
Improved Alzheimer’s Detection from Brain MRI via Transfer Learning
on Pre-Trained Convolutional Deep Models
Malek Jallali
1 a
, Raouia Mokni
2,3 b
and Boudour Ammar
1,4 c
1
Research Groups in Intelligent Machines(REGIM), National Engineering School of Sfax, University of Sfax, Tunisia
2
Advanced Technologies for Environment and Smart Cities(ATES Unit), University of sfax, Tunisia
3
The Higher Institute of Management of Gab
´
es(ISGG), University of Gabes, Tunisia
4
Department of Computer Management at the Higher Business School of Sfax (ESC Sfax), University of Sfax, Tunisia
Keywords:
Alzheimer’s Disease (AD), Convolutional Neural Network (CNN), Deep Learning (DL), MCNN, Transfer
Learning, Fine-Tuned, FT-VGGNet19, Brain MRI Images.
Abstract:
Alzheimer’s Disease (AD) presents a major challenge in modern healthcare due to its complex diagnosis and
management. Early and accurate detection is essential for improving patient care and enabling timely thera-
peutic interventions. Research suggests that neurodegenerative changes associated with AD may appear years
before clinical symptoms, highlighting the need for advanced diagnostic techniques. This study explores deep
learning models for classifying AD stages using MRI scans. Specifically, we propose a Modified Convolu-
tional Neural Network (MCNN) and a fine-tuned VGGNet19 (FT-VGGNet19) architecture. Both models were
evaluated on the Alzheimer’s Disease Neuroimaging Initiative (ADNI) dataset, leveraging data augmentation
to enhance generalization and mitigate dataset limitations. Experimental results show that data augmentation
significantly improves classification performance. The FT-VGGNet19 model achieved the highest accuracy,
reaching 90% on the original dataset and 92% with augmented data. This study highlights the strengths of
each model for clinical applications, emphasizing the role of optimized deep-learning frameworks in early AD
detection. The findings contribute to developing robust and scalable diagnostic systems, offering promising
advancements in neurodegenerative disease management.
1 INTRODUCTION
Alzheimer’s disease, the most common form of de-
mentia, is a progressive neurodegenerative disorder
that typically begins with subtle memory loss and ad-
vances to profound cognitive decline, ultimately im-
pairing a person’s ability to communicate coherently
or interact with their environment. The disease pri-
marily affects brain region responsible for memory,
language, and reasoning—such as the hippocampus
and cerebral cortex—disrupting neural pathways and
compromising an individual’s ability to perform rou-
tine daily tasks. Current estimates indicate that 1 in 9
adults aged 65 and older lives with Alzheimer’s, ac-
counting for approximately 11.4% of individuals in
this age group worldwide. Characterized by the accu-
mulation of beta-amyloid plaques and neurofibrillary
a
https://orcid.org/0009-0008-8167-765X
b
https://orcid.org/0000-0002-6652-5251
c
https://orcid.org/0000-0002-4934-1573
tau tangles, Alzheimer’s remains incurable, though
ongoing research seeks to unravel its mechanisms and
develop therapies to slow its devastating progression.
(Balasundaram et al., 2023). The primary cause of
Alzheimer’s disease is the abnormal buildup of pro-
teins in the brain, including beta-amyloid plaques and
tau tangles, which contribute to brain cell death and
the shrinkage of brain tissue. While ongoing research
continues to enhance our understanding, there is cur-
rently no cure for Alzheimer’s. However, medications
and lifestyle interventions can help manage symp-
toms and slow the disease’s progression. Medical
imaging, particularly Magnetic Resonance Imaging
(MRI), plays a crucial role in diagnosing Alzheimer’s
by providing detailed insights into brain structures
and identifying characteristic patterns associated with
the condition (Honig and Chin, 2001). Early detec-
tion and accurate classification of Alzheimer’s dis-
ease (AD) are crucial for improving therapeutic out-
comes and effectively managing cognitive decline.
The challenge lies not only in identifying the con-
Jallali, M., Mokni, R., Ammar and B.
Improved Alzheimer’s Detection from Brain MRI via Transfer Learning on Pre-Trained Convolutional Deep Models.
DOI: 10.5220/0013523000003967
In Proceedings of the 14th International Conference on Data Science, Technology and Applications (DATA 2025), pages 431-438
ISBN: 978-989-758-758-0; ISSN: 2184-285X
Copyright © 2025 by Paper published under CC license (CC BY-NC-ND 4.0)
431

dition in its early stages—when symptoms are mild
and neurological changes are subtle—but also in sys-
tematically categorizing it into distinct stages: Non-
Demented, Very Mild Demented, Mild Demented,
and Moderate Demented. Precise staging allows clin-
icians to implement targeted interventions tailored to
the severity of the disorder, significantly enhancing
patient care and outcomes(Rasmussen and Langer-
man, 2019). In this context, advanced deep learn-
ing methods, particularly those leveraging brain MRI
analysis, have proven to be effective tools for de-
tecting early signs of Alzheimer’s disease (AD) and
monitoring its progression. MRI is widely used due
to its unparalleled ability to capture detailed struc-
tural features, such as brain atrophy, a hallmark of
AD pathology (El-Latif et al., 2023a) (Mokni and
Haoues, 2022). The main purpose of this paper is
to investigate the application of deep learning mod-
els for the early detection of Alzheimer’s disease us-
ing MRI scans, classifying it into four stages: Non-
Demented, Very Mild Demented, Mild Demented,
and Moderate Demented. The key contributions of
this work are twofold: (1) the development of a modi-
fied CNN architecture (MCNN) tailored specifically
for Alzheimer’s diagnosis, and (2) the fine-tuning
of a pre-trained VGGNet19 model through transfer
learning to enhance classification accuracy and per-
formance. These approaches independently highlight
the effectiveness of CNN architectural modifications
and transfer learning techniques in improving the de-
tection and classification of Alzheimer’s disease. The
remainder of this paper is structured as follows: Sec-
tion 2 reviews related work, while Section 3 describes
the proposed methodology. Section 4 presents the ex-
perimental results, followed by a discussion and com-
parative evaluation with previous studies in Section
5. Finally, Section 6 concludes the paper and outlines
future research directions.
