
Gas Fuels for Engines of Internal Combustion with Spark Ignition
Lazizbek Daminov
1a
, Abdulla Mirzaev
1b
, Otabek Mirzaev
2c
and Yashnarbek Sharipov
3d
1
Tashkent State Technical University, 100095, University str. 2, Tashkent, Uzbekistan
2
Urgench State University, 220100, Kh.Alimdjan str. 14, Urgench, Uzbekistan
3
Shakhrisabz Branch of the Tashkent Institute of Chemical Technology, 181306, Shahrisabz str. 20, Shahrisabz, Uzbekistan
Keywords: Gas Fuels, Spark Ignition, Natural Gas.
Abstract: The article provides information about gas fuels currently used in internal combustion engines, their types
and composition. The analysis of the fuels used in these engines, as well as the types of fuels that meet today's
environmental requirements, is also presented. The analysis of scientific research work carried out on the
economic and environmental significance of the transition to natural gas fuel in the conditions of global
warming and reduction of oil reserves today is described.
1 INTRODUCTION
1.1 Overview of Fuels Used for
Internal Combustion Engines
Many authors describe the main types of fuels, which
are currently being used for automobile engines. They
are Erokhov V.I. (Erokhov, 2003), Khachiyan A.S.
(Khachiyan et al., 2008), Chernyshova N.D.
(Chebykin et al., 2024), and Zongyu Yue (Yue et al.,
2023) etc:
• Diesel fuel produced from petroleum; synthetic
diesel fuel produced from mineral oils; semi-
synthetic diesel fuel, consisting of various
ratios of the above fuels; gasoline with various
octane numbers;
• Fuels based on alcohols (wood, potato, corn,
etc.), mainly used in China, Brazil and
Argentina;
• Gas fuel based on various mixtures of propane
and butane, produced from oil;
• Fuel based on natural gas with methane content
from 80 to 99%, both in liquid and gaseous state
(Khachiyan et al., 2010);
• Fuel based on synthesis gas СН3 (ОН);
a
https://orcid.org/0000-0003-2179-9176
b
https://orcid.org/0000-0002-8894-9565
c
https://orcid.org/0009-0002-8386-8827
d
https://orcid.org/0000-0008-5289-8921
• Hydrogen fuels (Bryzgalov et al., 2009,
Khachiyan et al., 2008, Umerov et al., 2024);
In addition to fuels, electrical energy from storage
batteries, such as solar energy from cells, and
mechanical energy from flywheels are used. These
energies are not considered in this effort, since
vehicles with internal combustion engines are used
only for hybrid powertrains.
1.2 Promising Fuels for Vehicles
The most promising fuels in terms of environmental
requirements according to Erokhov V.I. (Erokhov et
al., 2011), Chernyshova N.D. (Chebykin et al., 2024
and others are natural gas, chemical formula CH
4
;
synthesis gas, chemical formula CH
3
(OH); hydrogen,
chemical formula H
2
.
To obtain zero toxicity of a car, hydrogen is
usually oxidized in a special device called a "fuel
cell", with the receipt of electrical energy used in
electric motors.
Internal combustion engines cannot compete with
electric motors in terms of zero toxicity (Cisek et al.,
2022), because even when hydrogen is burned in air
at a high temperature in the combustion chamber, in
addition to water, nitrogen oxides NOx are formed.
The absence of NOx emissions during combustion of
274
Daminov, L., Mirzaev, A., Mirzaev, O. and Sharipov, Y.
Gas Fuels for Engines of Internal Combustion with Spark Ignition.
DOI: 10.5220/0014261500004738
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 4th International Conference on Research of Agricultural and Food Technologies (I-CRAFT 2024), pages 274-280
ISBN: 978-989-758-773-3; ISSN: 3051-7710
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
hydrogen in an internal combustion engine is possible
only when pure oxygen is used as an oxidizer.
However, this is currently unacceptable due to the
impossibility of storing a sufficient amount of oxygen
on board the car. The use of hydrogen in road
transport on a large scale requires significant material
costs and new technologies, which have recently
received the prospect of further development.
The use of synthesis gas is associated with the
costs of obtaining it from natural gas and requires
material costs, both for the development of such units
and for the development of a network of filling
stations, which will restrain its further use.
