
Review and Analysis of Batteries for Electric Vehicles in the Central
Asian Climate
Jakhongir Mirzaabdullaev
a
, Rustam Tillahodjaev
b
, Oybek Daminov
c
and Rustamjon Mirzajonov
d
Tashkent State Technical University, Energy Engineering and Professional Education Department,
100095, Tashkent, Uzbekistan
Keywords: Electric Vehicles, Battery Analysis, Central Asian Climate.
Abstract: This article discusses a review and analysis of batteries for electric vehicles in the dry and hot climate of
Central Asia. Suitable battery types were selected for electric vehicles operating under these conditions.
1 INTRODUCTION
Recently, more and more cars are switching from
hydrocarbon fuel (gasoline, diesel fuel, methane,
propane-butane, alternative fuels of plant origin, etc.)
to electric traction. Of course, the latter is still far
from the classic internal combustion engines in terms
of its characteristics, but progress does not stand still,
and if gasoline and diesel engines still hold the palm,
then very soon there will be a mass transition to more
environmentally friendly and silent electric engines,
that is, electric vehicles (Daminov et al., 2022;
Daminov et al., 2022; Daminov et al., 2022; Daminov
et al., 2022).
At the moment, the weak point of an electric car
is the battery. Lithium-ion batteries are mainly used
now. Such batteries, with their advantages associated
with relatively small sizes, suffer more from the
process of deterioration of characteristics over time.
Most of these batteries cannot serve for more than
five years. The number of charge-discharge cycles
(short charge and discharge cycle time, the battery
can withstand up to 2000 cycles) does not affect the
resource as much as age. To achieve maximum
battery life, it is necessary to use currents equal to half
the capacity when charging. And it is undesirable to
exceed the limit of one capacity, as this leads to a
sharp reduction in service life (Uddin et al., 2016; De
Dudley et al., 2017; Sutter et al., 2018; Lai et al.,
a
https://orcid.org/0000-0002-1002-2946
b
https://orcid.org/0000-0003-4137-1273
c
https://orcid.org/0000-0001-7876-1289
d
https://orcid.org/0000-0008-5289-0001
2018; El Ghossein, et al., 2019; Fan et al., 2019; Fang
et al., 2019; Han et al., 2019; Harting et al., 2019).
2 MATERIALS AND METHODS
A battery is already a set of many elements. Several
cells are connected into a battery when the
characteristics of one cell are not enough. If
connected in series, the voltage increases according
to the battery connection formula. If connected in
parallel, the battery capacity increases. It can include
not only battery elements, but also auxiliary control
electronics.
Capacity is a battery characteristic measured in
ampere-hours (A·h). For example, a capacity of 2 A·h
means that the battery can deliver a current of 1 A for
two hours and 2 A for one hour. Ampere-hour (A·h)
is a non-systemic unit of measurement of electric
charge, used mainly to characterize the capacity of
electric batteries.
Figure 1: Voltage dependence on capacitance.
240
Mirzaabdullaev, J., Tillahodjaev, R., Daminov, O. and Mirzajonov, R.
Review and Analysis of Batteries for Electr ic Vehicles in the Central Asian Climate.
DOI: 10.5220/0014247500004738
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 4th International Conference on Research of Agricultural and Food Technologies (I-CRAFT 2024), pages 240-247
ISBN: 978-989-758-773-3; ISSN: 3051-7710
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
A charged battery with a declared capacity of 1
A·h is theoretically capable of providing a current of
1 ampere for one hour (or, for example, 10 for 0.1
hour, or 0.1 A for 10 hours). In practice, too high a
battery discharges current leads to less efficient
power output, which non-linearly reduces its
operating time with such a current and can lead to
overheating.
The derived unit milliampere-hour (mA·h) is also
often used, which is usually used to indicate the
capacity of small batteries (Barcellona & Piegari,
2017; Abdullaev, 2022; Ismatov et al., 2023).
The value in ampere-hours can be converted to the
system unit of charge measurement - coulomb. Since
1 C/s is equal to 1 A, then, converting hours to
seconds, we get that one ampere-hour will be equal to
3600 C.
The battery capacity also depends on the
discharge current. Usually, the higher it is, the smaller
the capacity. Battery manufacturers usually indicate
the capacity obtained during discharge with some
measly current of 100 mA.
The graphic diagram shows the characteristics
of a Li-ion battery, which is discharged at different
current strengths. The higher the current, the lower
the discharge curve (Tillaxodjayev & Juraboev, 2021;
Tillyahodjaev & Mirzaev, 2022; Umerov et al., 2022;
Umerov et al., 2024).