2 RELATED WORK
In this section, we summarize and review findings
from research studies on Alzheimer’s disease detec-
tion using deep learning models. Ibrahem M.M.
et al., (Madhat et al., 2024)developed an improved
method for detecting Alzheimer’s disease by combin-
ing DenseNet, VGG16, and ensemble learning. They
evaluated performance using accuracy, precision, re-
call, F1-score, and AUC-ROC on preprocessed MRI
scans divided into four disease stages. Techniques
like transfer learning, fine-tuning, and dropout regu-
larization were used. Using the Alzheimer’s Disease
Neuroimaging Initiative (ADNI) Dataset, the method
achieved The ensemble model reached 94% accu-
racy, outperforming standalone CNN (90%), VGG19
(89%), and DenseNet (86%), with better precision
and recall across all stages, highlighting its promise
for early and reliable Alzheimer’s diagnosis in clin-
ical practice. This highlights the potential of com-
bining advanced architectures with ensemble strate-
gies to improve early and reliable detection in clin-
ical settings. Araqi & Abbas (Araqi and Abbas,
2022) developed a CNN-based deep learning ap-
proach for Alzheimer’s detection using brain MRI,
achieving 90.83% accuracy. Their findings empha-
sized the importance of image preprocessing in sig-
nificantly improving model performance, suggesting
that combining data refinement with deep learning
enhances early and reliable detection. Ajagbe et
al.,(Ajagbe et al., 2021) designed a framework for
classifying Alzheimer’s disease (AD) stages via MRI.
Their model achieved 94.5% accuracy in distinguish-
ing AD from normal controls, surpassing traditional
diagnostic methods. This underscores the potential
of DCNNs for improving early AD detection in clin-
ical settings. Fathi et al., (Fathi et al., 2024) Fathi et
al.,(Fathi et al., 2024) introduced an ensemble CNN
method for early Alzheimer’s detection using MRI
scans. Tested on the ADNI dataset, their approach
reached 91.2% accuracy, demonstrating its reliability
for diagnosis. Table 1 provides an overview of past
research efforts focused on detecting Alzheimer’s dis-
ease.
Table 1: Summary of the related works focused on
Alzheimer’s disease classification.
Proposal Used
Dataset
Method Results
Ibrahem
M.M. et
al., (Mad-
hat et al.,
2024)
ADNI
dataset
CNN
VGG16
DenseNet
Ensemble
learning
90%
89%
86%
94%
Araqi
& Abbas
(Araqi and
Abbas,
2022)
alzheimers-
dataset-4-
class-of-
images
CNN 90.83%
Ajagbe et
al.,(Ajagbe
et al.,
2021)
alzheimers-
dataset-4-
class-of-
images
VGG-19 77.66%
Fathi et
al., (Fathi
et al.,
2024)
ADNI
Dataset
Ensemble
learning
CNN
91.2%
DATA 2025 - 14th International Conference on Data Science, Technology and Applications
432
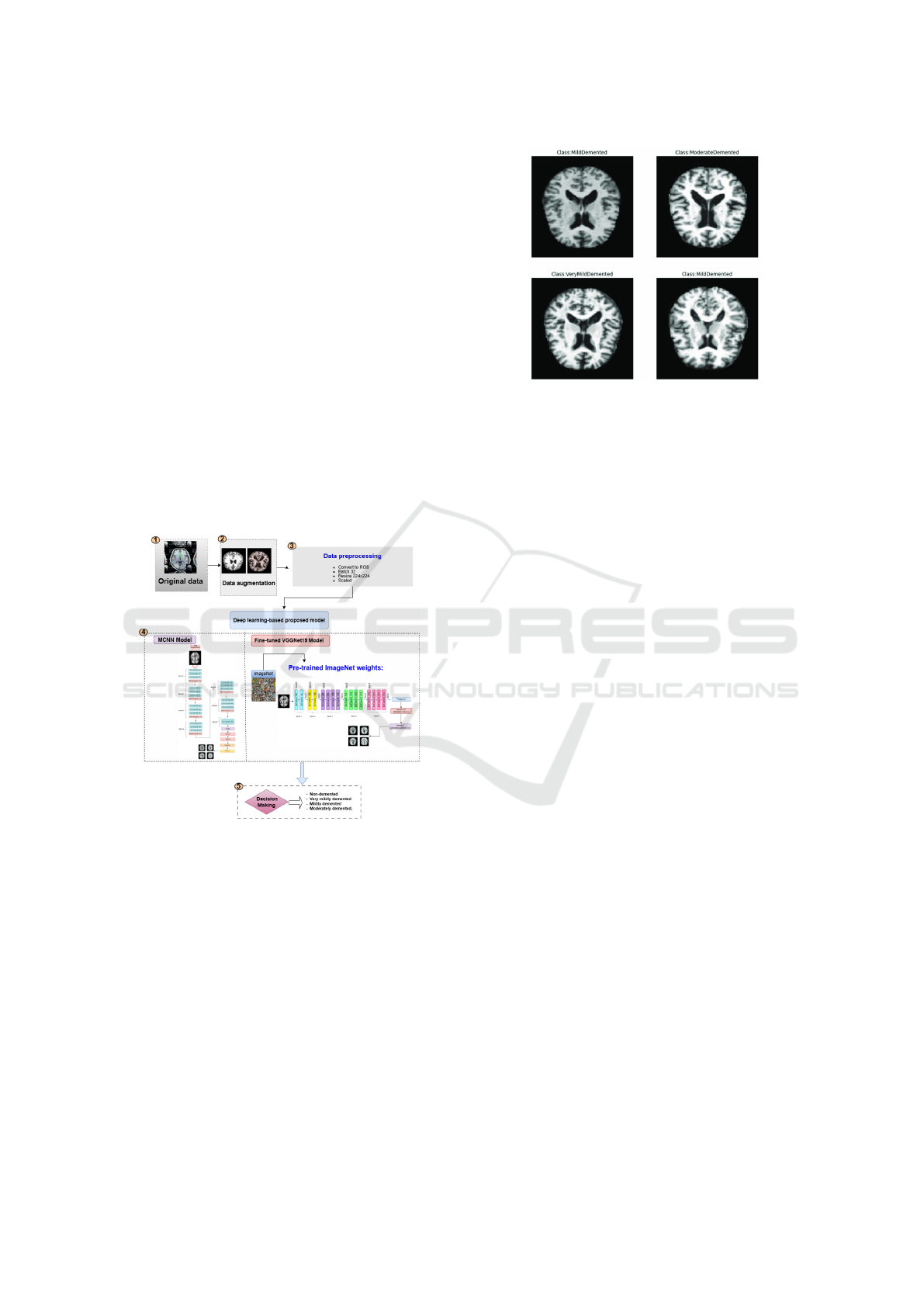
3 PROPOSED METHODOLOGY
As highlighted in Section 2, various studies have
explored Alzheimer’s Disease (AD) diagnosis us-
ing deep learning. Some approaches, such as those
combining DenseNet, VGG16, and ensemble learn-
ing, have demonstrated strong performance but in-
volve complex architectures. Others have employed
CNNs, Deep Convolutional Neural Networks (DC-
NNs), or ensemble learning of multiple CNNs. In
contrast, our approach focuses on a modified CNN
architecture (MCNN) and a fine-tuned VGGNet19
(FT-VGGNet19), aiming to enhance classification ac-
curacy through architectural modifications and fine-
tuning rather than relying on extensive ensemble
models. Our approach for classifying Alzheimer’s
disease (AD), as shown in Figure 1, involves five key
steps: (1) Dataset collection (2) Data augmentation,
(3) Data preprocessing, (4) Deep learning-based pro-
posed models, and (5) Decision Making to classify
AD into four stages.