Natural gas is the most promising in terms of both
economics and environmental performance. Natural
gas can produce both synthesis gas and hydrogen. So
that nowadays, natural gas is at the next step towards
achieving the lowest emissions of toxic substances
from internal combustion engines.
1.3 Changes in Environmental
Requirements for Car
Every year the fleet of automotive vehicles is growing
rapidly (Grigoriev et al., 1989). Accordingly, the
amount of pollutant emissions from the exhaust gases
of internal combustion engines is also growing. To
maintain the balance of the Earth's atmosphere,
harmful emissions should not exceed the capacity of
natural phenomena to neutralize them. In this case,
the bulk of the emissions should participate in the
cycle of chemical components in wildlife. Plants
using photochemical processes must absorb so all the
CO
2
released from automobile engines. They are
required to decompose it into carbon and oxygen. In
this case, carbon participates in the process of
building living organisms of nature, and oxygen is
released into the atmosphere. Substances such as CO,
CH, NO
x
undergo redox processes on board with
vehicles to transform them into naturally occurring
substances (CO
2
, H
2
O, N
2
). These substances in the
earth's atmosphere should not increase in absolute
and relative quantities. The process of tightening the
standards of exhaust gas toxicity is natural
(Fusshoeller et al., 2004).
2 MATERIALS AND METHODS
Reducing pollutant emissions can be achieved by
various technical means used both to reduce the total
consumption of hydrocarbon fuels, and for redox
processes on board a vehicle Musabekov et al., 2023).
Reducing the consumption of hydrocarbon fuels can
be carried out as follows:
• by switching to a new type of environmentally
friendly fuel or energy;
• improvement of energy conversion processes in
internal combustion engines;
• transition to hybrid energy conversion schemes.
Redox processes on board a vehicle are usually
carried out using the following:
• multicomponent neutralizers and collectors;
• chemical filters;
• electrical plasma converters.
Each change in environmental requirements in
tightening leads to a complication of the design of
both the engine itself and its systems: fuel supply, air
supply, exhaust gas, oil, cooling, neutralization and
control. All this together leads to a rise in the cost of
the vehicle.
Tables 1 and 2 show the main characteristics of
liquid and gaseous fuels.
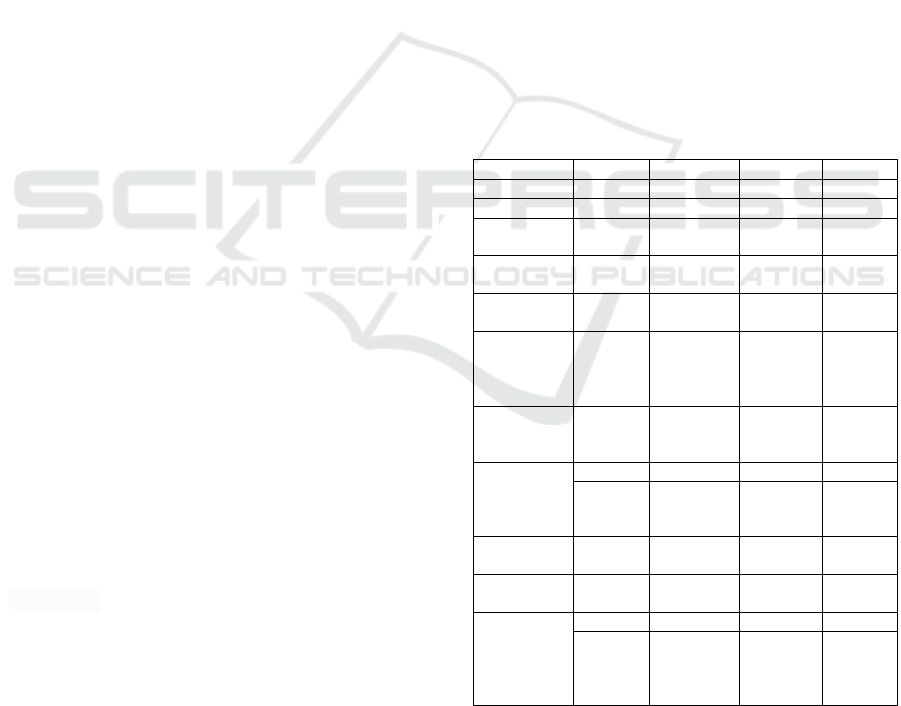
Table 1: Physical properties of isooctane, motor gasoline,
diesel fuel and liquefied propane as fuels for transport
Property Isooctane Petrol Diesel fuel Propane
Formula С
8
Н
18
С
4
...С
12
С
14
…С
22
С
3