C is a letter of the Latin alphabet that measures
the ratio of current strength to battery capacity, that
is, how many times the current exceeds the capacity.
If the battery has a capacity of 2 A·h and is discharged
at a current of 4 A, then we can say that it is
discharged at a current of 2 C. The thing is that the
greater the capacity of the battery, the easier it is to
give current to it, and therefore it is more convenient
to use such a characteristic than just amperes.
Energy is a characteristic that allows you to
compare batteries with different voltages. It is
measured in watt-hours and is roughly calculated by
multiplying the battery voltage by its capacity
(Musabekov et al., 2023; Musabekov et al., 2023;
Musabekov et al., 2023; Matmurodov et al., 2024).
Often, battery manufacturers indicate only the stored
charge in mAh (mA·h) in their technical
specifications, while others indicate only the stored
energy in W·h (W·h). Both characteristics can be
called "capacity" (not to be confused with electrical
capacity as a measure of a conductor's ability to
accumulate charge, measured in farads). Calculating
the stored energy from the stored charge is generally
not easy: it requires integrating the instantaneous
power supplied by the battery over the entire period
of its discharge. If high accuracy is not required, then
instead of integrating, you can use the average values
of voltage and current consumption, using the
formula that follows from the fact that
1 W = 1 V · 1 A:
1 W · h = 1 В · 1 А·h.
That is, the stored energy (in watt-hours) is
approximately equal to the product of the stored
charge (in ampere-hours) and the average voltage (in
volts):
E = q · U.
Example
The technical specification of the device states
that the “capacity” (stored charge) of the battery is 2.2
A·h, the operating voltage is 3.7 V. Then the
“capacity” (stored energy) is 2.2 A·h · 3.7 V = 8.14
W·h = 8.14 W · 3600 s = 29.304 kJ.
When identical batteries are connected in series,
the "capacity" remains the same, when connected in
parallel, it is added up. For example, for two batteries,
each with a voltage of 3.7 V and a stored charge of
2200 mAh, a series connection will create a source
with a voltage of 7.4 V and a stored charge of 2200
mAh, a parallel connection will create a source with
a voltage of 3.7 V and a stored charge of 4400 mAh.
Internal resistance of batteries - Li-ion battery
with a capacity of 2.2 A·h and a nominal voltage of
3.7 V. When fully charged, the battery has a voltage
of approximately U = 4 V. What current I will flow
through the battery if a resistor with a resistance of R
= 1 Ohm is connected to it? Based on the formula I =
U / R not 4 amperes, but slightly less - about 3.75 A.
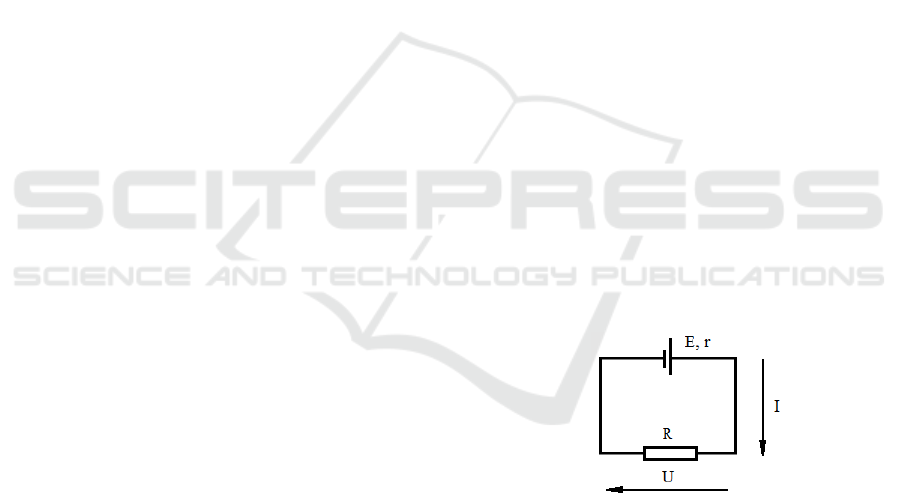
Figure 2: Battery resistance determination diagram.
Internal resistance of the battery if we measure the
voltage on the battery to which the resistor is
connected, we will see that it is approximately equal
to 3.75 V - the voltage on the battery has dropped due
to the fact that the diffusion rate of ions in the
electrolyte is not infinitely high.
The battery is represented as a two-terminal
network with EMF (electromotive force - voltage
without load) E and internal resistance r. It is assumed
that part of the battery EMF drops on the load, and the
other part - on the internal resistance of the battery. In
other words, it is assumed that the formula is correct:
Review and Analysis of Batteries for Electric Vehicles in the Central Asian Climate
241

E = (R + r) · I
We can roughly determine the internal resistance
of a 3.7V, 2.2Ah DC battery.
r = (E ‒ U) / I = (4В – 3,75V) / 2,2А = 0,11 Om.
