
Analysis of Technologies for Improving Steel Purity by Controlling
Slag Content
Nosir Saidmakhamadov
1a
, Nozimjon Kholmirzaev
2b
, Valida Bekchanova
1c
, Furkat Odilov
3d
,
Ibrokhimjon Tukhtaboev
1e
, Bokhodir Karimov
1f
and Murodilla Gaybullaev
1g
1
Namangan Engineering-Construction Institute, Technology of Machine Building Department,
160100, Namangan, Uzbekistan
2
Tashkent State Technical University, Foundry Technologies Department 100057, Tashkent, Uzbekistan
3
Andijan Institute of Economics and Construction, Civil Engineering Department 170100, Andijan, Uzbekistan
Keywords: Steel Purity, Slag Control, Casting Quality.
Abstract: This article analyzes the composition and reactions of slag formed during steel melting, as well as the
influence of liquid slag on the quality of cast products. The importance of using slag-forming fluxes and
accelerating the oxidation process of elements in the process of melting the alloy is mentioned. By adding
flux to the liquid metal, the slag was completely separated from the liquid metal by accelerating the formation
process. In addition, the quality of cast products was improved by separating them from the liquid metal, since
the addition of slag during the process of pouring liquid metal into the ladle and casting mold affects the
quality of the casting.
1 INTRODUCTION
Improving the quality of steel requires the reduction
of harmful elements, mirrors and gas pores in its
composition, as well as the prevention of liquid metal
fluidity and the formation of cracks, as well as
obtaining high-quality cast products. Improved
quality of bulk products ensures long – term use.
Generally, the steels used in most products have a
high Cr – C system content, and the steel grades
produced by the researchers are no exception, can be
seen in the table 1. Factors affecting the service life
of steel are: non-metallic inclusions, segregation, etc.
Among these factors, the effect of non-metallic
additives on steels has been studied using various
methods (Saidmakhamadov, 2024; Patrik, 1997;
Kholmirzaev et al., 2023; Nodir et al., 2021). In these
studies, non-metallic inclusions play the role of
a
https://orcid.org/0000-0002-7460-5972
b
https://orcid.org/0009-0008-9662-4796
c
https://orcid.org/0009-0003-2903-3914
d
https://orcid.org/0009-0001-0727-6451
e
https://orcid.org/0000-0002-1779-9435
f
https://orcid.org/0000-0003-1615-2757
g
https://orcid.org/0000-0008-2274-2571
fatigue concentration points and finally lead to the
initiation and growth of fatigue cracks
(Saidmakhamadov, 2024). Non – metallic inclusions
such as Al
2
O
3
, SiO
2
, TiO
2
, etc. can cause the casting
to crack. As shown in Fig. 1 – a, Al
2
O
3
and SiO
2
additions in steel reduced the service life of the
bearing steel. At the same time, the sulfide present in
the form of MnS as inclusions reduced the strength of
the casting due to the inclusions and therefore
negatively affected the working time of the part (see
Fig. 1. b). However, taking into account the general
harmful effects of sulfur, its presence in steel should
be limited to an appropriate range (Nosir & Bokhodir,
2023; Kholmirzaev et al., 2024a).
Saidmakhamadov, N., Kholmirzaev, N., Bekchanova, V., Odilov, F., Tukhtaboev, I., Karimov, B. and Gaybullaev, M.
Analysis of Technologies for Improving Steel Purity by Controlling Slag Content.
DOI: 10.5220/0014268600004738
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 4th International Conference on Research of Agricultural and Food Technologies (I-CRAFT 2024), pages 333-337
ISBN: 978-989-758-773-3; ISSN: 3051-7710
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
333
2 MATERIALS AND METHODS
Thefore, it is necessary to control the slag content to
remove Al
2
O
3
inclusions from steel. The adsorption
capacity of slag for Al
2
O
3
can be improved by
reducing the activity of Al
2
O
3
or the melting
temperature of the slag, which contributed to the mass
transport of Al
2
O
3
(Kholmirzaev, 2023; Nodir v et al.,
2022; Tursunbaev et al., 2023; Tursunbaev et al.,
2024; Kholmirzaev et al., 2024b).
