, pp. 85–90.
Sathiyamoorthi, V., Ilavarasi, A. K., Murugeswari, K.,
Thouheed Ahmed, S., Aruna Devi, B., Kalipindi, M.,
2021. A Deep Convolutional Neural Network Based
Computer Aided Diagnosis System for the Prediction
of Alzheimer’s Disease in MRI Images. Measurement,
171, 108838.
Zhao, Y., Ma, B., Jiang, P., Zeng, D., Wang, X., Li, S.,
2021. Prediction of Alzheimer’s Disease Progression
with Multi-Information Generative Adversarial
Network. IEEE J. Biomed. Health Inform., 25(3), 711–
719.
Bron, E. E., et al., 2021. Cross-Cohort Generalizability of
Deep and Conventional Machine Learning for MRI-
Based Diagnosis and Prediction of Alzheimer’s
Disease. NeuroImage: Clinical, 31, 102712.
Jung, W., Jun, E., Suk, H.-I., 2021. Deep Recurrent Model
for Individualized Prediction of Alzheimer’s Disease
Progression. NeuroImage, 237, 118143.
Balagopalan, A., Eyre, B., Robin, J., Rudzicz, F.,
Novikova, J., 2021. Comparing Pre-Trained and
Feature-Based Models for Prediction of Alzheimer’s
Disease Based on Speech. Front. Aging Neurosci., 13,
635945.
Lei, B., et al., 2022. Predicting Clinical Scores for
Alzheimer’s Disease Based on Joint and Deep
Learning. Expert Systems with Applications, 187,
115966.
Franzmeier, N., et al., 2020. Predicting Sporadic
Alzheimer’s Disease Progression via Inherited
Alzheimer’s Disease-Informed Machine-Learning.
Alzheimer’s & Dementia, 16(3), 501–511.
Salehi, A. W., Baglat, P., Sharma, B. B., Gupta, G.,
Upadhya, A., 2020. A CNN Model: Earlier Diagnosis
and Classification of Alzheimer Disease Using MRI. In
2020 International Conference on Smart Electronics
and Communication (ICOSEC), Trichy, India. IEEE,
pp. 156–161.
Nakagawa, T., et al., 2020. Prediction of Conversion to
Alzheimer’s Disease Using Deep Survival Analysis of
MRI Images. Brain Communications, 2(1), fcaa057.
INCOFT 2025 - International Conference on Futuristic Technology
122