Battery types
The electrical and operational characteristics of
the battery depend on the material of the electrodes
and the composition of the electrolyte. The following
batteries are currently the most common:
Li-ion ‒ lithium-ion. They have the highest
energy capacity. They discharge quickly when used
in the cold season.
Figure 3.
They deteriorate when discharged below 2.5 V.
They are explosive when overcharged above 4.2 V.
That is why many Li-ion batteries have a special
board under the case that switches off the current
when the voltage is below 2.5 V or above 4.2 V. Such
batteries have the word "protected" in the name.
Unprotected batteries without a special board cannot
be used in the battery. For more information on
protection - Li-ion batteries and their balancing, see
below. They lose capacity over time, even from
simply lying on a shelf. They lose capacity especially
quickly at high temperatures (Barcellona & Piegari,
2017; Venugopal et al., 2019). The easiest way to
avoid this problem is to use protected batteries. These
are the ones bought for all kinds of LED flashlights.
Protected batteries have a small board like this inside
the case:
Figure 4.
A popular size for lithium-ion batteries is 18650
(18mm wide and 65mm long).
Figure 5.
These are the ones used in laptop batteries. They
are located behind the plastic battery case. The same
ones are used in the Tesla Roadster electric sports car.
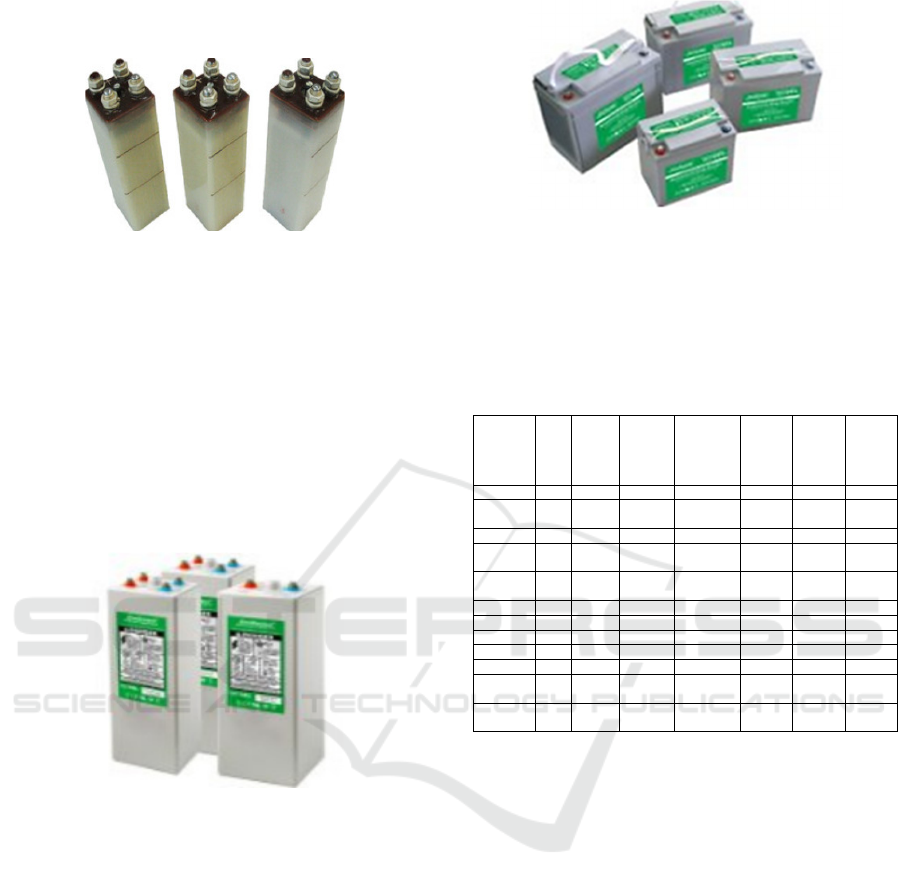
A nickel-cadmium battery (NiCd) is a
secondary chemical power source in which the
cathode is nickel oxide hydrate Ni(OH)2 with
graphite powder (about 5–8%), the electrolyte is
potassium hydroxide KOH with a density of 1.19–
1.21 with the addition of lithium hydroxide LiOH (to
form lithium nickelates and increase capacity by 21–
25%), and the anode is cadmium oxide hydrate
Cd(OH)2 or metallic cadmium Cd (in powder form).