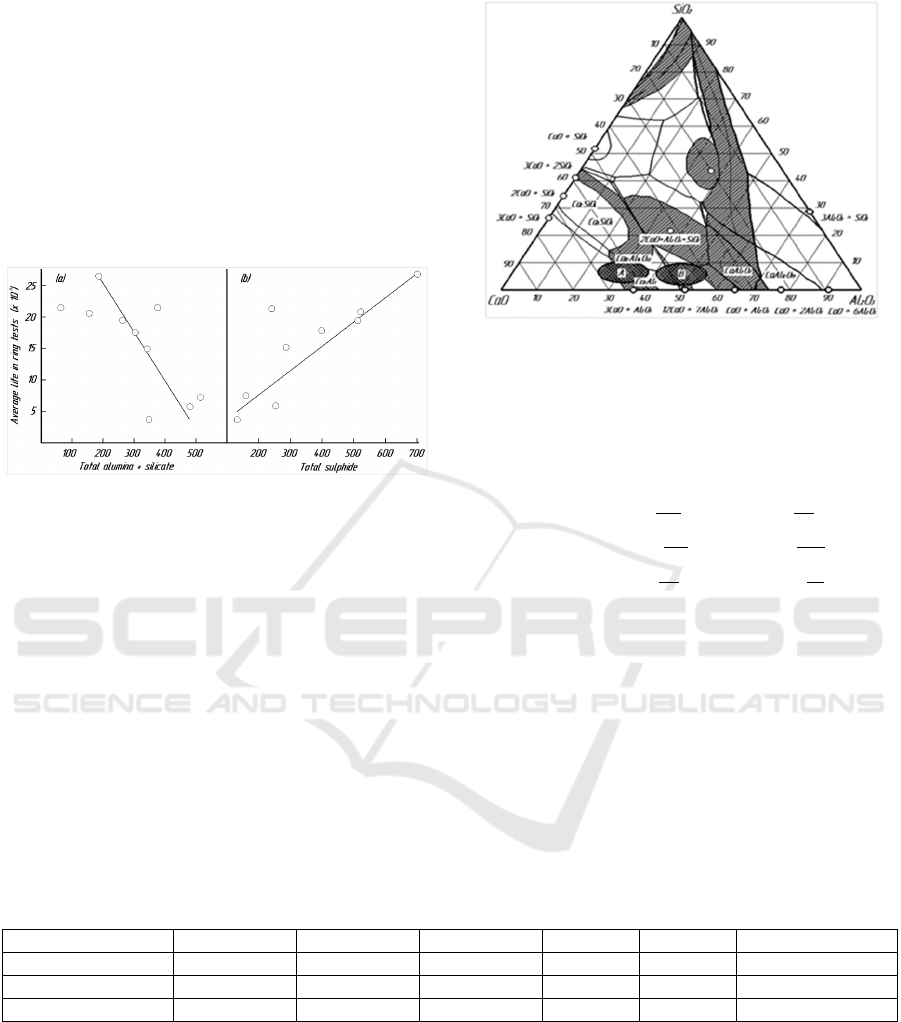
Figure 1: Correlation between mean lifetime and inclusion
content: calculations based on total inclusions (x 750)
observed in 516 fields with a total area of ~9 mm
2
.
The slag composition range satisfying these
conditions should be close to the Al2O3 – CaO binary
system. The composition of the slag used with steel
must also take into account the re – oxidation of the
steel being liquefied by the SiO2 in the slag. If the
former is considered more important, the relevant
composition range should be close to the saturation
region of CaO, marked as region A in Fig. 2.
However, if the latter is considered more important,
the composition should be located in region B. The
chemical composition of the tested steels as a sample
is presented in Table 1.
Figure 2: Control of slag content of steel.
