
A Streamlined Lesion Segmentation Method Using Deep Learning and
Image Processing for a Further Melanoma Diagnosis
Jinen Daghrir
1 a
, Wafa Mbarki
1
, Lotfi Tlig
1
, Moez Bouchouicha
2
, Noureddine Litaiem
3
,
Faten Zeglaoui
3
and Mounir Sayadi
1
1
Universit ´e de Tunis, ENSIT, Laboratory SIME, Tunisia
2
Universit ´e de Toulon, Aix Marseille Univ, CNRS, LIS, Marseille, France
3
University of Tunis El-Manar, Faculty of Medicine of Tunis, Department of Dermatology,
Charles Nicolle Hospital, Tunis, Tunisia
Keywords:
Deep Learning, u-Net, Image Processing, Skin Lesion Segmentation, Majority Voting, Medical Imaging.
Abstract:
Over the past two decades, the world has known a significant number of deaths from cancer. More specifically,
melanoma which is considered as the deadliest form of skin cancer causes a remarkable percentage of all can-
cer deaths. Therefore, the health and disease management community has exceedingly invested in creating
automated systems to help doctors better analyze such diseases. Correspondingly, we were interested in creat-
ing an automatic lesion detection task for further melanoma diagnosis. The lesion segmentation is considered
to be a critical step in a pattern recognition system. Our proposed segmentation technique consists of finding
lesions’ masks using a baseline, edge-based, and more sophisticated and state-of-the-art method: thresholding
using Otsu’s technique, morphological snakes, and a fully CNN (Convolutional Neural Network) model based
on the U-net architecture, respectively. These methods are commonly used when dealing with skin lesion seg-
mentation, and each one of them has its advantages and drawbacks. The U-net architecture is improved by the
use of the pre-trained encoder ResNet-50 on the ImageNet dataset. A majority voting is applied to generate
the final segmentation map using these three methods. The experiments were conducted using a benchmark
dataset and showed promising results compared to using these methods separately, the majority voting of the
three methods can significantly improve the segmentation task by refining the borders of the masks issued by
the Deep learning model.
1 INTRODUCTION
Skin cancer is considered one of the most frequent
types of cancer. Melanoma which is a particular
form of skin cancer, is the less common type but it
is considered as the most malignant one. This fa-
tal skin cancer can quickly spread to other parts of
the body causing about 60 000 cancer deaths in 2018
as reported in (Khazaei et al., 2019), it is consid-
ered as 0.7% of all cancer deaths. Statistics exam-
ining the incidence rate from 1973 to 2009 show a
rise in the number of cases (Heinzerling and Eigentler,
2021), which is particularly worrying in such a dis-
ease. Some types of skin lesions can be more dan-
gerous than others, due to their ability to spread to
other sites. About 5 to 15% of people having con-
genital nevi are more risked to develop melanoma.
a
https://orcid.org/0000-0002-1300-8939
About one-third of cases will be certainly affected
in their brains and can be associated with malforma-
tion of the central nervous system (Heinzerling and
Eigentler, 2021). The incidence and mortality rates
of melanoma differ from one country to another due
to the variety of ethnic and racial groups(Schadendorf
et al., 2018). The well-known definite risk factors for
melanoma include ultraviolet (UV) radiation, life in
low geographic latitudes, high alcohol consumption,
consuming fatty foods, the presence of melanocytic
or dysplastic naevi, a family or personal history of
melanoma, phenotypic characteristics including fair
hair, eye, and skin colors (Khazaei et al., 2019). In-
terestingly, it has been proved that the development
index (HDI) is quite related to the number of mortal-
ity of melanoma. The more the HDI index increases,
the more people have access to health services and
early detection of diseases, and the less mortality will
occur(Khazaei et al., 2019). UV protection is recom-
368
Daghrir, J., Mbarki, W., Tlig, L., Bouchouicha, M., Litaiem, N., Zeglaoui, F. and Sayadi, M.
A Streamlined Lesion Segmentation Method Using Deep Learning and Image Processing for a Further Melanoma Diagnosis.
DOI: 10.5220/0013472400003938
In Proceedings of the 11th International Conference on Information and Communication Technologies for Ageing Well and e-Health (ICT4AWE 2025), pages 368-376
ISBN: 978-989-758-743-6; ISSN: 2184-4984
Copyright © 2025 by Paper published under CC license (CC BY-NC-ND 4.0)

mended since it is proved that using protection will
lead to a decrease in the incidence rate. However,
the use of sunscreen does not provide enough pro-
tection against the development of melanocytic nevi
consequently the high risk of developing melanoma
(Heinzerling and Eigentler, 2021). One typical solu-
tion to decrease the annual number of deaths caused
by melanoma is diagnosed it in an early stage, where
the relative survival rate will be critical (Schaden-
dorf et al., 2018). Self-examination is so vital in this
case. Thus, people should examine their skin head
to toe regularly, looking for any lesions that might be
turned into melanoma. Self-exams can help people
identify skin cancers in an early stage when the odds
of curing them are completely high. Yet, physicians
encourage people to routinely do self-examination if
they notice any suspicious-looking lesions. ”when in
doubt, cut it out”, says Darrick Antell, MD, a board-
certified plastic and reconstructive surgeon practicing
in New York (Daghrir et al., 2020). Currently, the
health and disease management community has en-
tirely been interested in creating automated computer
systems for skin lesion inspection and characteriza-
tion. Thus, it becomes vital to use supportive imaging
to improve the diagnosis of this life-threatening skin
cancer. Conventionally, these systems use some vi-
sual cues to characterize the lesions as malignant or
not. One typical used clue is the ABCD signs. This
feature can differentiate malignant melanoma from
a benign skin lesion based on five defined charac-
teristics, namely asymmetry (A), border irregularity
(B), color variability (C), and diameter greater than 6
mm (D)(Maglogiannis and Doukas, 2009). To extract
information about the border, lesion segmentation
should be performed. This task is considered to be
a critical step in the whole process of melanoma de-
tection, it concerns the isolation of pathological skin
lesions from the surrounding healthy skin. The seg-
mentation step is usually done by experts, who try to
find the ROI (Region Of Interest). This method is ac-
curate since the experts apply their knowledge and ex-
periences to the segmentation process. Thought, this
process will be time-consuming and exigent, due to
the variability and reliability of the expert behavioral
observation. One straightforward method is to use
fully automatic systems where the segmentation pro-
cess is done using computer vision techniques without
the need for human intervention.