The EMF of a nickel-cadmium battery is about 1.37
V, and the specific energy is about 45–65 Wh/kg
(Burzy et al., 2019).
Figure 6.
Depending on the design, operating mode (long or
short discharges) and purity of the materials used, the
service life is from 100 to 900 charge-discharge
cycles. Modern (lamellar) industrial nickel-cadmium
batteries can serve up to 20-25 years.
Nickel-cadmium batteries (NiCd), along with
Nickel-Saline batteries, can be stored discharged,
unlike nickel-metal hydride (NiMH) and lithium-ion
batteries (Li-ion), which must be stored charged. A
nickel-metal hydride battery (Ni-MH or NiMH) is a
secondary chemical power source in which the anode
is a hydrogen metal hydride electrode (usually nickel-
lanthanum or nickel-lithium hydride), the electrolyte
is potassium hydroxide, and the cathode is nickel
oxide (Canals et al., 2018; Chin et al., 2018; Dambone
et al., 2018; Chen et al., 2024).
Figure 7.
I-CRAFT 2024 - 4th International Conference on Research of Agricultural and Food Technologies
242

Research into NiMH battery technology began in
the 1970s as an attempt to overcome the shortcomings
of nickel-cadmium batteries. However, the metal
hydride compounds used at that time were unstable
and the required characteristics were not achieved. As
a result, the development of NiMH batteries stalled.
New metal hydride compounds stable enough for use
in batteries were developed in 1980.
Since the late 1980s, NiMH batteries have been
steadily improving, primarily in terms of energy
density. Their developers have noted that NiMH
technology has the potential to achieve even higher
energy densities.
A lithium-polymer battery (lithium-ion
polymer battery; abbreviations: Li-pol, Li-
polymer, LIP, Li-poly, etc.) is an improved design
of a lithium-ion battery. A polymer material is used
as an electrolyte (Worwood et al., 2018), (Wu et al.,
2019), (Xia et al., 2021), (Yehorov et al., 2024),
(Zhang and Lyu, 2018). It is used in mobile phones,
digital equipment, radio-controlled models, etc.
Figure 8.
Fully compatible with Li-ion. Unlike Li-ion, they
can deliver strong currents - 10-40 C. They can be of
any thickness and shape. They are suitable for
powering very miniature devices, such as compact
micro-equipment. They are usually sold in an already
assembled battery, with protective boards and cables
for balancing. They work even worse in the cold.
Lithium iron phosphate battery (LiFePO4,
LFP) is a type of electric battery, which is a type of
lithium-ion battery that uses LiFePO4 as a cathode.
Further evolution of lithium batteries. Batteries of
the future. Unlike Li-ion, they: are not afraid of frost;
are not fire hazardous; deliver currents up to 50 C; can
be charged with a strong current in 15 minutes; have
a huge number of charge-discharge cycles (2000-
8000 up to a loss of 20% of capacity); are practically
not subject to capacity loss during storage (Tom,
2012; Noh et al., 2019; Osara & Bryant, 2019).
Figure 9.
Disadvantages compared to Li-ion: more
expensive and have lower capacity; have lower
energy capacity; are not compatible with
conventional Li-ion cells due to a different voltage
range of 2-3.65 V. And, just like Li-ion, require
compliance with their voltage range of 2-3.65 V.
Lanthanum fluoride battery (La-Ft) is a very
powerful chemical current source with a solid
electrolyte. The anode is metallic lanthanum or
cerium, the electrolyte is lanthanum fluoride with the
addition of barium fluoride (about 6%) or, in the case
of a cerium anode, cerium (III) fluoride with the
addition of strontium fluoride, the cathode is bismuth
or lead fluoride with the addition of potassium
fluoride (~ 6%).
Figure 10.
Additions of potassium and alkaline earth metal
fluorides promote disordering in the anion sublattice
of lanthanum/cerium fluorides, which ultimately
leads to a 6-8-fold increase in the specific
conductivity of the solid electrolyte.
Lithium titanate battery (Li4Ti5O12) is a
variant of lithium-ion batteries that uses lithium
titanate (Li4Ti5O12) as an anode. To increase the
area, the anode has a nanocrystalline structure.
Figure 11.
This solution allows for an anode surface area of
up to 100 m2/g, compared to 3 m2/g for carbon,
which allows for a significant increase in the recharge
rate and high current density (De et al., 2018; Jinlei et
al., 2019; Wei et al., 2024).
A silver-cadmium battery (Ag-Cd) is a chemical
current source in which the anode is cadmium, the
Review and Analysis of Batteries for Electric Vehicles in the Central Asian Climate
243
electrolyte is potassium hydroxide, and the cathode is
silver oxide.