Reoxidation of molten steel with low oxide in slag
As shown in the following equations, low-grade
oxides such as FeO and MnO in the slag reacted with
the oxide former in the molten steel to form non-
metallic inclusions.
3(FeO) + 2Al = (Al
2
O
3
) + 3Fe 1 a
3(MnO) + 2Al = (Al
2
O
3
) + 3Mn 1 b
3(SiO
2
) + 4Al = 2 (Al
2
O
3
) + 3Si 1 c
From the equations (1 a) and (1 b), when the
amount of FeO and MnO in the slag is high, non –
metallic inclusions are formed, so it is very important
to reduce the content of FeO and MnO in the slag.
However, as shown in Figure 3, the reoxidation
behavior of SiO
2
is different from that of FeO and
MnO because Si is a stronger oxidizing element than
Fe and Mn. Consequently, if the basicity of the
molten slag is controlled to some extent, reoxidation
of the molten steel by SiO
2
in the slag does not occur
and can therefore be prevented by controlling the slag
composition.
Table 1: Chemical composition of steel, weight – %.
Standard/grade C Si Mn P S Cr
J/SUJ1 0·95-1·10 0·15-0·35 <0·50 <0·025 <0·025 0·90-1·20
I/SUJ2 0·95-1·10 0·15-0·35 <0·50 <0·025 <0·025 1·30-1·60
S/SUJ3 0·95-1·10 0·40-0·70 0·90-1·15 <0·025 <0·025 0·90-1·20
Effect of oxygen content at end of blow in BOF
on steel cleanliness. Since blowing in BOF (basic
oxygen furnace) reduces the amount of oxygen in the
molten steel from the formation of non – metallic
inclusions, it affects the purity of the steel and should
therefore be taken into account. Table 2 shows the
production process of bearing steel in an electric arc
furnace. The process consists of BOF – ladle furnace
-RH degassing – continuous casting, and the
operation schemes of the technological units greatly
affected the purity of the bearing steel. In this study,
the following three tests were conducted to remove
non – metallic inclusions from molten steel: changing
the deoxidation method, controlling the slag
composition, and using the oxygen content
efficiently.
I-CRAFT 2024 - 4th International Conference on Research of Agricultural and Food Technologies
334
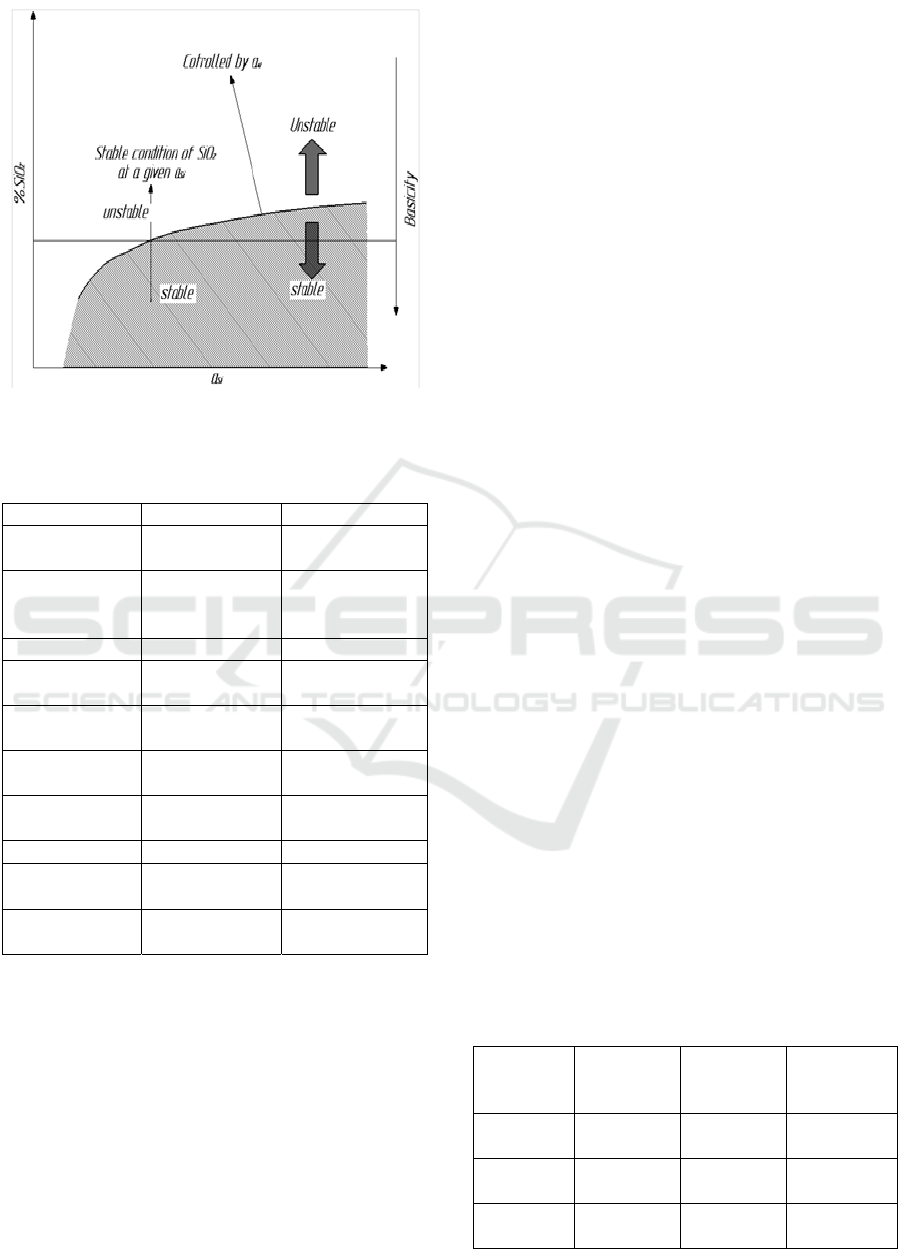
Figure 3:
Effect of slag base on SiO
2
and total oxygen
content of steel.
Table 2: Comparison of traditional and improved methods.
Process Traditional Improved
Chemical
process
Oxidation
Oxidizing
order: FeSi,
FeMn, Al
Oxidizing order:
Al, FeMn, FeSi
Slag formation
Electric arc
furnace
Temperature
control
Heating the
furnace
Heating the
furnace
Slag formation
Addition of
CaO, CaF
2
Addition of
CaO, Al
2
O
3
Remove
attachments
Degassing Usage: 25 min Usage: 25 min
Remove
attachments
Continuous
pouring
Cast formation Cast formation
Change of CaO/Al
2
O
3
value. Al
2
O
3
absorption
capacity of slag changed depending on its
composition. If the slag composition is saturated with
CaO, the activity of Al
2
O
3
additions decreases, and
thus improved thermodynamic conditions have
appeared. However, additives may be less effective
due to higher melting temperatures. When the slag
composition is at a low melting temperature, the
absorption capacity is increased, but the
thermodynamic equilibrium condition is worsened.
In the traditional method, the slag composition
was in the CaO saturation region, but the improved
method changed the composition to the low melting
temperature region, as shown in Table 3. In the
traditional method, before heating the arc in a slag
furnace, CaO and a small amount of CaF
2
are added
to accelerate the slag formation process. However,
despite the addition of CaF
2
, the slag was not
completely melted and most of the slag solidified
after arc heating. In this study, in order to solve this
problem, the following ratio was controlled between
1 · 7 and 1 · 8 by slightly increasing the addition of
%CaO/%Al
2
O
3
and decreasing the addition of CaO.
Change the oxidation method. In the traditional
method, FeSi, FeMn and Al were added sequentially
during the charge loading (see Table 2). In this case,
the oxides are 3(MnO) + 2Al = (The oxides formed
during the oxidation of Al were SiO
2
and MnO, but
when Al was added, the oxides changed to Al
2
O
3
due
to the closeness of Al to oxygen. However, the
composition of the oxides did not reach the state of
thermodynamic equilibrium.