In medical image processing, ROI segmentation is
defined as a semantic segmentation which is a pixel-
level recognition problem. It refers to the process of
assigning each pixel in the image to a particular label.
Particularly, in the case of skin lesion segmentation,
healthy skin is assigned a background label. How-
ever, the inspected lesion is assigned a foreground la-
bel. The result of this process is a segmentation map,
where the background and foreground regions are as-
signed with two different class labels. Commonly,
many classical image processing techniques are pro-
posed for Medical image segmentation (Mbarki et al.,
2020; Sharma and Ray, 2006). These techniques rely
only on low-level pixel processing and mathematical
modeling to construct rule-based systems. Thresh-
olding has been widely used since it is simple and
not time-consuming. It consists of dividing an im-
age into two regions: one corresponds to the ROI and
the other to the background. Thus, each pixel is as-
signed a class label if its intensity is greater than a
threshold. The threshold can be either globally or lo-
cally fixed according to a small set of pixels’ inten-
sities. The threshold is defined using statistical anal-
ysis or by optimizing a certain criterion. Thus, us-
ing only thresholding can often fail to produce accu-
rate results since it can generate disconnected objects.
However, Active contours or snakes can guarantee a
connected set of pixels for an ROI. These methods
are considered edge-based methods. An active con-
tour is formulated as an energy minimization prob-
lem, where the energy depends on the placement of
the curve surrounding the object to be segmented.
The initial contour is manually defined and then it-
eratively deformed to minimize the energy function,
which is typically based on the intensity differences
between the object and the background pixels. Obvi-
ously, these methods are very sensitive to noise and
the initial curve placement. The large variability of
the shape of substructures and organs in medical im-
ages is one of the most challenging problems for the
segmentation task. Therefore, there is no universal
segmentation technique. Some techniques do not use
only color information of pixels, but also much more
domain knowledge(Litjens et al., 2017). In order to
define and implement this knowledge, a considerable
amount of expertise and time is required. Thus, com-
pletely automated systems are proposed to model the
domain knowledge by training some algorithms with
labeled pixels. Recently, a significant trend in seg-
mentation tasks is the use of deep learning(DL)due
to its effectiveness and speediness. With the advance
of DL models, scientific papers presenting organ and
substructure segmentation have gradually increased in
the last few years(about 90 papers were published in
2017)(Litjens et al., 2017). The motivation for the
excessive use of DL is the ability to use one model
in many different domain applications and with dif-
ferent data resources, such as in(Alom et al., 2018),
authors have tested their proposed DL model on three
different benchmark datasets such as blood vessel in
A Streamlined Lesion Segmentation Method Using Deep Learning and Image Processing for a Further Melanoma Diagnosis
369

the retina, skin lesion, and lung lesion datasets. One
widely used architecture of DL is the CNN( Con-
volutional Neural Network) which ensures continued
good performance in many tasks, such as classifica-
tion, segmentation, detection, decision-making, etc.
The number of papers published in 2017 applying
CNNs in medical image analysis is over 200 papers,
and most of them are dedicated to dealing with sub-
structure segmentation(Litjens et al., 2017). Excep-
tionally, for semantic segmentation, a fully convolu-
tional network is required, which contains two op-
posite units, encoder and decoder. The encoder is
used to define the high-level information with a low-
resolution spatial tensor, while the decoder contains
also convolution layers but coupled with up-sampling
instead of down-sampling layers that increase the size
of the spatial tensor accordingly recapturing the seg-
mented image. In(Ronneberger et al., 2015), the au-
thors have proposed the U-net architecture that takes
an input image and outputs a segmentation map that
has the same dimension as the input. The U-net was
first used to detect cell boundaries in biomedical im-
ages. The contribution of such architecture is to use
skip connections, which connect opposite convolu-
tional layers in order to preserve low-level informa-
tion. Definitely, DL is efficiently and widely used
for many tasks, but the fact that it is time-consuming
and data-hungry makes it a little bit limited. The per-
formance of systems using DL will degrade if the
amount of input data is not big enough. In addi-
tion, the data can be only produced by an expert who
will label every input. Time and resources are re-
quired to properly do data labeling to build highly
accurate systems, but it is not a straightforward pro-
cess when it comes to huge datasets. For melanoma
diagnosis, the border-based features should be ex-
tracted after segmenting the skin lesion. Two main
automatic segmentation methods are presented in the
literature, region-based and contour-based methods.