Figure 1: The overview of the proposed Model.
3.1 Dataset Description
We used a publicly available MRI dataset for
Alzheimer’s disease (AD) (Dubey, 2024), sourced
from Kaggle. This dataset includes images from the
Alzheimer’s Disease Neuroimaging Initiative (ADNI)
and contains 6,400 MRI scans split into four cate-
gories: mild dementia (896 images), moderate de-
mentia (64 images), non-dementia (3,200 images),
and very mild dementia (2,240 images). Examples
from the dataset are shown in Figure 2 (El-Latif et al.,
2023b).
Figure 2: Sample MRI images.
3.2 Dataset Augmentation
Data augmentation is a key technique to improve how
well deep learning models classify Alzheimer’s dis-
ease (AD) using MRI scans. AD datasets are often
small due to limited patient availability, which can
cause models to overfit and perform poorly on new
data. Data augmentation tackles this by artificially ex-
panding the training dataset’s size and variety, helping
models learn stronger features and generalize better
to unseen cases (El-Assy et al., 2024). The original
dataset, from Kaggle’s open-access platform (Uran-
injo, 2024), contains 33,984 MRI scans grouped into
four categories: mild dementia (8,960 images), mod-
erate dementia (6,464 images), non-dementia (9,600
images), and very mild dementia (8,960 images). To
expand the dataset, we applied image transformations
(flipping, rotating...) while keeping key features rele-
vant to Alzheimer’s disease intact. The augmentation
techniques employed include:
-Rotation: Random rotations within a range of [-20°,
20°] to simulate different head orientations.
-Scaling: Size adjustments were applied to account
for MRI dimensional variability.
-Flipping: Horizontal flips were applied to increase
dataset variability while preserving the diagnostic fea-
tures.
-Brightness Adjustment: Variations in brightness
were incorporated to simulate the impact of different
lighting conditions during the image capture process.
-Zoom Transformation: Images were randomly
zoomed within a range of ±20% to simulate variations
in scale. Using these augmentation techniques, we
expanded the dataset and boosted the performance of
our deep learning models in classifying Alzheimer’s
disease (AD). Figure 3 shows the distribution of
Alzheimer’s disease categories within the Mild De-
mented, Moderate Demented, Non-Demented, and
Very Mild Demented.
Improved Alzheimer’s Detection from Brain MRI via Transfer Learning on Pre-Trained Convolutional Deep Models
433
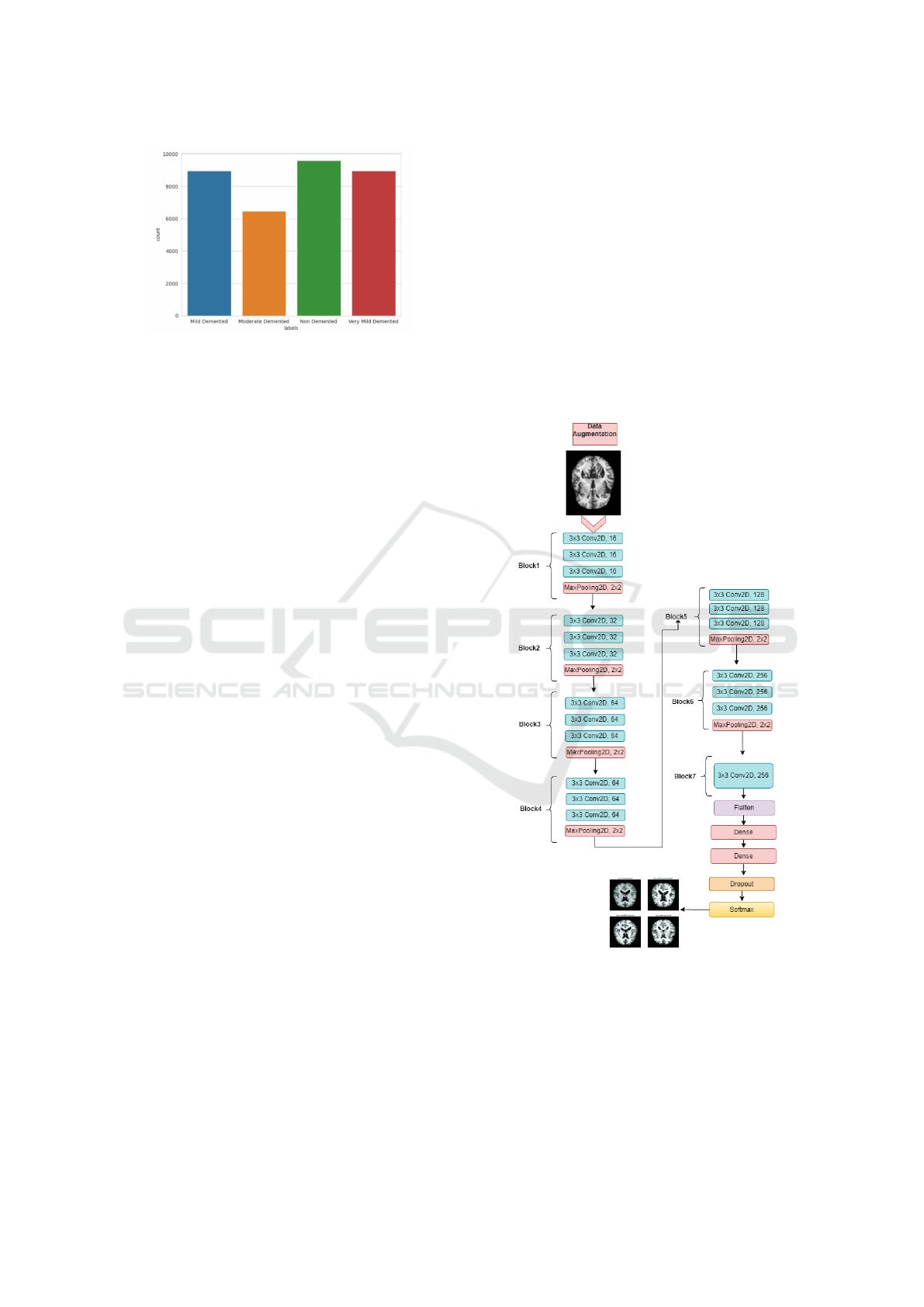
Figure 3: The distribution of Alzheimer’s diseases after data
augmentation.
3.3 Dataset Preprocessing
This section describes the essential preprocessing
steps taken to prepare the data for model training, en-
suring efficient learning on a potentially imbalanced
dataset. First, all images were turned to RGB color.
Next, we bundled them into batches of 32, reshaped
them to 224x224 pixels, and scaled their pixel val-
ues. For better model performance and generaliza-
tion, the dataset was split into three subsets: 70% of
the data was used for training, 20% for testing, and
10% for validation. This three-way split ensures that
the model is evaluated on unseen data, helping to mit-