Н
8
A
tom ratio H/C 2,25 2,03 1,63 2,67
Density at 15°
С, kg/m
3
690,2208 746,54 880,75 579,9772
Net calorific
v
alue Hu, kJ/k
g
44411,848 42912,076 40610,1 46411,54
E
nergy density,
MJ/m
3
30,653981 32,035581 35,767346 26,917635
Stoichiometric
number L
0
air/fuel (in
mass numbers)
15,1 14,7 13,9 15,7
Octane number 100 80-98
Cetane
number 40-
60
105
Stoichiometric
energy mixes
E, kJ / kg
Ev, MJ / m
3
2757,720 2732,142 2725,167 2780,972
1,903436 2,039653 2,400191 1,6129
Evaporation
temperature,
K
398,15 302,6...477,6 458,1.610,9 230,93
Saturated vapo
r
pressure (kPa)
55,16 45-105 1,38 1420,33 (1)
Flammable
limits lower (%
by volume)
upper (% by
volume)
1,4 1,4 1 2,2
7,6 7,6 6 9,5
Stoichiometric mixture energy, kJ / kg
stoichiometric mixture:
E=Hu/(1+L
0
) or in kJ/m
3
stoichiometric mixture
E
v
=E
p
, where p is the density in kg/m
3
.
Gas Fuels for Engines of Internal Combustion with Spark Ignition
275
1. The critical temperature of propane is
369.817 K, the critical pressure is 4247.2 kPa.
The important parameters of gasoline as a fuel are:
the amount of energy (net calorific value) per unit of
mass and unit of volume, volatility and octane
number (Enomoto et al., 2023). Mass and volume
indicate the capacity of the fuel needs to be
conveniently placed on board the vehicle.
The volatility of the fuel is important in a vehicle
during cold start of the engine in order to obtain the
required air-fuel mixture. Currently, fuel volatility is
critical for exhaust emissions and fuel evaporation
from tanks in warm weather.
The octane number is indicative of the ability of
the fuel to prevent the occurrence of premature
ignition (detonation) of the air-fuel mixture in the
engine cylinders when the mixture is compressed in
the engine cylinders and, consequently, to its
efficiency.
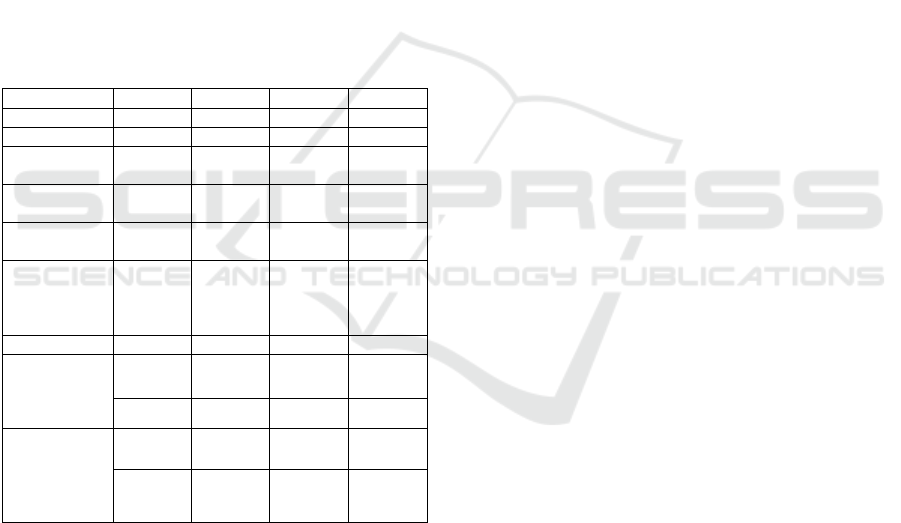
Table 2: Physical properties of ethanol, methanol, methane
and hydrogen as alternative fuels for transport
Property Ethanol Methanol Methane Hydrogen
Formula С
2
Н
5
ОН СН
3
ОН СН
4
Н
2
Atom ratio H/C 3 4 4 -
D
ensity at 15° С,
kg/m
3
788,4814 790,878 0.672756 0.0864972
Net calorific
value, Нu, kJ/kg
26707,564 19903,948 50108,655 120028,25
Energy density,
MJ/m
3
21,058 15,742 21,256 8,486
Stoichiometric
number L
0
air/fuel (mass
number)
9,0 6,5 17,3 34,5
Octane numbe
r
101 100 129 60
Stoichiometric
energy mixture
E, kJ/kg
Ev, MJ/m
3
2690,288 2669,361 2750,744 3380,881
2,121 2,111 1,167 0,239
Flammable
limits lower (%
by volume)
upper (% by
volume)
4,3 7,3 5,3 4,1
19 36 15 74
3 RESULTS AND DISCUSSION
Ethanol can be used straight as a motor fuel in three
ways:
• 100% ethanol;
• from 5 to 15% ethanol mixed with gasoline;
• Hydro-ethanol, consisting of 95% ethanol and
5% water.