Other hybrid methods are proposed which use both
color transformation and contour detection techniques
(Maglogiannis and Doukas, 2009). These methods
are also classified into two groups: Methods that use
classical image processing with analysis techniques
and those that involve Artificial Intelligence(AI) con-
cepts. The most usual and basic techniques that are
used for lesion segmentation, are thresholding and
region-growing techniques. These methods are clas-
sified as region-based approaches since they involve
the differentiation of pixels’ intensities of both ma-
lignant and healthy skin regions. Another type of
method is contour-based which detects the region of
interest by finding its edge. However, with the ad-
vent of more powerful techniques, these techniques
seem to be very naive. For that, hybrid approaches
are proposed in the literature (Jamil et al., 2019)
that use both color transformation and edge detec-
tion, snakes or active contours are considered to be
the state-of-the-art of lesion segmentation before the
extensive use of machine learning(Maglogiannis and
Doukas, 2009). Other methods involving AI tech-
niques like fuzzy borders, K-means segmentation, or
Deep Learning (DL) techniques are considered nowa-
days as the state-of-the-art for most image segmenta-
tion problems (Jadhav et al., 2019). Melanoma de-
tection at an early stage can highly extend the life
expectancy, thus it becomes vital to use CAD to en-
sure a quick and accurate diagnosis of a huge number
of people. Due to the sensitivity of the lesion seg-
mentation process which plays an imperative role in
melanoma diagnosis. Many research papers are pro-
posed in the literature dealing with lesion segmenta-
tion, although research is still trending up sharply due
to a lot of challenges.
In this matter, we were interested in creating a
new method for lesion segmentation that combines
three different methods: thresholding using Otsu’s
technique, morphological Snakes, and a deep convo-
lutional neural network based on U-net. The afore-
mentioned techniques are used independently to gen-
erate three lesion masks. Then, using majority voting
a new lesion segmentation map is generated. Major-
ity voting defines the class of each pixel whether it
belongs to the lesion or to the background based on
the other decisions. This paper is organized as fol-
lows. In the following section, we extendedly intro-
duce our proposed method. All the conducted exper-
iments and the results evaluating the performance of
the proposed method are given in the third section. At
the end, we highlight and discuss the basic concept of
our proposed method.
2 PROPOSED METHOD
One critical process in melanoma diagnosis is lesion
segmentation. It consists of identifying the lesion’s
pixels among the others belonging to the healthy
skin. In this paper, a new method has been proposed
that combines three different methods using major-
ity voting. The fusion of the three results issued by
thresholding using Otsu’s technique, morphological
snakes, and CNN-based U-Net architecture, signifi-
cantly ameliorates the segmentation mask by refining
the lesion’s border.
ICT4AWE 2025 - 11th International Conference on Information and Communication Technologies for Ageing Well and e-Health
370

2.1 Thresholding Method
Thresholding has been widely used in skin cancer due
to its effectiveness and integrity. Researchers have
proposed many local adaptative and global threshold-
ing techniques, such as Kapur et al.’s Entropy tech-
nique(Armato et al., 2002), Abutaleb’s entropy tech-
nique(Ashwin et al., 2012), and Kittler and Illing-
worth’s minimum error technique(Bae et al., 2005).
However, Otsu’s algorithm has been extensively ap-
plied in many systems. Thresholding is done by find-
ing one single threshold that classifies pixels into two
classes without image preprocessing. The threshold is
determined by minimizing intra-class intensity vari-
ance σ
w
, which is defined as the weighted sum of
class variances:
σ
w
(t) = w
C
0
(t)σ
2
C
0
(t) + w
C
1
σ
2
C
1
(t) (1)
w
C
0
and w
C
1
are the probabilities of the two classes
separated by a threshold t, and σ
2
C
0
and σ
2
C
1
are the
variances of the two classes. After computing the
probabilities of each class using the histogram, w
i
(0)
and σ
2
i
(0) are initially set. Using different values of
threshold t from 1 to the maximum pixel’s intensity,
w
i
(t) and σ
2
i
(t) will be computed to get the intra-
class intensity variance. The global threshold will be
the one that minimizes the σ
w
(t). This threshold is
used then to differentiate the foreground which is the
pathological skin lesion and the background. The seg-
mentation map is found by assigning the value 1 to all
the pixels having an intensity greater than the thresh-
old t and the value 0 to all the other pixels. Two steps
are performed to filter the unwanted objects. First, an
opening is used twice, the morphological opening is
an erosion followed by dilation using a disk as a struc-
turing element. The erosion of the image helps to re-
move the small objects. Consecutively, dilation helps
in ameliorating the background region by closing all
the gaps. For further filtering, big regions having a
centroid that does not belong to 75% of the image area
will be eliminated. This process ensures that only the
skin lesion mask will stand. Fig.1 illustrates the result
of using thresholding and filtering the segmentation
map.
2.2 Morphological Snake Method
Active contours, also known as Snakes are one of
the most used techniques in computer vision, more
specifically for image segmentation. Frequently,
these methods are edge-based which requires clean
boundaries indicting and the absence of artifacts and
noises. These problems were then handled by the
invention of more practical approaches such as the
Active Contours Without Edges (ACWE)(Marquez-
Neila et al., 2013). These approaches are known
as morphological snakes since they use morpholog-
ical operators over a binary array. The morpholog-
ical ACWE can accurately segment the object when
the pixels of the region of interest and those of the
background have different averages. In this work, the
Chan-Vese algorithm(Chan and Vese, 1999) which is
a form of the snake curve is adopted. It is an energy
minimization method that is widely used in medical
applications. This method does not require defining
the contours to separate the heterogeneous objects and
it is sensitive to noise. This algorithm can also ac-
curately detect the objects and increase the conver-
gence speed when using a good initial curve place-
ment. Since this method can detect more than one
region of interest and obviously the skin lesion will
be placed in the center of the image, we have used the
initial curve presented in Fig.2. The result of using
the snakes is shown in Fig.2. The final segmentation
mask is generated after postprocessing the output of
the Snake process. We have applied the morphologi-
cal opening to refine the segmentation map.