Figure 12.
Compared to a silver-zinc battery, it has lower
specific characteristics (EMF 1.6 V, 45-90 Wh/kg),
but at the same time a significant service life (over
3000 charge-discharge cycles, which is explained by
the fact that during charging, due to the lower
solubility of cadmium in alkali, cadmium dendrites
do not form).
NMC - one of the most successful options for the
implementation of a lithium-ion electrochemical
system is a combination of nickel, manganese and
cobalt.
Figure 13.
NMC battery in the 18650 cell size for moderate
load has a capacity of 2800 mAh and can provide a
current of 4-5 A. The capacity can be increased to
4000 mAh. But the maximum current is 20 A. Over
2000 charge-discharge cycles (Hildebrand et al.,
2018), (Kovtun et al., 2024), (Kuo et al., 2019),
(Wang et al., 2004).
LiCoO2 is a lithium-cobalt battery in the 18650
cell size with a capacity of 2400 mAh can be charged
and discharged with a current not exceeding 2400
mAh.
Figure 14.
Charge-discharge cycles 500-1000. Performance
at low and high temperatures.
Below are the parameters of batteries that can be
used in electric vehicles.
Table 1: Average values obtained in the experiment.
Designatio
n
EMF
(V)
Energy
capacity
(Wh/kg)
Energy
density
(Wh/dm3)
Operating
temperature
(°С)
Number
of
charge/
discharge
cycles
Self-
discharge
per year
(%)
Fast
charge
time
(minutes)
NiC
d
1,37 237 50-150 -50…+40 100-900 10 1,5
Ni-MH or
NiMH
1,25 300 150 -60…+50 300-500 100 1,5
Li-
p
ol 3,7 161 120 -20…+60 1000 60 1,5
LiFePO4 3,3 90-250 220-350 -30…+50 2000-
8000
50 -
La-Ft 2.3-
2.7
290-350 1330 +400…+50
0
- - -
Li4Ti5О12 3,7 30-110 177 -30...+60 15000 5 7
Ag-C
d
1,6 45-90 120 -30…+50 3000 - -
Li-ion 3,7 110-250 - -20…+60 1000 - 60
Lea
d
-Acid 2,1 135 1250 -40…+40 200-300 36-120 480-720
Ni-Zn 1,65 60 255 -30…+40 250-370 - 120
NMC 3,6-
3,7
150-220 - +210 1000-
2000
- 180
LiCoО2 3,0-
4,2
150-240 - +150 500-1000 - 180
3 RESULTS AND DISCUSSION
But whatever the layout, batteries are at the top of this
list. Electric vehicles use batteries assembled into a
single block with a series, parallel and mixed
connection. Since when choosing the optimal
connection scheme, you can achieve a positive effect
by reducing the number of batteries and, accordingly,
the weight of the battery block (Daminov et al.,
2022a; Daminov et al., 2022b; Yehorov et al., 2024).
The battery of the TESLA electric car has been of
interest to specialists in this field for some time, who
have sought to learn the secret of the connection. In
principle, this is not a secret, but a competent choice
of batteries with high energy capacity (Wh/kg),
energy density (Wh/dm3) and the number of
charge/discharge cycles.
These justifications can be summarized in the
following simple arithmetic solutions.
I-CRAFT 2024 - 4th International Conference on Research of Agricultural and Food Technologies
244

A lithium-ion battery has the following
parameters. 3.7 V and 2.2 Ah.
Power of one battery;
3,7 · 2,2 = 8,14 WA/h
If you connect 100 cells in parallel or in series, or
even combine them, the result is obtained according
to the formula;
1003,72,2 = 814 WA/h
Tesla model S has 7104 batteries with a capacity
of 85,000 W (A/h). According to the data, these
batteries are manufactured by Panasonic.
The parameters of the battery are NCR 18650B,
voltage 3.6 V and current 3.4 Ah. It consists of 16
modules of 5300 W (A/h). One module has 444
elements. There are 6 groups of 74 batteries. These
batteries are connected in parallel, and the power of
the groups is calculated using the formula;
3,6 3,4 7104=86952 WA/h
We have considered different ways of connecting
batteries. In turn, we can draw the following
conclusions. Regardless of how you connect the
batteries, the maximum value of w·h (running time in
hours) will remain unchanged. In particular, among
different manufacturers of electric vehicles, the same
w·h (power hour) remains unchanged when analyzing
the Tesla Model C, which has a battery capacity of 85
kWh. When calculating losses of approximately ≈85
kW (A/h), these calculations show that the range of a
Tesla electric car has nothing to do with the
connection of the battery.