igate the risk of overfitting and ensuring that it per-
forms well on new, unseen data.
3.4 Application of Deep Learning-Based
Proposed Models
This study explores the application of deep learning-
based models for Alzheimer’s disease classification,
proposing a novel modified Convolutional Neural
Network (MCNN) and leveraging transfer learning
with pre-trained models. Specifically, we fine-tuned
VGGNet19 to develop the FT-VGGNet19 model, op-
timizing its performance for AD classification.
3.4.1 Modified-CNN
A Convolutional Neural Network (CNN) is a deep
learning model specifically designed to process struc-
tured grid-like data, such as images. Its architec-
ture typically consists of multiple layers, each serv-
ing a distinct purpose: convolutional layers extract
meaningful features, pooling layers reduce dimen-
sionality and computational complexity, fully con-
nected layers make high-level decisions, and acti-
vation functions introduce non-linearity to enhance
learning capabilities. Together, these components en-
able CNNs to efficiently capture patterns and im-
prove model performance (Awarayi et al., 2024) (Fki
et al., 2024). In this study, we propose a novel archi-
tecture called Modified-CNN (MCNN), which intro-
duces several enhancements to the standard CNN de-
sign. The MCNN consists of seven blocks, with the
first six containing four layers each: three Conv2D
layers followed by a MaxPooling2D layer. The final
block includes an additional Conv2D layer to improve
feature extraction. After these blocks, the model is
compressed, followed by two fully connected (dense)
layers with 512 units each and ReLU activation. A
dropout layer with a rate of 0.3 is applied before the
final dense layer, which consists of four units and a
softmax activation function for classification. Figure
4 provides a detailed overview of the MCNN archi-
tecture.
Figure 4: Proposed MCNN Model.
3.4.2 Fine-Tuned VGGNet 19 Model
In this paper, we investigate the use of transfer
learning and fine-tuning the pre-trained VGGNet 19
model, called Fine-Tuned VGGNet 19 model (FT-
VGGNet 19). Initially, we leverage the VGG19
model, pre-trained on the ImageNet dataset to obtain
DATA 2025 - 14th International Conference on Data Science, Technology and Applications
434
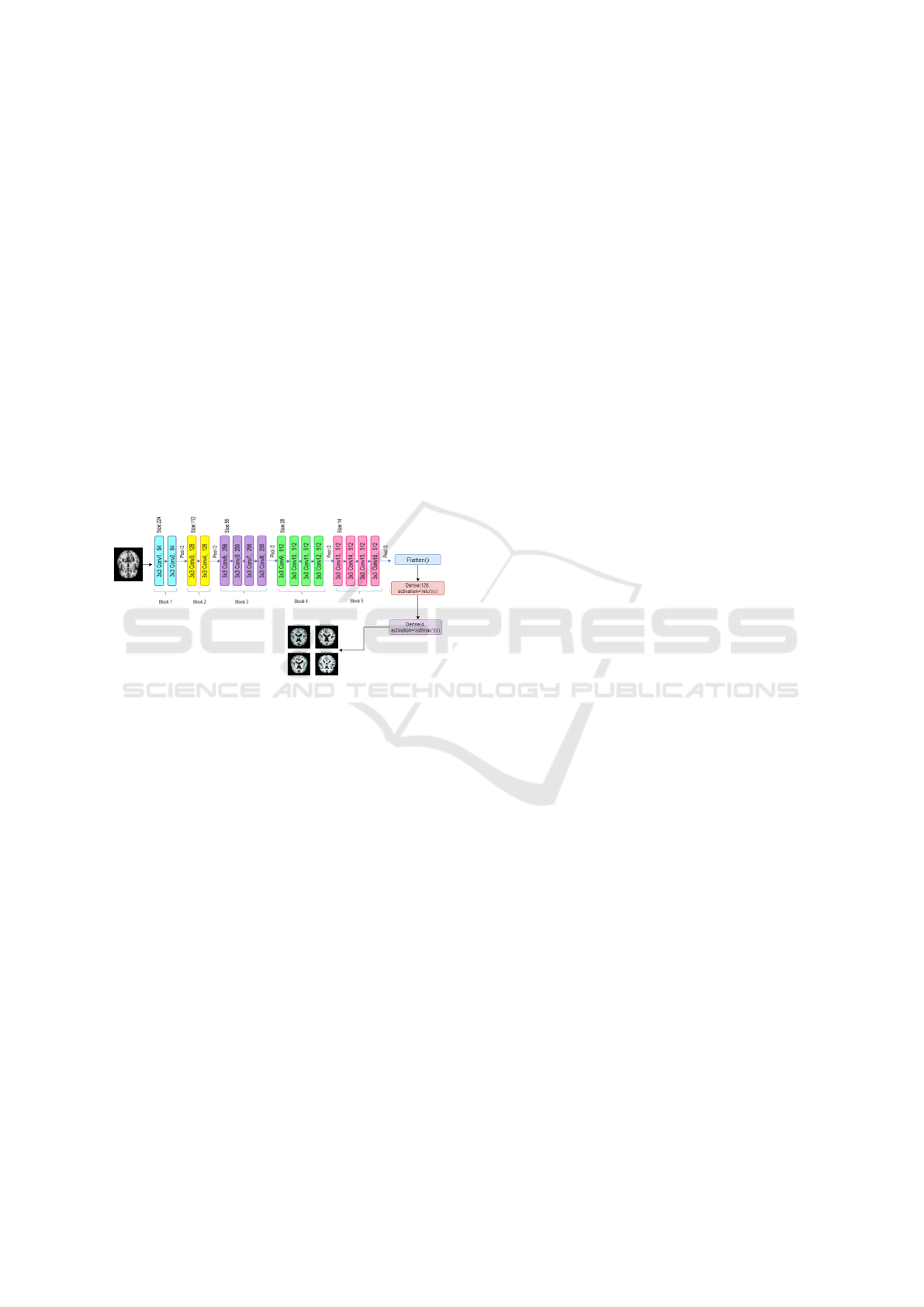
the trained weights parameters that are used to ex-
tract the pertinent features. It consists of 19 layers,
including convolutional, pooling, and fully connected
layers. The network employs 3×3 convolution filters
with small strides to extract features from input im-
ages while pooling layers reduce the spatial dimen-
sions of feature maps. The fully connected layers per-
form object classification, with a softmax output layer
handling multi-class classification. In this study, the
model’s pre-trained weights remain frozen through-
out the training process to maintain the integrity of
these learned features. After that, we created a trans-
fer learning method by modifying the architecture by
firstly flattening, followed by adding a dense layer
and introducing a softmax activation function to clas-
sify the images into four distinct categories related to