Direct use of ethanol, in the above three ways, as
a vehicle fuel has both advantages and disadvantages.
Because the high latent heat of vaporization of
ethanol results in a lower combustion temperature in
the engine cylinder.
It should be noted that alcohols methanol and
ethanol are not desirable to be used as additives in
gasoline, because they increase the saturated vapor
pressure of the mixture more than that of pure
gasoline. This increases the emission of gases into the
atmosphere. However, the gains in exhaust gas
emission reductions that can be achieved with these
alternative blends of gasoline and alcohols are very
small compared to those that can be achieved with
hydrogen or natural gas (Norouzi et al., 2021).
3.1 Natural Gas as Fuel for Vehicles
Natural gas (Erokhov et al., 2003) is a gaseous
mixture of light hydrocarbons, mainly methane and
ethane, and other gases found in the atmosphere of
our planet. In the early stages of the development of
the oil and gas industry, natural gas was obtained as a
by-product from the production of liquid fuels and
hydrocarbons for the chemical industry from oil.
Currently, from 3 to 5% of natural gas is obtained in
the above-mentioned industries, the rest consists of
approximately 10 to 20% obtained from oil wells and
from 75 to 87% from individual wells. Natural gas
found with oil can contain pentane and gasoline
constituents as well as propane and butane. This gas
is classified as a "wet" gas containing heavy
hydrocarbons. In the course of processing, the gas
condensate part is separated from it and "dry" gas is
obtained. The composition of natural gas varies
considerably from field to field.
Natural gas contains: propane, butane, pentane,
heavy hydrocarbons, hydrogen, oxygen, carbon
dioxide, nitrogen and helium (rarely found in small
quantities). Propane and butane are known as
liquefied petroleum gas (LPG or LPG), while pentane
and heavy hydrocarbons are known as natural
gasoline. Carbon dioxide, nitrogen and helium - inert
gases must be removed in order to improve the energy
value of natural gas.
When the fuel is ignited in the cylinder, hydrogen
creates active centers that improve the combustion
process. Therefore, the hydrogen content in natural
gas should be optimal from 6 to 8%, according to the
data by Bryzgalov A.A., Smolensky V.V. and Shaikin
A.P. (Bryzgalov et al., 2009, Umerov et al., 2024).
Natural gas, which is an odorless gas, is odorized
prior to distribution to the consumer to provide a
distinctive aroma that warns customers of potential
leaks. At normal atmospheric pressure, the density of
natural gas is too low, and therefore the energy supply
I-CRAFT 2024 - 4th International Conference on Research of Agricultural and Food Technologies
276

in this case will not be sufficient on board the vehicle.
To ensure the required supply of natural gas on board
the vehicle, it is necessary to compress (compress) to
20 either MPa or 80 MPa and place it in a high-
pressure vessel, or cool it to a liquid state and fill it
into a cryogenic tank.
One of the positive characteristics of natural gas
as a vehicle fuel is its high octane number from 125
to 130, which depends on the chemical composition
of the natural gas. This allows it to be used in engines
with high compression ratios from 11.5: 1 to 15: 1.