4 CONCLUSIONS
We conducted our research, considering from the
small list which is provided above it is difficult to
choose what met the requirements of the electric
vehicle manufacturers. The parameters are not
unambiguous and go to the optimal solution.
Therefore, based on the climate of the Central Asian
region for electric vehicles, it is necessary to select
the appropriate battery suitable for operation in a hot
and dry environment. The most suitable elements
from the above list are lithium-titanate batteries
(Li
4
Ti
5
O
12
). The battery is more optimal in
parameters and meets the minimum requirements
presented for the battery for electric vehicles such as
a long service life based on charge-discharge cycles
and the shortest charging time (Tillaxodjayev et al.,
2021; Daminov et al., 2022).
Currently, the most pressing problem for many
electric vehicle manufacturers, including Tesla
electric vehicles, is to increase the mileage. The
power reserve depends on some characteristics of the
electric vehicle, in particular, on the power of the
main source of electricity in it - batteries, which can
be provided by choosing more advanced batteries.
And the aspect associated with the problem of
environmental safety associated with the disposal of
used batteries can be compensated for by batteries
with a long service life and rational use of battery
capacity.
ACKNOWLEDGMENT
The management of the Tashkent State Technical
University named after Islam Karimov and the staff
of the department of “Energy Engineering and
Vocational Education” express deep gratitude to the
World Bank for the allocated grant and the Academic
Innovation Fund under the Ministry of Higher
Education, Science and Innovation of the Republic of
Uzbekistan as well as the Islamic Development Bank
for the assistance provided in the implementation of
this project.
REFERENCES
Abdullaev I.Y. The importance of electric vehicles around
global areas. Scientific and Technical journal of
Namangan Institute of Engineering and Technology.
Tom 7 Volume 4, 2022 year. ISSN 2181-8622 pp 204-
210.
Barcellona, S.; Piegari, L. Lithium ion battery models and
parameter identification techniques. Energies 2017, 10,
2007.
Bazarov, B., Axmatjanov, R., Tojiyev, J., Azimov, A. The
concept of improving the performance indicators of
gas-cylinder vehicles. E3S Web of Conferences, 2023,
434, 02008
Burzy´nski, D.; Pietracho, R.; Kasprzyk, L.; Tomczewski,
A. Analysis and modeling of the wear-out process of a
lithium-nickel manganese-cobalt cell during cycling
operation under constant load conditions. Energies
2019, 12, 3899.
Canals Casals, L.; Igualada, L.; Corchero, C. The effect of
building energy management systems on Battery
Aging. E3S Web Conf. 2018, 61, 00014.
Chen, W., Cheng, Z., Wen, Q., ... Volkova, O., Liu, J. Ya
High-Strength Nonoriented Electrical Steel with
Excellent Magnetic Properties Accomplished by Cu–Ni
Multialloying. Steel Research International, 2024
Chin, C.; Gao, Z.; Chiew, J.; Zhang, C. Nonlinear
temperature-dependent state model of cylindrical
LiFePO4 battery for open circuit voltage, terminal
Review and Analysis of Batteries for Electric Vehicles in the Central Asian Climate
245

voltage and state-of-charge estimation with extended
Kalman filter. Energies 2018, 11, 2467.
Dambone Sessa, S.; Tortella, A.; Andriollo, M.; Benato, R.
Li-ion battery-flywheel hybrid storage system:
Countering Battery Aging during a grid frequency
regulation service. Appl. Sci. 2018, 8, 2330.
Daminov O., Mirzajonov R., Turdiev J., Usmonov J.
Improving energy efficiency of electric vehicles.
International Conference on Electrical Facilities and
informational technologies 2022 (ICEF 2022). "New
Intelligence Technology: Past, Present and Future".
Turin polytechnic university in Tashkent, Uzbekistan.
August 10-13, 2022. pp. 61-64.
Daminov O.O., Juraboev A.Z., Daminov L.O., Kurbanov
A.M. Analysis of energy sources for electric vehicles.
International Conference on Electrical Facilities and
informational technologies 2022 (ICEF 2022). "New
Intelligence Technology: Past, Present and Future".
Turin polytechnic university in Tashkent, Uzbekistan.
August 10-13, 2022. pp. 37-41.
Daminov O.O., Mirzajonov R.I. Indicators of electric car
batteries. Actual problems of materials science,
innovative technologies of materials acquisition and
welding production - 2022. Republican scientific and
technical conference. A set of materials. November 19,
2022. - T.: 2022. - pp. 270-271.