Alzheimer’s disease. This adjustment effectively tai-
lors the model for Alzheimer’s disease image classi-
fication. Figure 5 provides a detailed overview of the
FT-VGGNet19 architecture.
Figure 5: Proposed Fine-Tuned VGGNet19 Model.
4 EXPERIMENTAL RESULTS
In our study, the processing and classification tasks
were performed using the Python programming lan-
guage. The models were trained on Google Colab,
leveraging a graphics processing unit (GPU) to en-
hance computational efficiency. To investigate the
contribution of deep learning models in classifying
Alzheimer’s disease, we conducted several experi-
ments using the proposed models: Modified CNN
(MCNN) and Fine-Tuned VGGNet19. Table 2 sum-
marizes the evaluation results for Alzheimer’s dis-
ease classification, highlighting the impact of dif-
ferent original data methods and augmentation tech-
niques on accuracy and robustness. The results pre-
sented in Table2 highlight the impact of data augmen-
tation on the performance of deep learning models
for Alzheimer’s disease classification. Both the Fine-
Tuned VGGNet19 (FT-VGGNet19) and the Modified
CNN (MCNN) models demonstrate improved accu-
racy when trained with data augmentation. Specif-
ically, FT-VGGNet19 achieves an accuracy of 92%
with data augmentation compared to 90% without,
while MCNN shows a significant improvement from
73% to 90%. Similarly, the F1-score for MCNN im-
proves significantly from 64% to 90% with data aug-
mentation, demonstrating a substantial enhancement
in the model’s overall classification performance, par-
ticularly in balancing precision and recall. These find-
ings emphasize the effectiveness of data augmenta-
tion in boosting model robustness and classification
performance, particularly for the MCNN model. In
this study, we conducted two experiments: the first
focused on classifying Alzheimer’s disease using the
original data, while the second involved applying data
augmentation to improve classification performance
from MRI images.
4.1 The First Experiment
In this section, a deep learning model was used
to classify MRI images into four categories: Mild
Demented, Moderately Demented, Non-Demented,
and Very Mild Demented, using the original dataset.
Model performance was evaluated with a confusion
matrix, comparing predicted labels to actual ones
(Vengala, 2024) and the training and validation ac-
curacy and curves. Figure 6 presents the results of the
confusion matrix for the classification of Alzheimer’s
diseases according to four categories obtained from
MCNN and FT-VGGNet19 using the original dataset.
As illustrated in this Figure, FT-VGGNet19 achieved
the highest accuracy, outperforming MCNN in terms
of classification effectiveness. Diagonal elements
indicate correct predictions, while off-diagonal val-
ues represent misclassifications. FT-VGGNet19 cor-
rectly classifies 161 Non Demented and 122 Very
Mild Demented cases, with misclassifications mainly
occurring between similar classes. Figure 7 pres-
nts the Training /Validation Accuracy curves results
for the classification of Alzheimer’s diseases accord-
ing to four categories obtained from MCNN and FT-
VGGNet19 over 15 epochs using the original dataset.