Due to this, the combustion efficiency improved with
obtaining maximum work, the power indicators of the
engine can be even higher than when running on
gasoline from 3 to 5%.
The main advantages of natural gas and
methane as motor fuels (Malyshev et al., 2008):
• reducing the wear of the connecting rod-piston
group of the engine increases the engine
resource;
• increase in engine overhaul mileage;
• an increase in the resource of spark plugs;
• increase in the service life of engine oil;
• lack of detonation;
• reduction of emissions of carbon dioxide and
toxic components in exhaust gases;
• a decrease in the vibration component of the
engine due to a decrease in the combustion rate
of methane and, accordingly, a decrease in the
rate of pressure rise in the combustion chamber.
Problems hindering the rapid introduction of natural
gas in modern transport, in contrast to gasoline and
diesel fuel:
• internal unavailability of the natural gas
infrastructure for the possibility of replacing
existing motor fuels (insufficient number of
CNG filling stations);
• insufficient supply of natural gas on board the
vehicle;
• lack of mass serial production of components
for gas equipment that meets modern safety
requirements, gas fuel vapor and exhaust gas
toxicity;
• lack of mass serial production of vehicles
running on natural gas;
• consequences for the environment during the
transportation and use of natural gas;
• economic aspects of natural gas as a fuel.
3.2 Economic Feasibility of Switching
to Natural Gas
The economic efficiency of natural gas is shown in
many works by Erokhov V.I. (Erokhov et al., 2003),
Kapustina A.A. (Kapustin et al., 2011), V.V.
Malysheva. (Llotko et al., 2000), D.V. Pasechnika.
(Matmurodov et al., 2024), Pevneva N.G. (Pevnev,
2010, Pevnev et al., 2010), Rovner G. (Rovner, 2006),
Teremyakina P.G., Khachiyan A.S., (Teremyakin et
al., 2011), Chernyshova N.D. (Chebykin et al., 2024)
and others. However, this has not yet become the
impetus for its mass introduction in transport.
Economic efficiency is determined by the price of
natural gas and its restrictions by government
agencies. The price of gas is made up of costs: for
prospecting a field, its appraisal, drilling a well,
building compressor stations for pumping gas,
building pipelines for transporting and distributing
gas, etc. These costs are significantly lower than the
costs of oil extraction and processing into motor fuels.
The second obstacle to the slow introduction of
natural gas in transport as a vehicle fuel is the high
cost of components for storing it on board. These
components include high pressure cylinders and
cryogenic tanks. The price of the rest of the fuel
equipment is commensurate with the price of
equipment for other types of fuels and does not
significantly affect the use of natural gas.
4 CONCLUSIONS
At present, when the price of natural gas is limited to
half the cost of gasoline with an octane rating of 76,
it allows the consumer to achieve savings from 3 to
3.5 times per 1 km of vehicle mileage. With such a
difference, even with the high cost of cylinders, the
payback period for light vehicles is from 25 to 30
thousand km and for trucks and buses about one year.
The advantages of natural gas in relation to other
types of fuel at the present stage of development:
• low price;
• the density is less than that of air, this ensures
the safety of its use due to its escape into the
upper layers of the atmosphere;
• the ratio of hydrogen atoms to carbon atoms is
4: 1, which, in comparison with gasoline,
reduces CO2 and CO emissions in exhaust
gases by up to 28%;
• the high value of the net calorific value
provides, in comparison with gasoline and
diesel fuel, the preservation and, in some cases,
Gas Fuels for Engines of Internal Combustion with Spark Ignition
277

an increase in engine power and torque (with an
optimal compression ratio, a high degree of
filling the cylinders with a fuel mixture, optimal
ignition parameters);
• low burning rate, in comparison with gasoline,
ensures smooth operation of the engine and,
accordingly, increases its resource;
• high knock value from 125 to 130 ensures
reliable engine operation in all modes with high
efficiency;
• is an environmentally friendly, renewable fuel
using plant residues for its production;
• when used in a compressed state (CNG), a
pump and an evaporator are not required to feed
the internal combustion engine into the
combustion chamber. When using wet gas, a
minimum heating of the reducer valve pair is
required to reduce the pressure.