2.3 U-Net Based Resnet50
The U-Net architecture is a fully convolutional neu-
ral network that was mainly developed for biomed-
ical image segmentation. It consists of two blocks:
encoder and decoder. CNNs are commonly used for
classification tasks, the input image is fed to the net-
work and downsampled into a low-resolution tensor
containing high-level information which can then be
classified using a fully connected layer. However, to
generate a segmentation map that has the same size as
the input, the output of the encoder should pass by an
upsampling path( decoder). This makes the network
layout has a U shape.
The left side of the U follows the typical archi-
tecture of a convolutional network. It consists of the
repeated application of two 3× 3 convolutions, which
is followed by a normalization layer and a rectified
linear unit (ReLU) activation function. Dropout and
2 × 2 max-pooling layers are used to downsample the
feature map. At each downsampling step, the num-
ber of feature channels is increased. The purpose of
the encoder is to capture the high-level information of
the input image to do the segmentation process. To
prevent losing the low-level information, the decoder
should have connections with the encoder layers, that
is known as skip connections.
Many CNNs are previously trained for image clas-
sification tasks, that contain meaningful information
about how to perform predictions effectively. These
A Streamlined Lesion Segmentation Method Using Deep Learning and Image Processing for a Further Melanoma Diagnosis
371
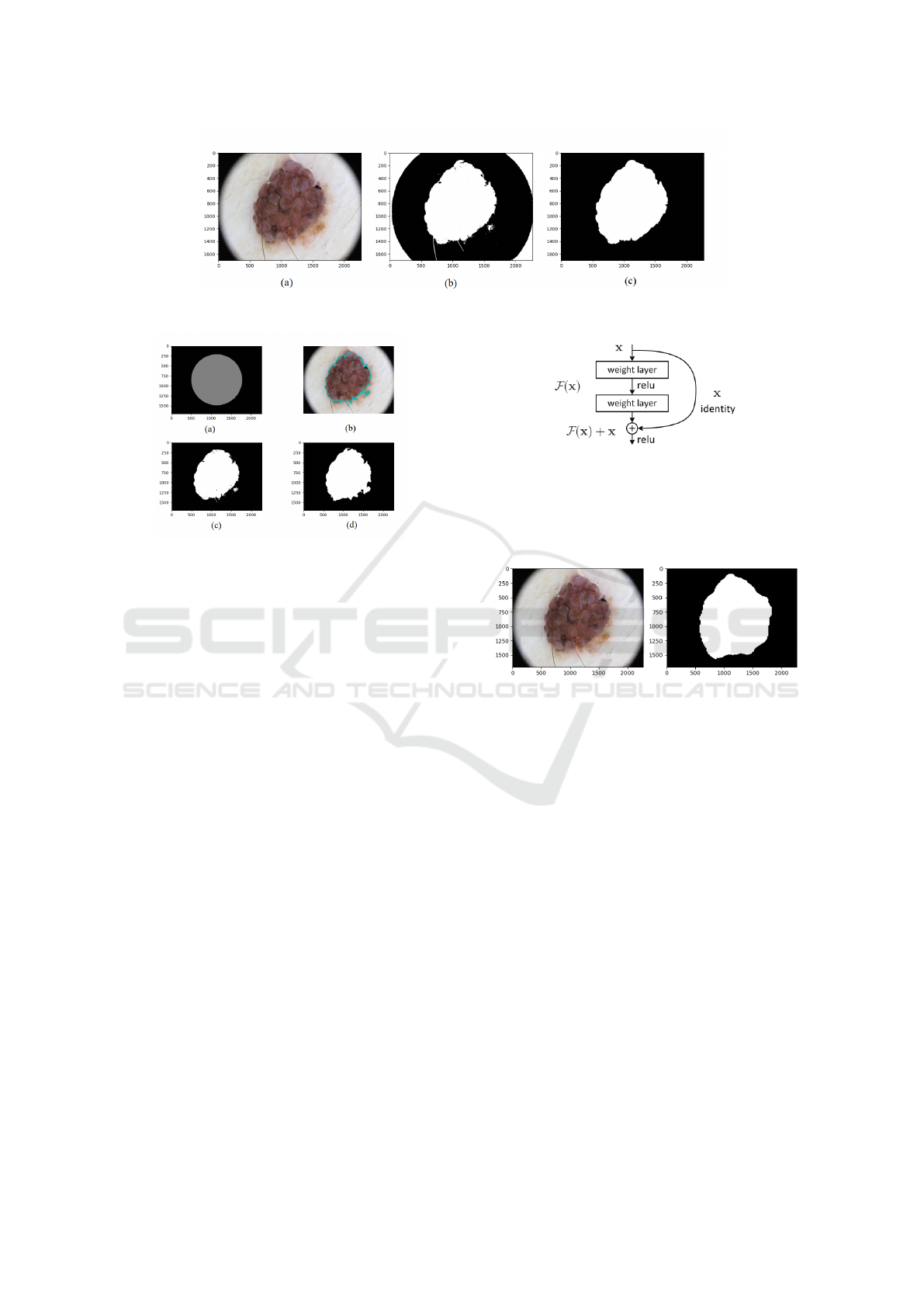
Figure 1: Segmentation result of the thresholding process: (a) Original image, (b) segmentation map using Otsu’s algorithm,
and (c) segmentation map after filtering.