Therefore, the composition of SiO
2
and MnO
higher than the thermodynamic equilibrium state and
the slag alkalinity was low and the slag oxidation
level was high. Al, then FeMn and FeSi were added
in the new method to improve the basis and degree of
oxidation. By reversing the addition order, Al
2
O
3
oxides are formed first and then only a small fraction
of the oxide composition should be Al
2
O
3
. As a result,
the basicity is higher and the oxidation level of the
slag is lower than in the traditional method.
A decrease in the amount of oxygen during
infusion. Reducing the amount of oxygen in the liquid
metal during casting to improve steel purity has
reduced gas voids in the casting. Because it can be the
main source of additives. However, when the amount
of oxygen decreased, the dephosphorylation process
was disrupted.
Therefore, liquid metal dephosphorization was
required. In this study, liquid metal
dephosphorization was performed in a liquid metal
pretreatment station, before the BOF (basic oxygen
furnace) operation, to reduce the oxygen content
during casting.
Table 3: Temperature change.
Softening
temperatur
e
Melting
temperatur
e
Liquefactio
n
temperature
Traditiona
l
0
C
1468 1505 >1510
Improved
0
C
1347 1349 1368
Difference
, K
121 156 ~142
Analysis of Technologies for Improving Steel Purity by Controlling Slag Content
335
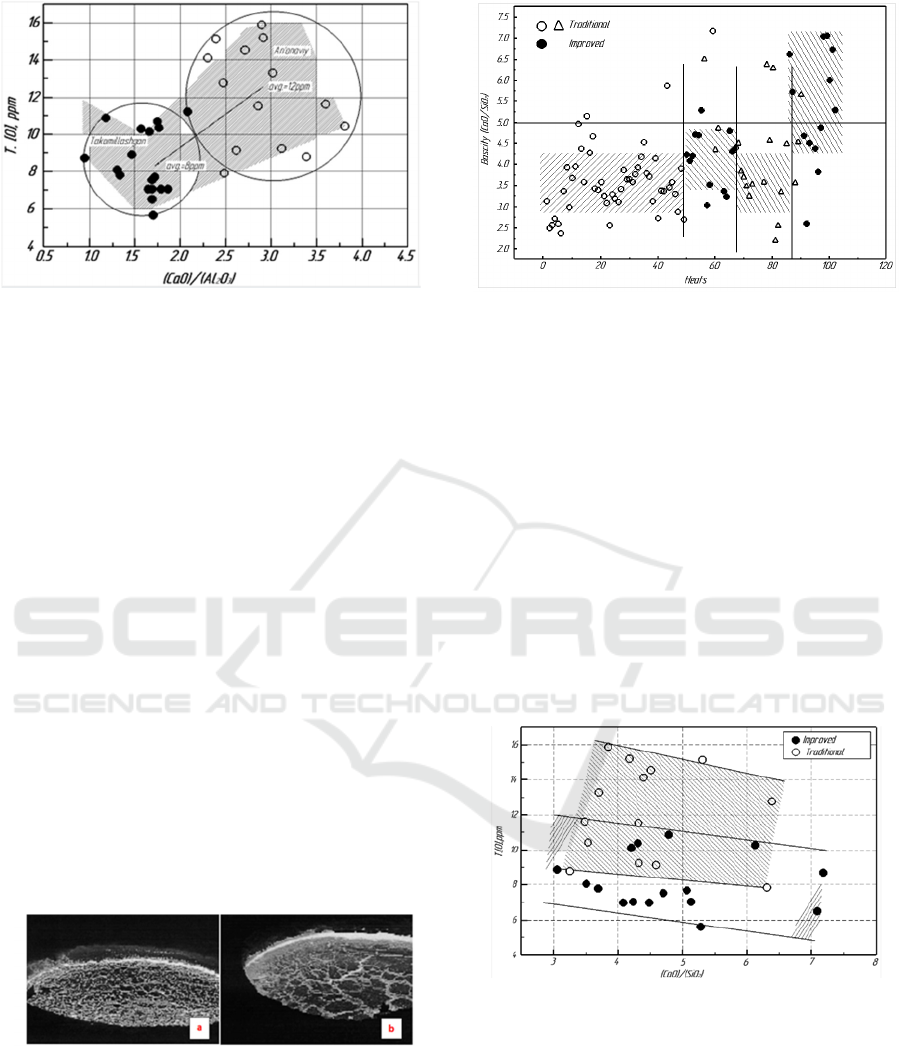
Figure 4: Comparison of total oxygen T.[O] between
improved and conventional methods of liquefaction.
