
Effects of Different LED Light Spectra on the Shelf Life and
Nutritional Quality of Hydroponically Grown Lettuce in a Plant
Factory
Abdullah Aldiyab and Hayriye Yildiz Dasgan
*
Department of Horticulture, Faculty of Agriculture, University of Cukurova, Adana 01330, Turkey
Keywords: Indoor Vertical Farming, Postharvest Quality, Shelf Life, Hydponic Culture, Lactuca sativa var. crispa L.,
Antioxidants.
Abstract: A study was conducted to assess the shelf life of lettuce grown under three different LED light spectra using
hydroponic techniques in a plant factory. The Batavia-type cultivar ‘Capira’ was used, and seedlings were
transferred to an ebb-and-flow system 12 days after sowing. The plant factory was maintained at 25 °C (day),
20 °C (night), with a 12-hour photoperiod, ~400 ppm CO₂, and 60–70% relative humidity. Lettuce was grown
under three LED spectra: LED1 = 70% red (610-720 nm) + 30% blue (450-495 nm), LED2 = Full PAR
spectrum (400-700 nm), LED3 = 65% red (610-720 nm) + 25% blue (450-495 nm) + 5% white (400 475 nm)
+ 5% far red (700-760).. After 30 days of growth, the lettuce was harvested and stored at 8 °C and 60–70%
humidity for 14 days. Post-storage evaluations included weight loss, chlorophyll degradation, phenolic and
flavonoid contents, nitrate levels, and mineral composition. Weight loss ranged from 1.54% to 2.80%,
chlorophyll degradation from 8.1% to 11.3%, phenolic decline from 20.2% to 23.2%, and flavonoid loss from
11.68% to 22.6%. Nitrate reduction varied from 26.0% to 47.3%. These results highlight how different LED
light spectra influence the postharvest quality and shelf life of hydroponically grown lettuce.
1 INTRODUCTION
The increasing demand for minimally processed
fruits and vegetables has drawn significant attention,
especially regarding changes in their phytochemical
properties during storage. Consumers, informed by
scientific research, are becoming more selective,
valuing not only sensory qualities like taste, aroma,
and texture but also the nutritional content, including
vitamins and minerals, when choosing fresh produce
(Özgen & Tokbaş, 2007). A substantial body of
research indicates that diets rich in fruits and
vegetables reduce the risk of chronic diseases (Block
et al., 1992). This protective effect is largely
attributed to the abundance of antioxidants and
flavonoids in these foods, which play a crucial role in
promoting health (Hertog et al., 1993). Multiple
studies have demonstrated an inverse relationship
between fruit and vegetable intake and the incidence
of certain cancers (Steinmetz & Potter, 1996; Kaur &
Kapoor, 2001). Consequently, identifying
*
Corresponding author
phytochemical profiles and evaluating antioxidant
capacities are essential steps in advancing clinical
research on specific cancer types (Özgen &
Scheerens, 2006). However, antioxidant levels in
fruits and vegetables are sensitive to various factors,
such as species differences, cultivation methods,
storage conditions, and pre-treatment processes that
affect bioactive compounds (Price et al., 1998; Del
Caro et al., 2004). This variability has generated
growing interest in assessing the antioxidant
capacities of foods consumed regularly (Sağlam,
2007). In recent years, pre-processed fruits and
vegetables have gained popularity among consumers
due to their convenience in reducing preparation time.
Maintaining high-quality standards throughout
production and storage is crucial for these products
(Martinez et al., 2008).
In this study, three types of LED lighting were
used to grow lettuce, which was harvested after 30
days of cultivation. Following harvest, the lettuce was
stored for 14 days, with chemical properties analyzed
158
Aldiyab, A. and Dasgan, H. Y.
Effects of Different LED Light Spectra on the Shelf Life and Nutritional Quality of Hydroponically Grown Lettuce in a Plant Factory.
DOI: 10.5220/0014224200004738
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 4th International Conference on Research of Agricultural and Food Technologies (I-CRAFT 2024), pages 158-168
ISBN: 978-989-758-773-3; ISSN: 3051-7710
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

at three intervals: on the first, seventh, and fourteenth
days of storage. These analyses aimed to assess the
effects of storage duration and different lighting
conditions on the lettuce’s chemical composition. The
study provides insight into how pre-harvest
environmental conditions, such as light quality, and
post-harvest treatments influence the nutritional and
phytochemical stability of leafy greens during
storage.
The quality of freshly cut fruits and vegetables
depends on attributes such as texture, appearance,
nutritional value, and flavor (Witkowska &
Woltering, 2013). However, this quality degrades
over time due to simultaneous biological processes
(Witkowska & Woltering, 2014). These processes are
influenced by the plant’s morphological and
physiological characteristics, which are shaped by
pre-harvest environmental conditions (Watada et al.,
1996; Zou et al., 2019). Therefore, optimizing the
growth environment to preserve post-harvest quality
is essential for the food industry (Watada et al., 1996;
Fanourakis et al., 2016). Proper storage temperatures
(0–5°C) are crucial for maintaining the quality of both
whole and freshly cut produce (Tian et al., 2014;
Tsaniklidis et al., 2014).