Figure 2: Segmentation result of using morphological
snakes: (a) initial curve, (b) evolution of the curve, (c) fi-
nal segmentation map, and (d) final segmentation map after
applying morphological operations.
models can be re-used to vastly speed up the train-
ing process, this concept is called transfer learn-
ing. Further, the convolution layers of the pre-
trained models can be re-used in the encoder lay-
ers of the segmentation model. In this work, a
Resnet50 is adopted which was pre-trained on the
ImageNet dataset(Russakovsky et al., 2015). This
dataset is widely used when dealing with medical
imaging problems since it contains a repository of
annotated clinical (radiology and pathology) images.
Thus, the U-net model will use the weights of a
pre-trained ResNet50 model systematically having
knowledge about the disposed features.
CNN has known many improvements over time,
one common trend in research was to add more regu-
larization layers to overcome overfitting. AlexNet has
five convolutional layers, however, other architectures
have gone deeper to ameliorate their performance,
such as VGG and GoogleNet networks. Therefore,
increasing the convolutional layers does not regularly
improve the performance, contrarily, its performance
can get saturated due to vanishing gradient issues.
Thus, ResNet was introduced to overcome this issue
by adding identity shortcut connections and skipping
one or more convolutional layers as shown in Fig.3
(He et al., 2016a). Then the residual block has been
ameliorated generating a pre-activation variant of the
residual block where the gradient can flow through
Figure 3: The residual block.
the shortcut connections to any other earlier convolu-
tional layer(He et al., 2016b).
Fig.4 illustrates the segmentation process using
the U-Net-based ResNet50 encoder.
Figure 4: Semantic segmentation result when using the U-
net architecture based ResNet50 encoder: Original image,
and segmentation map.
2.4 Majority Voting
After applying the three techniques separately, a fu-
sion method on the three segmentation maps is used.
The fusion of multiple result predictions has been
used by many researchers as an accurate strategy to
ameliorate the performance of many pattern recog-
nition systems, rather than relying on one single
model prediction Many techniques have been pro-
posed such as majority voting, bayesian decision
fusion(Yue et al., 2012), or Dempster-Shafter the-
ory(Kang et al., 2020) for fuzzy information fusion.
In this paper, we used the majority voting method
which is one of the basic and intuitive approaches.
It assigns a sample based on the most frequent class
assignment(Ballabio et al., 2019). For semantic seg-
mentation, each pixel in the output of the major-
ity voting process is assigned a background or fore-
ground class based on the three other correspond-
ing pixels of the three segmentation maps issued by
ICT4AWE 2025 - 11th International Conference on Information and Communication Technologies for Ageing Well and e-Health
372

thresholding, snakes, and U-Net-based ResNet.
3 EXPERIMENTS AND RESULTS
Experiments used to evaluate the performance of
DL models are not only an academic practice but
even when creating commercial systems, experiments
are necessary to create the most performant models.
Thus, to demonstrate the performance of our pro-
posed method, we have tested the methods separately
using different strategies and then we have reported
the result of fusing them. The experiments were con-
ducted using the ISIC2017 ( International Skin Imag-
ing Collaboration) dataset(Codella et al., 2018).
3.1 Technical Materials
Implementing these methods was done using Keras,
Sklearn, OpenCV, and TensorFlow frameworks. The
Keras and TensorFlow are used on a single machine
with an NVIDIA GEFORCE GTX-1050 Ti.
3.2 Dataset Representation
The experiments were conducted using a public
dataset which is collected from the ISIC archive, the
dataset was taken from a competition on skin lesion
segmentation that held in 2017(Codella et al., 2018).
The images were collected from a dermatoscopy tool,
and it contains about 2000 images of malignant and
benign lesions with their corresponding segmentation
maps. The dermoscopic lesion images are in JPEG
format, the size of almost all images is 1022×767
pixels. Their ground truth images are mask images
in PNG format. They are encoded as grayscale(8-
bit), where each pixel is 0 for pixels belonging to the
healthy skin(Background) and 255 for pixels belong-
ing to the suspicious lesion.
3.3 Evaluation Strategy
Our proposed method consists of fusing different
types of methods, those that are trainable and those
that use classical image processing methods. To
demonstrate the performance of our proposed method
the data set is split into two groups: a testing set con-
taining 600 images and 1400 images used for build-
ing the DL model. The selected test images are used
to test the DL model and to evaluate the performance
of the two other methods, as well. The normal pro-
cedure when evaluating a hypothesis is to validate it.
The validation helps to formulate the hypothesis to
see other proprieties and relations that can make it
better understood. Thus, this process will be itera-
tively repeated to find a better version of the hypoth-
esis, also the same process is needed when creating
ML and DL models. While training the DL model,
a validation step should take place at each epoch. It
is necessary to validate its performance to report how
well the model is learning. Yet, a part of the train-
ing set will be held out for validation, and when the
experiments seem to be successful, the final evalua-
tion will be done on the testing set. Therefore, split-
ting the training set into two sets one for training and
the other for validation may fail in accurately building
the best machine learning model due to the reduction
of the number of samples used for learning. Many
validation strategies are introduced for evaluating the
model(James et al., 2013), such as k-fold,leave-one-
out Cross-Validation, etc. The latter strategy consists
of validating one image using the model that has been
trained on the other remaining images. However, the
k-fold Cross-Validation is more interesting and rele-
vant since it minimizes the number of validation pro-
cesses by dividing the N data points into k subsets.