3 RESULTS AND DISCUSSION
Effect of %CaO/%Al
2
O
3
ratio on casting quality. The
change in slag composition after RH degassing is
shown in Figure 4. The %CaO/%Al
2
O
3
(C/A) value
in the conventional method was 2 · 0 to 4 · 4, but the
C/A ratio was 1 · 2 to 2 · 0 in the improved method.
Total oxygen value was minimum at C/A=1 · 7
regardless of T. [O] method. Therefore, in steels, a
C/A ratio of 1 · 7 gave the best results for improving
steel purity. This is because lowering the slag melting
temperature is more effective than controlling the slag
composition to reach the CaO saturated region.
Figure 15 shows a comparison of slag formation
between conventional and improved methods after
arc heating in an electric arc furnace. As shown in this
picture, in the traditional method, most of the slag is
solidified, but in the improved method, the molten
slag is spread uniformly over the top of the furnace.
During operation (cycling and storage) LIA the
most significant changes occur on electrodes made of
lithium-manganese spinals.
Figure 5: Comparison of slag condition after treatment in
an electric arc furnace. a) solid slag, traditional method; b)
liquid slag, improved method.
Therefore, it can be confirmed that the melting
temperature of the slag has decreased and as a result,
the absorption capacity of the inclusion has increased
with the improved method.
Figure 6: Modifying slag basicity by improving reoxidation
method and slag control.
Total oxygen T.O can be reduced from 12 to 8
ppm using the improved method over the
conventional method (see Figure 4).
Changing the composition of slag with a new
reoxidation method. The slag composition of
traditional and improved methods can be compared in
Figure 6. In the conventional reoxidation process, the
slag basicity was in the range of 3 ·5 – 4 · 5 due to
oxidation of Si and Mn, but in the improved method,
the basicity was 4 · 5 – 7 · 0 because Al was oxidized
before Si and Mn. Figure 7 shows the relationship
between slag basicity and T.[O] for solidification.
Although T.[O] decreased slightly after increasing
basicity, slag basicity had little effect on T.[O]
compared to other factors such as C/A ratio.
Figure 7: Rectangle.
To investigate these processes, the SiO
2
stability
zone was calculated using the bearing steel
composition with the following equations.
3(SiO
2
) + 4Al=2(Al
2
O
3
) + 3Si (2 a)
lo
g
K=
–
6 · 947 + 47645/T (2 b)
Calculation – the composition of the steel used in
the books is 0 · 99C – 0 · 3Mn – 1 · 3Cr (wt – %) and
I-CRAFT 2024 - 4th International Conference on Research of Agricultural and Food Technologies
336

the activity coefficient was calculated from these
values: the results are shown in figure 1.7. The
soluble Al and Si content of steel, i.e. 0 ·02 – 0 · 03
wt – % and 0 · 20 wt – %, respectively, fall into the
rectangle shown in Fig. 7. In this area, if the basicity
is greater than 4, SiO
2
does not act as an oxygen
source. Therefore, basicity does not affect the purity
of steel.