Daminov O.O., Juraboev A.Z., Kersten S. Working process
of cells and batteries. Journal: Technical science and
innovation. 2022, №2. - P. 134-140
https://unilibrary.uz/articles/303207
De Hoog, J.; Jaguemont, J.; Abdel-Monem, M.; Van Den
Bossche, P.; Van Mierlo, J.; Omar, N. Combining an
electrothermal and impedance aging model to
investigate thermal degradation caused by fast
charging. Energies 2018, 11, 804.
De Sutter, L.; Berckmans, G.; Marinaro, M.; Smekens, J.;
Firouz, Y.; Wohlfahrt-Mehrens, M.; Van Mierlo, J.;
Omar, N. Comprehen sive aging analysis of volumetric
constrained lithium-ion pouch cells with high
concentration silicon-alloy anodes. Energies 2018, 11,
2948.
Dudley, G.; Blake, R.; Lucas, L. Mars Express Lithium Ion
batteries performance analysis. E3S Web Conf. 2017,
16, 06002.
El Ghossein, N.; Sari, A.; Venet, P. Lifetime prediction of
lithium-ion capacitors based on accelerated aging tests.
Batteries 2019, 5, 28.
Fan, J.; Zou, Y.; Zhang, X.; Guo, H. A novel state of health
estimation method for lithium-ion battery in electric
vehicles. J. Phys. Conf. Ser. 2019, 1187, 022014.
Fang, Q.; Wei, X.; Lu, T.; Dai, H.; Zhu, J. A state of health
estimation method for lithium-ion batteries based on
voltage relaxation model. Energies 2019, 12, 1349.
Han, X.; Feng, X.; Ouyang, M.; Lu, L.; Li, J.; Zheng, Y.;
Li, Z. A comparative study of charging voltage curve
analysis and state of health estimation of lithium-ion
batteries in Electric Vehicle. Automot. Innov. 2019, 2,
263–275.
Harting, N.; Wolff, N.; Röder, F.; Krewer, U. State-of-
health diagnosis of lithium-ion batteries using nonlinear
frequency response analysis. J. Electrochem. Soc. 2019,
166, A277.
Hildebrand, S.; Rheinfeld, A.; Friesen, A.; Haetge, J.;
Schappacher, F.M.; Jossen, A.; Winter, M. Thermal
analysis of lini0.4co0.2mn0.4o2/mesocarbon
microbeads cells and electrodes: State-of-charge and
state-of-health influences on reaction kinetics. J.
Electrochem. Soc. 2018, 165, A104.
Ismatov, J., Aripdjanov, M., Mirzaabdullaev, J., ... Mirzaev,
A., Viarshyna, H. Operating indicators in diesels with
reduced heat and energy losses. IOP Conference Series:
Earth and Environmental Science, 2023, 1142(1),
012021
Jinlei, S.; Lei, P.; Ruihang, L.; Qian, M.; Chuanyu, T.;
Tianru, W. Economic Operation Optimization for 2nd
use batteries in Battery Energy Storage Systems. IEEE
Access 2019, 7, 41852–41859.
Jutt V.E., Stroganov V.I. Electric vehicles and vehicles with
a combined power plant. Calculation of speed
characteristics: textbook. Allowance / V.E. Jutt and V.I.
Stroganov. - Moscow: MADI, 2016 - 108 p. (11-35 p.)
Kovtun, O., Levchenko, M., Höntsch, S., ... Gräbner, M.,
Volkova, O. Recycling of iron-rich basic oxygen
furnace dust using hydrogen-based direct reduction.
Resources, Conservation and Recycling Advances,
2024, 23, 200225
Kuo, T.J.; Lee, K.Y.; Chiang, M.H. Development of a
neural network model for SOH of LiFePO4 batteries
under different aging conditions. IOP Conf. Ser. Mater.
Sci. Eng. 2019, 486, 012083.
Lai, X.; Qiao, D.; Zheng, Y.; Yi, W. A novel screening
method based on a partially discharging curve using a
genetic algorithm and back-propagation model for the
Cascade Utilization of retired lithium-ion batteries.
Electronics 2018, 7, 399.