Figure 7 (B) tracks training and validation accuracy
obtained from FT-VGGNet19 across 15 epochs. A
well-trained model shows both curves rising and sta-
bilizing. A divergence where training accuracy in-
creases but validation accuracy stagnates suggests
overfitting, while low accuracy in both indicates un-
derfitting. The validation curve is crucial for assess-
ing real-world performance.
Improved Alzheimer’s Detection from Brain MRI via Transfer Learning on Pre-Trained Convolutional Deep Models
435
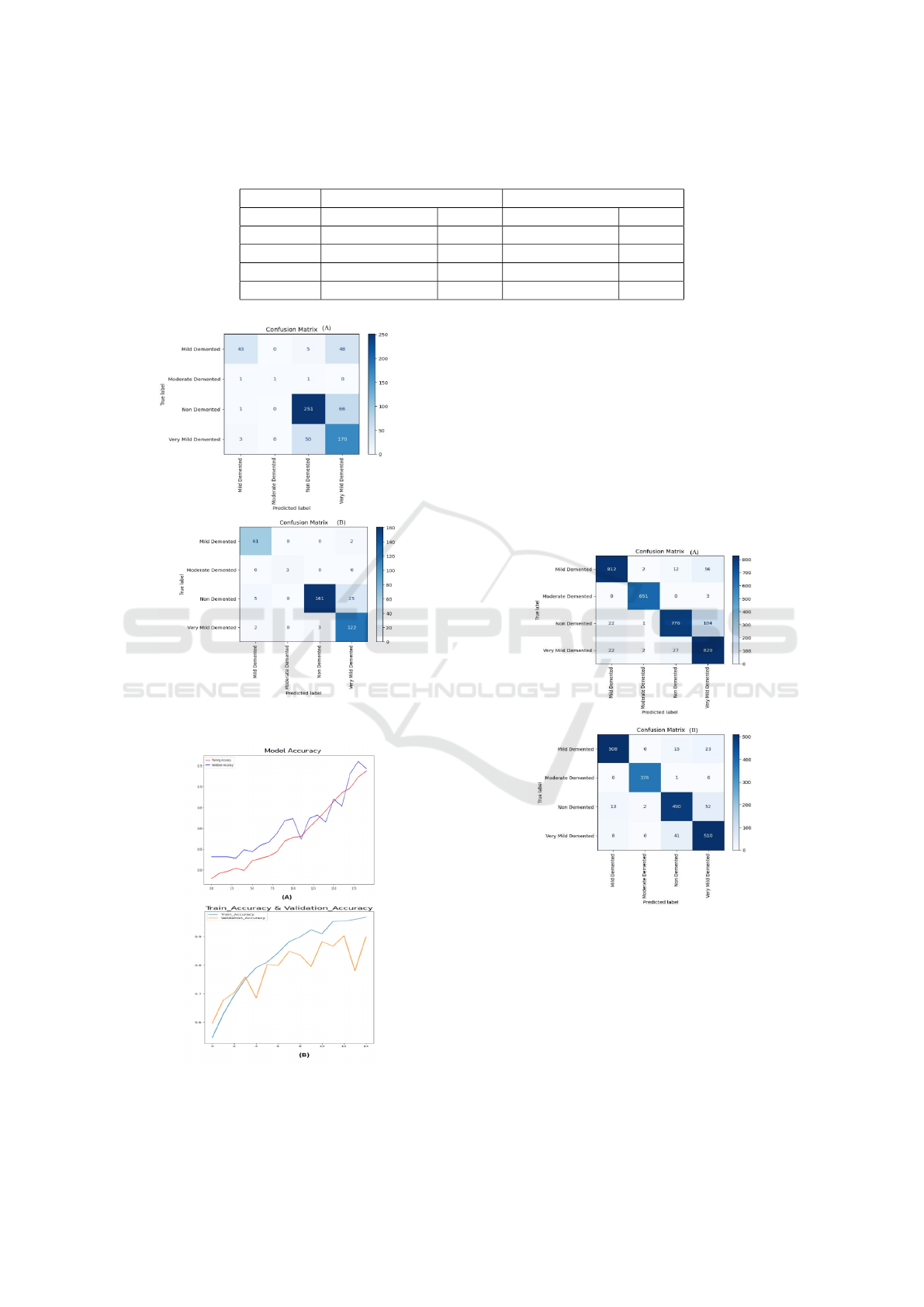
Table 2: Evaluation of Alzheimer’s Disease Classification Using the Proposed MCNN Model and Fine-Tuned VGGNet19
Models with Data Augmentation Techniques and Original Data.
Model Data Augmentation Original Data
FT-VGG-Net19 MCNN FT-VGG-Net19 MCNN
Accuracy 92% 90% 90% 73%
Precision 91% 91% 92% 83%
Recall 92% 90% 94% 58%
F1 Score 91% 90% 93% 64%
Figure 6: Confusion Matrices obtained by (A) MCNN (B)
FT-VGGNet19 with original data.
Figure 7: Training and Validation Accuracy curves obtained
by (A) MCNN (B) FT-VGGNet19 with original data.
4.2 The Second Experiment
In this section, the proposed MCNN and Pretrained
FT-VGGNet19 was implemented to classify MRI
scans into four categories: Mild Demented, Moder-
ately Demented, Non-Demented, and Very Mild De-
mented using the augmented dataset to enhance the
model’s robustness and generalization. The model’s
effectiveness was assessed through the confusion ma-
trix, which compares predicted labels with actual
ground truth labels and the training and validation ac-
curacy curves.
Figure 8: Confusion Matrices obtained by (A) MCNN (B)
FT-VGGNet19 with data augmentation.