Disadvantages of natural gas as a fuel and ways to
minimize them:
• storage on board a vehicle under high pressure
or at cryogenic temperatures, or in an adsorbent
increases the weight of cylinders or tanks, fuel
equipment and its fasteners;
• low combustion temperature, in comparison
with gasoline, increases the heating time of the
collector or neutralizer;
• causes the greenhouse effect in the earth's
atmosphere, for which it is required to
minimize its leakage from fuel systems and
emission from the combustion chamber during
the start of an internal combustion engine;
• when used in a liquefied state in cryogenic
cylinders, drainage into the atmosphere is
required to prevent an increase in pressure in
the cryogenic cylinder above the working one.
To reduce the weight of fuel equipment cylinders,
new materials have recently been used: aluminum
alloys, resin-impregnated fibrous synthetic materials,
stainless steels and super-strong steels, titanium
alloys - to obtain the required margin of safety. In
addition, the use of one cylinder of the same volume,
instead of several on the vehicle, also reduces their
overall weight.
To accelerate the heating of the catalyst, several
methods are currently used, these are:
• reducing the distance between the catalyst and
the exhaust valves of the engine;
• warming up the engine after starting is carried
out on lean mixtures to increase the
afterburning temperature of natural gas
(excessive depletion in the warm-up mode can
lead to a significant increase in NOx emissions
in the exhaust gases);
• an increase in the content of hydrogen
molecules in natural gas, which increases the
number of active ignition centers, and therefore
increases the combustion rate and temperature
of the exhaust gases;
• decomposition of methane molecules into
radicals of hydrogen and carbon, and oxygen
molecules into radicals of oxygen atoms before
ignition, which also increases the combustion
rate of the fuel mixture and its temperature;
• increasing the pressure in the combustion
chamber before ignition, by increasing the
compression ratio, leads to an increase in the
initial ignition temperature and, accordingly,
the combustion temperature.
To minimize methane emissions into the atmosphere,
the following measures are applied:
•special seals of pipelines and fittings, reducing
the likelihood of depressurization;
• use of a gas leakage monitoring system, which
signals about malfunctions and automatically
gives a command to shut off the gas valve to
stop gas fuel leaks;
• the use of catalysts for the oxidation of methane
to CO2 and H2O and excluding its release into
the atmosphere;
• optimization of gas consumption at engine start
and depletion of the mixture during its warming
up reduces methane emissions through exhaust
valves for engines with simultaneously open
intake valve;
• direct injection of gas into the combustion
chamber during compression, when both valves
(intake and exhaust) are closed;
• increasing the completeness of natural gas
combustion with the help of: various
constructive measures for the combustion
chamber, for the fuel mixture preparation
system and for the ignition system;
• reduction of misfire and inefficient combustion
in combustion chambers by increasing the
energy given off by the ignition coils.
To reduce the amount of methane emitted through the
drainage of the cryogenic cylinder, it is necessary:
• a catalyst is installed in the drain for the
oxidation of methane to CO2 and H2O and to
prevent it from entering the atmosphere;
• increase the degree of evacuation of the volume
of the cryogenic cylinder insulation;
I-CRAFT 2024 - 4th International Conference on Research of Agricultural and Food Technologies
278

• increase the degree of thermal insulation of the
inner vessel using special construction
materials (materials with low thermal
conductivity, mirror multilayer foil, etc.);
• increase the strength of the walls of the inner
vessel to increase the working pressure and
increase the time without drainage storage.
ACKNOWLEDGMENT
The management of the Tashkent State Technical
University named after Islam Karimov and the staff
of the department of “Energy Engineering and
Vocational Education” express deep gratitude to the
World Bank for the allocated grant and the Academic
Innovation Fund under the Ministry of Higher
Education, Science and Innovation of the Republic of
Uzbekistan as well as the Islamic Development Bank
for the assistance provided in the implementation of
this project.
REFERENCES
Bryzgalov, A.A. Adding hydrogen to the methane-air
mixture of a gas engine / A.A. Bryzgalov, A.P. Shaikin
// Materials of the International Scientific Symposium
"Automotive Tractor-2009", March 25-26, 2009. Book
2. Moscow: MSTU "MAMI", 2009. - pp. 25-33.
GOST 27577 Compressed natural fuel gas for internal
combustion engines. Interstate Council for
Standardization, Metrology and Certification. - Minsk,
2000 .7 p.