Matmurodov, F.M., Daminov, O.O., Sobirov, .Sh.,
Abdurakxmanova, M.M., Atakhanov, F.U.M. Dynamic
simulation of force loading of drives of mobile power
facilities with variable external resistance. E3S Web of
Conferences, 2024, 486, 03001
Musabekov, Z., Daminov, O., Ismatov, A. Structural
solutions of the supercharged engine in the output and
input system. E3S Web of Conferences, 2023, 419,
01015
Musabekov, Z., Ergashev, B., Daminov, O., Khushnaev,
O., Kurbanov, A., Kukharonok, G. Efficiency and
environmental indicators of diesel engine operation
when using water injection. IOP Conference Series:
Earth and Environmental Science, 2023, 1142(1),
012024
https://iopscience.iop.org/article/10.1088/1755–1315
Musabekov, Z., Tillahodjaev, R., Mirzayev, A.,
Yangibayev, A., Uralova, H. Increasing energy
efficiency as a result of the influence on the operation
of a Sano automobile engine with the adding of
hydrogen as an additive to primary fuel. IOP
Conference Series: Earth and Environmental Science,
2023, 1284(1), 012038
Noh, T.-W.; Ahn, J.-H.; Lee, B.K. Cranking capability
estimation algorithm based on modeling and online
I-CRAFT 2024 - 4th International Conference on Research of Agricultural and Food Technologies
246

update of model parameters for Li-ion SLI Batteries.
Energies 2019, 12, 3365.
Osara, J.; Bryant, M. A thermodynamic model for lithium-
ion battery degradation: Application of the degradation-
entropy generation theorem. Inventions 2019, 4, 23.
Optimization of the resource of defining operating modes
of traction batteries of electric vehicles: Dissertation for
the degree of Candidate of Technical Sciences / B.K.
Ospanbekov. - Moscow: MADI, 2017 - 170 p. (10-46
p.)
Tom Denton. Automobile Electrical and Electronic
Systems. Fourth Edition. – New York: Routledge,
2012. 703 p.
Tillaxodjayev R.R., Juraboev A.A. Analysis of batteries for
electric vehicles and their calculations. International
scientific and technical journal “Innovation technical
and technology”, 2021, vol. 2, no. 1, pp. 99-103.
Tillyahodjaev R.R., Mirzaev A.A. Efficiency of using solar
collectors in energy saving and saving energy
resources. Technical science and innovation №4/2022
year.
Uddin, K.; Perera, S.; Widanage, W.; Somerville, L.;
Marco, J. Characterising lithium-ion battery
degradation through the identification and tracking of
electrochemical battery model parameters. Batteries
2016, 2, 13.
Umerov F.Sh., Inoyatkhodjaev J.Sh., Asanov S.E.
The prospects for the development of electric vehicles
in Uzbekistan. Журнал Acta of Turin Polytechnic
University in Tashkent ВЕСТНИК, 2022, 30, – С. 65-
68. Published Online June 2022 in Acta TTPU
(http://www.acta.polito.uz/)
Umerov, F., Daminov, O., Khakimov, J., Yangibaev, A.,
Asanov, S. Validation of performance indicators and
theoretical aspects of the use of compressed natural gas
(CNG) equipment as a main energy supply source on
turbocharged internal combustion engines vehicles.
AIP Conference Proceedings, 2024, 3152(1), 030017
Venugopal, P.; Vigneswaran, T. State-of-health estimation
of Li-ion batteries in electric vehicle using INDRNN
under variable load condition. Energies 2019, 12, 4338.
Wang X. et al. // J. Crystal Growth. 2004. – vol. 267. –
p.184.
Wei, X., Zhao, W., Ilatovskaia, M., ... Ma, G., Volkova, O.
Effect of Calcium Addition on the Aluminum Alloy
A201 Foam. Advanced Engineering Materials, 2024,
26(10), 2302093
Worwood, D.; Algoo, R.; McGlen, R.J.; Marco, J.;
Greenwood, D. A study into different cell-level cooling
strategies for cylindrical lithium-ion cells in automotive
applications. Int. J. Powertrains 2018, 7, 199.
Wu, Y.; Li, W.; Wang, Y.; Zhang, K. Remaining useful life
prediction of lithium-ion batteries using neural network
and bat-based particle filter. IEEE Access 2019, 7,
54843–54854.
Xia, B.; Chen, G.; Zhou, J.; Yang, Y.; Huang, R.; Wang,
W.; Lai, Y.; Wang, M.; Wang, H. Online parameter
identification and joint estimation of the State of charge
and the state of health of lithium-ion batteries
considering the degree of polarization. Energies 2019,
12, 2939. Energies 2021, 14, 8072 32 of 33
Yehorov, A., Wei, X., Mazepa, S., Sherstneva, A.,
Volkova, O. Oxidation Behavior of MgO-C
Refractories Containing Metallic Aluminum, Calcium
Magnesium Aluminate Aggregates, and Carbores P.
Steel Research International, 2024
Zhang, L.; Lyu, C. Decomposition study of degradation
reasons for licoo2-based 14500 lithium-ion batteries
using a nondestructive method. IEEE Access 2018, 6,
44417–44432.
Review and Analysis of Batteries for Electric Vehicles in the Central Asian Climate
247