4 CONCLUSIONS
1. When removing inclusions from bearing steel
containing (wt. – %) 0 · 99C – 0 · 3Mn – 1 · 3Cr
with a slag basicity higher than 4, steel purity is
not affected because SiO
2
is stable and oxygen
does not act as a source.
2. Controlling the %CaO/%Al
2
O
3
ratio is the most
effective method for removing inclusions from
bearing steels, and its value was most effective at
1 · 7 – 1 · 8. Using this method, total oxygen
T.[O] can be reduced from 10 – 12 ppm to 5 – 8
ppm.
3. T.[O] of the bearing steel is reduced due to the
reduction of oxygen content due to the moving
contact of the liquid metal during casting.
However, when %CaO/%Al
2
O
3
> 2 · 0, the
amount of oxygen at impact does not have a
significant effect because the equilibrium T.[O]
is too high to reach equilibrium during
steelmaking.
REFERENCES
Kholmirzaev, N. , Turakhodjaev, N., Saidmakhamadov, N.,
Khasanov, J., Bektemirov, A., 2024a. Effects of
titanium (Ti) contents on the wear resistance of low-
alloy steel alloys. E3S Web of Conferences, EDP
Sciences, 525, 03003.
Kholmirzaev, N., Khasanov, J., Abdullayev, B.,
Saidkhodjaeva, S., Bektemirov, A., Sadikova, N., ... &
Nurdinov, Z., 2024b. Improving the technology of
obtaining highquality castings from steel in sand-clay
molds. E3S Web of Conferences, 525, 03012.
Kholmirzaev, N., Turakhodjaev, N., Saidmakhamadov, N.,
Khasanov, J., Saidkhodjaeva, S., & Sadikova, N., 2023.
Development of Technology of Making Shafts from
Steel Alloy 35XGCL. Lecture Notes in Networks and
Systems, 762. https://doi.org/10.1007/978-3-031-
40628-7_18.
Nodir, T., Nosir, S., Shokhista, S., Furkat, O., Nozimjon,
K., & Valida, B., 2021. Development of 280x29nl alloy
liquefaction technology to increase the hardness and
corrosion resistance of cast products. International
Journal of Mechatronics and Applied Mechanics,
1(10), 154–159.
https://doi.org/10.17683/IJOMAM/ISSUE10/V1.19
Nodir, T., Sarvar, T., Kamaldjan, K., Shirinkhon, T.,
Shavkat, A., Mukhammadali, A., 2022. International
Journal of Mechatronics and Applied Mechanics, (11),
52–56. https://doi.org/10.17683/ijomam/issue11.7
Nosir, S., & Bokhodir, K., 2023. Development of
Liquefaction Technology 280X29NL to Increase the
Strength and Brittleness of Castings. Lecture Notes in
Networks and Systems, 534.
https://doi.org/10.1007/978-3-031-15944-2_10.
Patrik, O.L.J., 1997. Hungarian Mining and Metallurgical
Society/The Institute of Materials, 2, 137.
Saidmakhamadov, N., Turahodjaev, N., Tursunbaev, S.,
Zokirov, R., Tadjiev, N., Abdullaev, K., Hamroev, V.,
Rakhmanov, U., Juraev, J., 2024. Improving the design
of the lining of the ball mill used to improve the quality
of grinding. E3S Web of Conferences, 525, 02017.
Tursunbaev, S., Turakhodjaev, N., Mardonakulov, S., &
Toshmatova, S., 2024. Effect of germanium oxide on
the properties of aluminum casting details in
agricultural machinery. BIO Web of Conferences, 85.
https://doi.org/10.1051/bioconf/20248501024.
Tursunbaev, S., Turakhodjaev, N., Odilov, F.,
Mardanokulov, S., & Zokirov, R., 2023. Change in
wear resistance of alloy when alloying aluminium alloy
with germanium oxide. E3S Web of Conferences, 401.
https://doi.org/10.1051/e3sconf/202340105001.
Analysis of Technologies for Improving Steel Purity by Controlling Slag Content
337