The training and the validation are then repeated k it-
erations, in each iteration one of the subsets is used
for validation and the remaining for training. The er-
ror is also averaged over all the k trials. Basically, k
can be either 5 or 10. Thus, we have used this strat-
egy for the validation of the DL model. We have also
evaluated its performance by splitting the dataset into
two sets: 1250 sample images for training and 150
for validation. This is the same conventional splitting
strategy used in(Codella et al., 2018).
3.4 Experimental Results
Fine-tuning the parameters depends on the nature of
the ML model. For deep learning, generally, the num-
ber of epochs will vary, simultaneously the training
and validation accuracy will be computed. This ex-
periment shows how the DL model is proceeding at
different epoch numbers. Regarding the experiments
done by Md Zahangir Alom et al.(Alom et al., 2018),
the training and validation accuracy becomes slightly
stable when using more than 50 epochs. Thus, this
number is adopted in this work.
We have trained the U-Net based Resnet encoder
with 1250 images, the 150 images will be used for
validation during training with a batch size of 12,
we have used the ADAM optimizer technique. Also,
the images were resized to 256 × 256 pixels and aug-
mented by applying many transformations such as ro-
tation, scale, color, and flipping. Using these trans-
formations on the images will increase the size of
the dataset, automatically ameliorating the training
A Streamlined Lesion Segmentation Method Using Deep Learning and Image Processing for a Further Melanoma Diagnosis
373
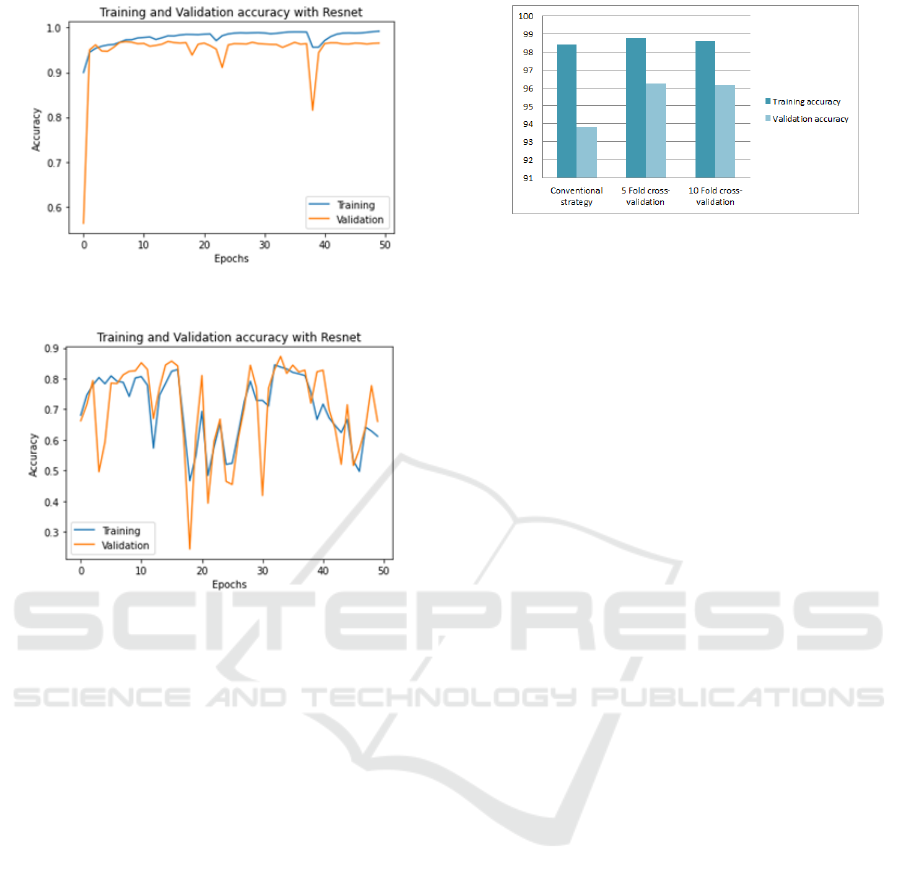
Figure 5: Validation and training accuracy using U-net
based ResNet50.
Figure 6: Validation and training accuracy using
DeepLabv3+ based ResNet50.
performance. Fig5 shows both the training and val-
idation accuracy when using 50 epochs. The accu-
racy is stable for both validation and training. We
have also tested the DeepLabv3+ based Resnet50 en-
coder(Chen et al., 2018). The training and validation
accuracy are shown in Fig.6 which shows many oscil-
lations compared to Fig.5. It is caused by the use of
a small batch. It is observed that when using a small
batch for some models, it will appear a huge degra-
dation in the training process. Thus, the batch size
is an important hyperparameter that leads to either
sharp or flat minimizers. We have used many strate-
gies for evaluating the performance of the proposed
method. For that, using the U-Net based ResNet50
encoder with a batch size of 12 is good enough for
accurately training the model and for minimizing the
amount of memory consumption. For a deeper study
of the U-net architecture, we have used more than
one validation strategy with 20 epochs. First, training
and validation were done using the conventional split-
ting strategy. Validation was performed using ran-
domly selected 150 images. Another strategy is the
k-fold cross-validation was performed. Using the k-
fold strategy can notably increase the diversity of data
used for both validation and training. The model will
Figure 7: Validation and training accuracy using U-net
based ResNet50 with different validation strategies.
be more generalized and unbiased. The training accu-
racy for both k-fold strategies is slightly higher than
using the conventional strategy (see Fig.7). However,
the validation accuracy is considerably greater when
using k-fold cross-validation for k=5 and k=10. Evi-
dently, models were stable in their predictions due to
the effectiveness of the training phase and the diver-
sity of validation samples.