Figure 8 shows the results of the confusion ma-
trix for the classification of Alzheimer’s disease ac-
cording to four categories obtained from MCNN
and FT-VGGNet using the augmented dataset. As
shown in this figure, using the augmented dataset,
FT-VGGNet19 also achieved the highest accuracy
compared to MCNN. FT-VGGNet19 correctly clas-
sifies 508 Mild Demented, 376 Moderate Demented,
DATA 2025 - 14th International Conference on Data Science, Technology and Applications
436
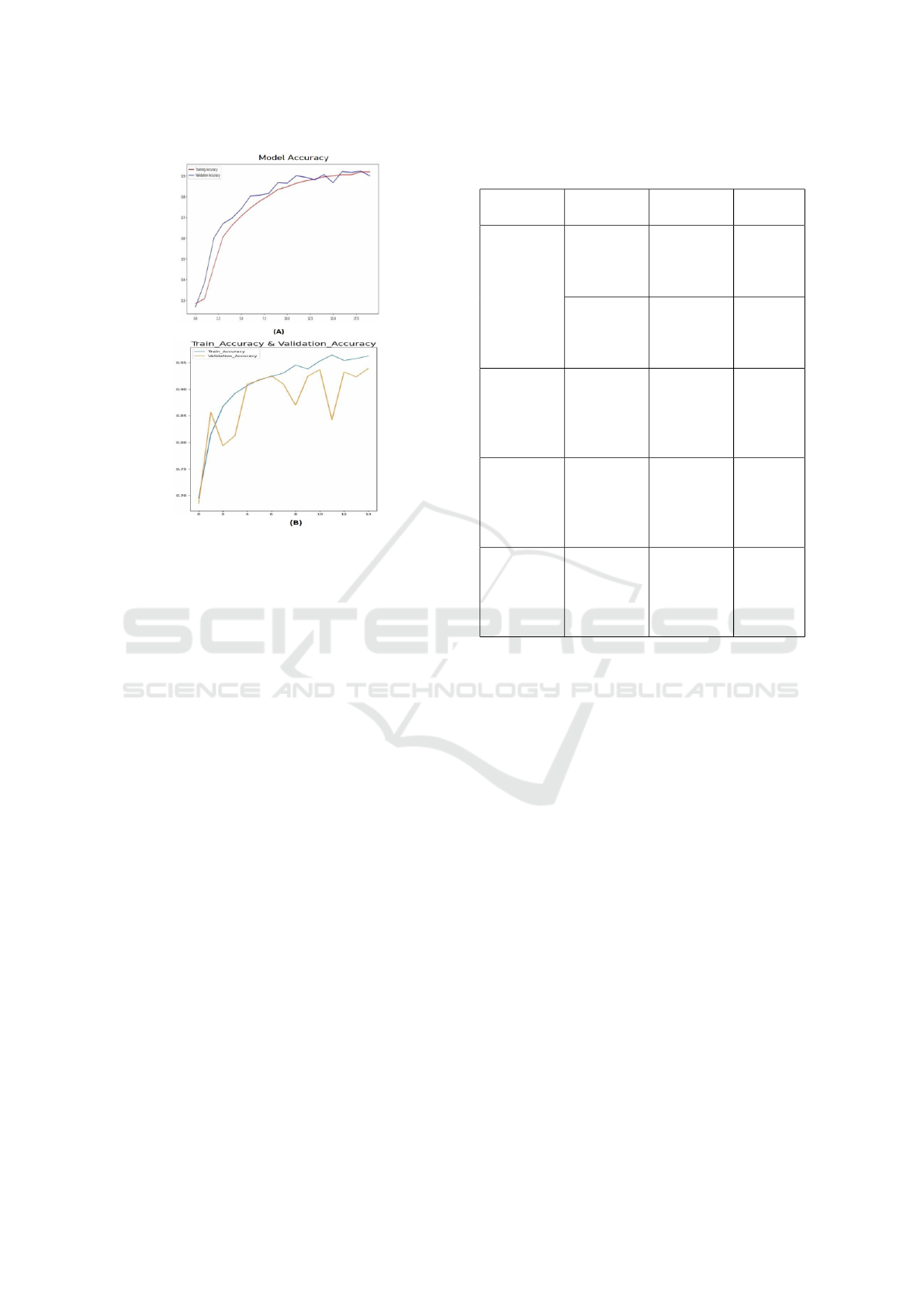
Figure 9: The Training and Validation Accuracy curves ob-
tained by (A) MCNN (B) FT-VGGNet19 with data augmen-
tation.
490 Non-Demented, and 510 Very Mild Demented
cases, with misclassifications mainly occurring be-
tween similar classes.
Figure 9 illustrates the training and validation ac-
curacy curves for Alzheimer’s disease classification
into four categories using MCNN and FT-VGGNet19
over 15 epochs with the augmented dataset. Specifi-
cally, Figure 9 (B) depicts the accuracy trends for the
proposed FT-VGGNet19 model.
A well-trained model exhibits steadily increasing
and stabilizing curves, indicating effective learning.
However, if the validation accuracy plateaus with no-
ticeable fluctuations while the training accuracy con-
tinues to rise, it suggests overfitting.
5 DISCUSSION AND
COMPARATIVE EVALUATION
This section presents the results of classifying
Alzheimer’s disease into four categories—non-
demented, very mildly demented, mildly demented,
and moderately demented—using various architec-
tures. We modified a basic CNN and fine-tuned a pre-
trained VGGNet19, evaluating them on both original
and augmented datasets. As shown in Table 2, data
augmentation had a significant positive impact.
The FT-VGGNet19 maintained high performance
Table 3: Comparative analysis of proposed work with pre-
vious works.
Authors Datasets Models Accuracy
(%)
Proposed
alzheimers-
dataset-4-
class-of-
images
FT-
VGGNet19
90%
Augmented
Alzheimer
MRI
Dataset
FT-
VGGNet19
92%
Ajagbe.
et al.,
(Ajagbe
et al.,
2021)
alzheimers-
dataset-4-
class-of-
images
VGG-19 77.66%
Ibrahem
M.M. et
al., (Mad-
hat et al.,
2024)
MRI
dataset
CNN
VGG16
DenseNet
Ensemble
90%
89%
86%
94%
Araqi
& Abbas
(Araqi and
Abbas,
2022)
alzheimers-
dataset-4-
class-of-
images
CNN 90.83%
across both datasets, while the modified CNN ben-
efited most from augmentation. These results high-
light the value of data augmentation, especially for
custom architectures when data is limited. To the best
of our knowledge, no prior work has been conducted
using augmented data for Alzheimer’s disease classi-
fication with this dataset. Therefore, we compare our
results with previous studies that used the same orig-
inal dataset. Table 3 highlights the effectiveness of
our proposed model in classifying Alzheimer’s dis-
ease compared to previous studies, including those
by Ajagbe et al. (Ajagbe et al., 2021) and Araqi &
Abbas (Araqi and Abbas, 2022), which were con-
ducted on the original Alzheimer’s dataset (4-class
classification). As illustrated in Table 3, our model
achieved an accuracy of 90%, outperforming Ajagbe
et al. (Ajagbe et al., 2021) (77.66%) with an improve-
ment of 12.34%, and yielding comparable results to
Araqi & Abbas (Araqi and Abbas, 2022) (90.83%).