Grigoriev, E.G. Gas-cylinder cars / E.G. Grigoriev, B.D.
Kolubaev, V.I. Erokhov, A.A. Zubarev. -M.:
Mechanical engineering, 1989. - 216 p.
GOST R 41.49-2003 (UNECE Regulation No. 49) Uniform
provisions concerning the approval of compression
ignition engines and engines fueled by natural gas and
positive ignition engines fueled by liquefied petroleum
gas (LPG), and vehicles equipped with compression
ignition engines, natural gas engines and positive
ignition engines fueled by LPG in relation to the
pollutants they emit. -M.: State Standard of Russia,
2004. - 146 p.
Erokhov, V.I. Physicochemical and motor properties of gas
fuel / V.I. Erokhov // Auto and Gas Refueling Complex
+ Alternative Fuel. International scientific and technical
journal. - 2003. - No. 5 (11). - S. 64-69.
Erokhov, V.I. Injection systems for gasoline engines.
Design, calculation, diagnostics: a textbook for
universities. / IN AND. Erokhov – M. Hotline-
Telecom. - 2011 .553 p.
Kapustin, A.A. Natural gas internal combustion engine
power supply system. Kapustin, A.L. Penkin // Auto
and Gas Refueling Complex + Alternative Fuel.
International scientific and technical journal. - 2011. -
No. 1 (55). S. 16-99.
Kovtun, O., Levchenko, M., Höntsch, S., ... Gräbner, M.,
Volkova, O. Recycling of iron-rich basic oxygen
furnace dust using hydrogen-based direct reduction.
Resources, Conservation and Recycling Advances,
2024, 23, 200225
Llotko, V. The use of alternative fuels in internal
combustion engines / V. Llotko, V.N. Lukanin, A.S.
Khachiyan. -M., 2000 .310 p.
Malyshev, V.V. Prospects for the use of alternative fuels in
aviation / V.V. Malyshev, V.I. Solozobov // Transport
on alternative fuel. International scientific and technical
journal. - 2008. - No. 1. - S. 62-64.
Matmurodov, F.M., Daminov, O.O., Sobirov, .Sh.,
Abdurakxmanova, M.M., Atakhanov, F.U.M. Dynamic
simulation of force loading of drives of mobile power
facilities with variable external resistance. E3S Web of
Conferences, 2024, 486, 03001
Pevnev, N.G. Increasing the efficiency of operation of gas-
cylinder vehicles with combined fuel injection / N.G.
Pevnev, I.M. Knyazev, M.G. Levashov // Auto and Gas
Refueling Complex + Alternative Fuel. International
scientific and technical journal. - 2006. - No. 5 (29). -
S. 20-23 / AGZK + AT. - 2010. - No. 1. - S. 2-5.
Pevnev, N.G. Experience in operating gas-cylinder vehicles
with engines equipped with a gasoline injection system
/ N.G. Pevnev, M.G. Levashov, S. Bukharov // Auto
and Gas Refueling Complex + Alternative Fuel.
International scientific and technical journal. - 2006. -
No. 6 (30). - S. 75-78 / AGZK + AT. - 2010. - No. 5
(53). - S. 3-6.
Rovner, G. Creation and first results of operation of
automobile gas fuel systems of CNG for a maximum
working pressure of 32 MPa / G. Rovner, Ya.
Mkrtychan, S. Batyushkov, V.А. Shcherbinin //
AutoGas Refueling Complex + Alternative Fuel.
International scientific and technical journal. - 2006. -
No. 2 (26). - S. 27-29.
Teremyakin, P.G. The main factors affecting the cost of
owning a gas-cylinder vehicle with a subordinate
control system / P.G. Teremyakin // Transport on
alternative fuel. International scientific and technical
journal. - 2011. - No. 2 (20). - S. 60-64.
Khachiyan, A.S. The use of hydrogen as a motor fuel for
automobile internal combustion engines. Khachiyan,
V.F. Vodeyko // Transport on alternative fuels.
International scientific and technical journal. - 2008. -
No. 3 (3). - S. 57-61.