After training the U-Net based Resnet50 model
using 1250 images during the 50 epochs, the network
weights are saved when the validation accuracy is at
its highest. These weights will be used then to make
predictions on the 600 testing images. Each one of
the three techniques will result in an output segmen-
tation mask. Table1 reports the quantitative results
of the experiments. The accuracy and IoU scores are
shown for each technique as well as for fusing them.
Fig.8 highlights some of the output examples of our
proposed method, it is obvious that the fifth column
contains the most accurately segmented skin lesions
compared to the output of the DL model predictions.
Fig.8 shows that the DL model segments the le-
sion accurately when thresholding fails in segment-
ing it. However, using majority voting to fuse the
three techniques can refine the segmentation result.
The irregularity is an important border-based feature
to recognize Melanoma, the proposed method can
perfectly draw attention to sharp borders contrarily
to the DL model. The ISIC2017 dataset has been
proposed in the 2017 skin lesion segmentation chal-
lenge. Yading Yuan has proposed a framework based
on a deep fully convolutional-deconvolutional neu-
ral network(CDNN) to automatically detect skin le-
sions(Yuan, 2017). This method yielded a Mean IoU
of 0.784 on the testing set, our proposed method pro-
vides a testing Mean IoU of 0.912 with a high accu-
racy of 0.983.
ICT4AWE 2025 - 11th International Conference on Information and Communication Technologies for Ageing Well and e-Health
374

Figure 8: Experimental results for skin lesion segmentation: (a) original lesion image, the segmentation map from: (b)
thresholding, (c) snakes, (d) U-net based Resnet50, (e) the proposed method, and (f) ground truth segmentation mask.
Table 1: Testing accuracy of the different methods and fusing them using majority voting.
Method Thresholding Snakes ResNet Majority Voting
Accuracy 87.61% 87.45% 96.64% 98.31%
Mean IoU 66.18% 66.66% 89.01% 91.29%
4 CONCLUSIONS
Statistics on melanoma incidence rates show that it
can be a non-life-threatening cancer if detected early.
Early diagnosis and treatment significantly improve
the chances of successful recovery. The healthcare
community has been highly invested in creating au-
tomatic systems that can help doctors and patients
quickly and accurately inspect melanoma. These sys-
tems are built based on image processing and machine
learning techniques. One typical process is segment-
ing the skin lesion that will be inspected and then rec-
ognizing it as malignant or benign. In this work, we
have proposed a new method for lesion segmentation
that fuses three different methods: thresholding using
Otsu’s algorithm, morphological snake, and U-Net-
based Resnet50. Data fusion, specifically decision
fusion combines the decisions of multiple decisions
into a common one. The majority voting was used
to generate the final segmentation map. Using other
decision fusion techniques can be adapted further to
ameliorate the performance of the proposed system.
The U-Net-based Resnet50 can be also explored us-
ing data fusion in the encoder or decoder units.
ACKNOWLEDGEMENTS
The authors would like to appreciably thank all the
members of LIS laboratory, for their direct help and
collaboration. This research was done during an in-
ternship at LIS laboratory, SeaTech, University of
Toulon.
REFERENCES
Alom, M. Z., Hasan, M., Yakopcic, C., Taha, T. M.,
and Asari, V. K. (2018). Recurrent residual con-
volutional neural network based on u-net (r2u-net)
for medical image segmentation. arXiv preprint
arXiv:1802.06955.
Armato, S. G., Li, F., Giger, M. L., MacMahon, H., Sone,
S., and Doi, K. (2002). Lung cancer: performance
of automated lung nodule detection applied to can-
cers missed in a ct screening program. Radiology,
225(3):685–692.
Ashwin, S., Ramesh, J., Kumar, S. A., and Gunavathi,
K. (2012). Efficient and reliable lung nodule detec-
tion using a neural network based computer aided di-
agnosis system. In 2012 International Conference
on Emerging Trends in Electrical Engineering and
Energy Management (ICETEEEM), pages 135–142.
IEEE.
A Streamlined Lesion Segmentation Method Using Deep Learning and Image Processing for a Further Melanoma Diagnosis
375

Bae, K. T., Kim, J.-S., Na, Y.-H., Kim, K. G., and Kim,
J.-H. (2005). Pulmonary nodules: automated detec-
tion on ct images with morphologic matching algo-
rithm—preliminary results. Radiology, 236(1):286–
293.
Ballabio, D., Todeschini, R., and Consonni, V. (2019). Re-
cent advances in high-level fusion methods to classify
multiple analytical chemical data. Data Handling in
Science and Technology, 31:129–155.
Chan, T. and Vese, L. (1999). An active contour model
without edges. In International Conference on Scale-
Space Theories in Computer Vision, pages 141–151.
Springer.
Chen, L.-C., Zhu, Y., Papandreou, G., Schroff, F., and
Adam, H. (2018). Encoder-decoder with atrous sepa-
rable convolution for semantic image segmentation. In
Proceedings of the European conference on computer
vision (ECCV), pages 801–818.