6 CONCLUSION AND FUTURE
WORK
Alzheimer’s disease (AD) is a major neurodegenera-
tive disorder, where early diagnosis is crucial for ef-
Improved Alzheimer’s Detection from Brain MRI via Transfer Learning on Pre-Trained Convolutional Deep Models
437

fective intervention. Traditional diagnostic methods
rely on clinical expertise, which can lead to delays
and inconsistencies. Advances in deep learning, par-
ticularly in medical imaging, have significantly im-
proved diagnostic accuracy using brain MRI scans.
This study proposes a modified CNN (MCNN) and
a fine-tuned VGGNet19 (FT-VGGNet19) for classi-
fying Alzheimer’s disease into four categories—non-
demented, very mildly demented, mildly demented,
and moderately demented, evaluating both on original
and augmented datasets. The FT-VGGNet19 consis-
tently maintained high performance, achieving 92%
accuracy with data augmentation and 90% without. In
contrast, the MCNN benefited significantly from aug-
mentation, demonstrating notable improvement. Ad-
ditionally, we assessed the models using precision, re-
call, and F1-score, further validating their effective-
ness. Overall, this study underscores the potential
of deep learning in improving AD diagnosis through
MRI analysis. Future work will explore advanced
augmentation techniques and explainable AI frame-
works to enhance model interpretability and clinical
applicability.
ACKNOWLEDGEMENTS
The research leading to these results has received
funding from the Ministry of Higher Education and
Scientific Research of Tunisia under grant agreement
number LR11ES48.
REFERENCES
Ajagbe, S. A., Amuda, K. A., Oladipupo, M. A., Oluwaseyi,
F. A., and Okesola, K. I. (2021). Multi-classification
of alzheimer disease on magnetic resonance images
(mri) using deep convolutional neural network (dcnn)
approaches. International Journal of Advanced Com-
puter Research, 11(53):51.
Araqi, Z. S. and Abbas, H. H. (2022). Alzheimer’s disease
detection using deep learning on mri images. arXiv,
abs/2204.00068.
Awarayi, N. S., Twum, F., Hayfron-Acquah, J. B., and
Owusu-Agyemang, K. (2024). A bilateral filtering-
based image enhancement for alzheimer disease clas-
sification using cnn. Plos one, 19(4):e0302358.
Balasundaram, A., Srinivasan, S., Prasad, A., Malik, J., and
Kumar, A. (2023). Hippocampus segmentation-based
alzheimer’s disease diagnosis and classification of mri
images. Arabian Journal for Science and Engineer-
ing, 48(8):10249–10265.
Dubey, S. (2024). Search results for alzheimer’s disease
datasets. https://www.kaggle.com/datasets/tourist55/
alzheimers-dataset-4-class-of-images. Accessed:
2024-05-22.
El-Assy, A. M., Amer, H. M., Ibrahim, H. M., and Mo-
hamed, M. A. (2024). A novel cnn architecture for ac-
curate early detection and classification of alzheimer’s
disease using mri data. Scientific Reports, 14(1):3463.
El-Latif, A. A. A., Chelloug, S. A., Alabdulhafith, M.,
and Hammad, M. (2023a). Accurate detection of
alzheimer’s disease using lightweight deep learning
model on mri data. Diagnostics, 13(7):1216.
El-Latif, A. A. A., Chelloug, S. A., Alabdulhafith, M.,
and Hammad, M. (2023b). Accurate detection of
alzheimer’s disease using lightweight deep learning
model on mri data. Diagnostics, 13(7).
Fathi, S., Ahmadi, A., Dehnad, A., Almasi-Dooghaee,
M., Sadegh, M., and for the Alzheimer’s Dis-
ease Neuroimaging Initiative (2024). A deep
learning-based ensemble method for early diagnosis
of alzheimer’s disease using mri images. Neuroinfor-
matics, 22(1):89–105.
Fki, Z., Ammar, B., and Ayed, M. B. (2024). Towards au-
tomated optimization of residual convolutional neural
networks for electrocardiogram classification. Cogni-
tive Computation, 16(3):1334–1344.
Honig, L. S. and Chin, S. S. (2001). Alzheimer’s dis-
ease. Science of Aging Knowledge Environment,
2001(1):dn2–dn2.
Madhat, M. I., Kadhim, K. N., Mohamed, F., Mohd Rahim,
M. S., Najjar, F. H., and Ramadhan, A. J. (2024). Di-
agnosing alzheimer’s disease severity: A comparative
study of deep learning algorithms. In BIO Web of Con-
ferences, volume 97, page 00102. EDP Sciences, EDP
Sciences.
Mokni, R. and Haoues, M. (2022). Cadnet157 model:
fine-tuned resnet152 model for breast cancer diagno-
sis from mammography images. Neural Computing
and Applications, 34(24):22023–22046.
Rasmussen, J. and Langerman, H. (2019). Alzheimer’s dis-
ease - why we need early diagnosis. Degenerative
Neurological and Neuromuscular Disease, 9:123–
130. eCollection 2019.
Uraninjo (2024). Search results for alzheimer’s disease
augmentation datasets. https://www.kaggle.com/
datasets/uraninjo/augmented-alzheimer-mri-dataset.
Accessed: 2024-06-22.
Vengala, A. (2024). Classification of mild, very mild,
moderate, and non-demented alzheimer’s disease mri
scans with svm.
DATA 2025 - 14th International Conference on Data Science, Technology and Applications
438