Khachiyan, A.S. Modeling of indicators and characteristics
of engines powered by natural gas / A.S. Khachiyan,
V.V. Sinyavsky, I.G. Shishlov, D.M. Karpov //
Transport on alternative fuel. International scientific
and technical journal. - 2010. - No. 3 (15). - S. 14-19.
Chebykin, D., Heller, H.-P., Schulz, K., Endo, R., Volkova,
O. Viscosity, Density, Surface Tension, and
Crystallization Behavior of Commercial Mold Fluxes.
Steel Research International, 2024
Umerov, F., Daminov, O., Khakimov, J., Yangibaev, A.,
Asanov, S. Validation of performance indicators and
Gas Fuels for Engines of Internal Combustion with Spark Ignition
279

theoretical aspects of the use of compressed natural gas
(CNG) equipment as a main energy supply source on
turbocharged internal combustion engines vehicles.
AIP Conference Proceedings, 2024, 3152(1), 030017
Auf der Suche nach der Grenze 90 g / km CO2 bai mehr als
1500 kg Fahrzeuggewicht? - MTZ. Extra, 2005,
Oktober. - p. 36-38.
Bazarov, B., Magdiev, K., Axmatjanov, R., ... Vasidov, B.,
Usmanov, I. Assessment of Environmental and Energy
Usage of Alternative Motor Fuels. AIP Conference
Proceedings, 2022, 2432, 020001
Musabekov, Z., Ergashev, B., Daminov, O., Khushnaev,
O., Kurbanov, A., Kukharonok, G. Efficiency and
environmental indicators of diesel engine operation
when using water injection. IOP Conference Series:
Earth and Environmental Science, 2023, 1142(1),
012024
https://iopscience.iop.org/article/10.1088/1755–1315
Fusshoeller, B., Jauss, A. EOBD-fähige Motorsteuerung für
die monovalenten Erdgasfahrzeuge von Opel. MTZ
Motortech Z 65, 904–909 (2004).
https://doi.org/10.1007/BF03227229
Zongyu Yue, Haifeng Liu. Advanced Research on Internal
Combustion Engines and Engine Fuels. Energies 2023,
16(16), 5940; https://doi.org/10.3390/en16165940 -
Wei, X., Zhao, W., Ilatovskaia, M., ... Ma, G., Volkova, O.
Effect of Calcium Addition on the Aluminum Alloy
A201 Foam. Advanced Engineering Materials, 2024,
26(10), 2302093
Delavarrafiee, M.; Frey, H.C. Real-world fuel use and
gaseous emission rates for flex fuel vehicles erated on
E85 versus gasoline. J. Air Waste Manag. Assoc. 2018,
68, 235–254.
Cisek, J.; Leśniak, S.; Borowski, A.; Przybylski, W.;
Mokretskyy, V. Visualisation and Thermovision of
Fuel Combustion Affecting Heat Release to Reduce
NO
x
and PM Diesel Engine Emissions. Energies 2022,
15, 4882.
Enomoto, H.; Nakagawa, R. Reduction in CO Emission
from Small Reciprocating Engine Operated with Wood
Gasifier by Mixture LHV Changing. Energies 2023, 16,
2563.
Norouzi, A.; Heidarifar, H.; Shahbakhti, M.; Koch, C.R.;
Borhan, H. Model Predictive Control of Internal
Combustion Engines: A Review and Future Directions.
Energies 2021, 14, 6251.
Yue, Z.; Som, S. Fuel property effects on knock propensity
and thermal efficiency in a direct-injection spark-
ignition engine. Appl. Energy 2021, 281, 114221.
Leach, F.; Kalghatgi, G.; Stone, R.; Miles, P. The scope for
improving the efficiency and environmental impact of
internal combustion engines. Transp. Eng. 2020, 1,
100005.
IEA. World Energy Outlook 2022; IEA: Paris, France,
2022; Available online:
https://www.iea.org/reports/world-energy-outlook-
2022 (accessed on 1 August 2023).
Musabekov, Z., Daminov, O., Ismatov, A. Structural
solutions of the supercharged engine in the output and
input system. E3S Web of Conferences, 2023, 419,
01015
I-CRAFT 2024 - 4th International Conference on Research of Agricultural and Food Technologies
280