Codella, N. C., Gutman, D., Celebi, M. E., Helba, B.,
Marchetti, M. A., Dusza, S. W., Kalloo, A., Liopy-
ris, K., Mishra, N., Kittler, H., et al. (2018). Skin
lesion analysis toward melanoma detection: A chal-
lenge at the 2017 international symposium on biomed-
ical imaging (isbi), hosted by the international skin
imaging collaboration (isic). In 2018 IEEE 15th In-
ternational Symposium on Biomedical Imaging (ISBI
2018), pages 168–172. IEEE.
Daghrir, J., Tlig, L., Bouchouicha, M., and Sayadi, M.
(2020). Melanoma skin cancer detection using deep
learning and classical machine learning techniques: A
hybrid approach. In 2020 5th International Confer-
ence on Advanced Technologies for Signal and Image
Processing (ATSIP), pages 1–5. IEEE.
He, K., Zhang, X., Ren, S., and Sun, J. (2016a). Deep resid-
ual learning for image recognition. In Proceedings of
the IEEE conference on computer vision and pattern
recognition, pages 770–778.
He, K., Zhang, X., Ren, S., and Sun, J. (2016b). Iden-
tity mappings in deep residual networks. In Euro-
pean conference on computer vision, pages 630–645.
Springer.
Heinzerling, L. and Eigentler, T. K. (2021). Skin cancer
in childhood and adolescents: Treatment and implica-
tions for the long-term follow-up. In Late Treatment
Effects and Cancer Survivor Care in the Young, pages
349–355. Springer.
Jadhav, A. R., Ghontale, A. G., and Shrivastava, V. K.
(2019). Segmentation and border detection of
melanoma lesions using convolutional neural network
and svm. In Computational Intelligence: Theories,
Applications and Future Directions-Volume I, pages
97–108. Springer.
James, G., Witten, D., Hastie, T., and Tibshirani, R. (2013).
An introduction to statistical learning, volume 112.
Springer.
Jamil, U., Sajid, A., Hussain, M., Aldabbas, O., Alam, A.,
and Shafiq, M. U. (2019). Melanoma segmentation
using bio-medical image analysis for smarter mobile
healthcare. Journal of Ambient Intelligence and Hu-
manized Computing, 10(10):4099–4120.
Kang, B., Zhang, P., Gao, Z., Chhipi-Shrestha, G., Hewage,
K., and Sadiq, R. (2020). Environmental assessment
under uncertainty using dempster–shafer theory and
z-numbers. Journal of Ambient Intelligence and Hu-
manized Computing, 11(5):2041–2060.
Khazaei, Z., Ghorat, F., Jarrahi, A., Adineh, H., Sohrabi-
vafa, M., and Goodarzi, E. (2019). Global incidence
and mortality of skin cancer by histological subtype
and its relationship with the human development in-
dex (hdi); an ecology study in 2018. World Cancer
Res J, 6(2):e13.
Litjens, G., Kooi, T., Bejnordi, B. E., Setio, A. A. A.,
Ciompi, F., Ghafoorian, M., Van Der Laak, J. A.,
Van Ginneken, B., and S
´
anchez, C. I. (2017). A survey
on deep learning in medical image analysis. Medical
image analysis, 42:60–88.
Maglogiannis, I. and Doukas, C. N. (2009). Overview of ad-
vanced computer vision systems for skin lesions char-
acterization. IEEE transactions on information tech-
nology in biomedicine, 13(5):721–733.
Marquez-Neila, P., Baumela, L., and Alvarez, L. (2013). A
morphological approach to curvature-based evolution
of curves and surfaces. IEEE Transactions on Pattern
Analysis and Machine Intelligence, 36(1):2–17.
Mbarki, W., Bouchouicha, M., Frizzi, S., Tshibasu, F.,
Farhat, L. B., and Sayadi, M. (2020). Semi-automatic
segmentation of intervertebral disc for diagnosing her-
niation using axial view mri. In 2020 5th International
Conference on Advanced Technologies for Signal and
Image Processing (ATSIP), pages 1–6. IEEE.
Ronneberger, O., Fischer, P., and Brox, T. (2015). U-net:
Convolutional networks for biomedical image seg-
mentation. In International Conference on Medical
image computing and computer-assisted intervention,
pages 234–241. Springer.
Russakovsky, O., Deng, J., Su, H., Krause, J., Satheesh, S.,
Ma, S., Huang, Z., Karpathy, A., Khosla, A., Bern-
stein, M., et al. (2015). Imagenet large scale visual
recognition challenge. International journal of com-
puter vision, 115(3):211–252.
Schadendorf, D., van Akkooi, A. C., Berking, C.,
Griewank, K. G., Gutzmer, R., Hauschild, A., Stang,
A., Roesch, A., and Ugurel, S. (2018). Melanoma.
The Lancet, 392(10151):971–984.
Sharma, N. and Ray, A. K. (2006). Computer aided seg-
mentation of medical images based on hybridized ap-
proach of edge and region based techniques. In Proc.
Int. Conf. Math. Biol, pages 150–155.
Yuan, Y. (2017). Automatic skin lesion segmentation with
fully convolutional-deconvolutional networks. arXiv
preprint arXiv:1703.05165.
Yue, D., Guo, M., Chen, Y., and Huang, Y. (2012). A
bayesian decision fusion approach for microrna target
prediction. BMC genomics, 13(8):1–11.
ICT4AWE 2025 - 11th International Conference on Information and Communication Technologies for Ageing Well and e-Health
376