In many developing countries, maintaining the
cold chain is challenging due to high costs and
unreliable electrical infrastructure (Mercier et al.,
2017). For example, only 15% of perishable food
products in China are transported using refrigerated
trucks (USDA, 2008), and India has only recently
developed a fully refrigerated supply chain (Dharni &
Sharma, 2015). Inadequate temperature control
affects not only transportation but also storage at
retail outlets and during commercial processing
(Likar & Jevšnik, 2006; Tian et al., 2014; Mercier et
al., 2017). Therefore, exploring ways to preserve
product quality under near-ambient conditions
remains a priority, especially in regions where cold
chain infrastructure is limited.
The shelf life of fruits and vegetables containing
chlorophyll is often constrained by the yellowing
caused by chlorophyll degradation (Tay & Perera,
2004). Browning, another common issue, results
from the oxidation of phenolic compounds (Fan &
Mattheis, 2000; Degl’Innocenti et al., 2005).
Phenylalanine ammonia-lyase (PAL) is the first key
enzyme involved in the biosynthesis of phenolics and
flavonoids via the phenylpropanoid pathway. PAL is
induced by stress, and the resulting phenolic
compounds offer antioxidant benefits for both plant
defense and human health (Fan & Mattheis, 2000;
Iakimova & Woltering, 2015; Tsaniklidis et al.,
2017). However, excessive phenolic accumulation
activates polyphenol oxidase (PPO), which catalyzes
the oxidation of phenols into quinones, leading to
undesirable browning. While PPO contributes to
plant defense against biotic stress, it also accelerates
post-harvest deterioration, affecting both the visual
appearance and nutritional value of produce
(Degl’Innocenti et al., 2005).
UVA radiation (320–400 nm), a primary
component of solar UV light, lies outside the spectral
range required for photosynthesis (Hogewoning et al.,
2012). As a result, its use in indoor cultivation has
been limited (Zhang et al., 2020). However, recent
from our laboratory shows that supplementing indoor
cultivation with UVA light (10 μmol m⁻² s⁻¹)
increases biomass and metabolite accumulation in
lettuce plants (Chen et al., 2019). Before commercial
application, it is essential to further evaluate the
impact of UVA light on post-harvest processes,
particularly its influence on shelf life.
The primary aim of this study is to investigate the
effects of different LED light treatments on the
quality, nutritional content, and shelf life of lettuce
during a 14-day storage period. Specifically, the study
seeks to evaluate key parameters such as weight loss,
pH, electrical conductivity, titratable acidity,
phenolic and flavonoid content, and nitrate levels
under varying LED light conditions. The objective is
to determine whether specific LED treatments can
enhance the post-harvest stability of lettuce,
preserving both its biochemical and sensory
attributes. It is hypothesized that certain LED light
treatments will be more effective in minimizing
nutrient losses and maintaining product quality, thus
extending the shelf life of lettuce. This research aims
to provide valuable insights into how LED lighting
can be optimized as part of post-harvest management
strategies, aligning with the growing demand for
high-quality, ready-to-eat produce in the modern food
industry.
2 MATERIALS AND METHODS
The experiment was conducted in March 2024 at the
Department of Horticulture, Faculty of Agriculture,
Çukurova University. A climate-controlled plant
growth chamber, measuring 5.0 m in length, 3.0 m in
width, and 2.6 m in height, was designed to function
as a plant factory for this study. The chamber was
equipped to regulate environmental factors essential
for optimal plant growth, including temperature,
humidity, lighting, CO₂ levels, and air circulation.
Effects of Different LED Light Spectra on the Shelf Life and Nutritional Quality of Hydroponically Grown Lettuce in a Plant Factory
159

These conditions were meticulously controlled to
provide an ideal environment for plant development.
Lettuce plants were cultivated in a vertical
farming system within the climate-controlled
chamber. The system consisted of three stacked tiers
made from galvanized steel, with 40 cm of space
between each shelf, maximizing space efficiency.
The plants were grown using the Ebb-Flow
hydroponic technique, also known as the "Med-
Cezir" technique in Turkish. This method
periodically floods the plant roots with nutrient-
enriched water, followed by drainage, ensuring the
roots receive both optimal nutrition and aeration. The
model plant selected for the study was green-leaf
lettuce, a widely cultivated leafy vegetable, chosen
for its suitability for indoor farming and sensitivity to
controlled environmental conditions, making it ideal
for hydroponic research. Twelve days after sowing,
the lettuce seedlings were transferred to the Ebb-Flow
hydroponic system (Figure 1). Environmental
conditions in the plant factory were carefully
managed to ensure optimal growth. Daytime
temperatures were set at 25°C and nighttime
temperatures at 20°C, with a 12-hour light/12-hour
dark photoperiod. CO₂ levels were maintained at
approximately 400 ppm, and relative humidity was
kept between 60-70%. Lettuce plants were exposed to
three distinct LED lighting configurations:
LED1 = 70% red (610-720 nm) + 30% blue (450-
495 nm)
LED2 = Full PAR spectrum (400-700 nm)
LED3 = 65% red (610-720 nm) + 25% blue (450-
495 nm) + 5% white (400 475 nm) + 5% far red
(700-760).
The lettuce plants were cultivated under these
controlled conditions using hydroponic techniques
for 30 days before being harvested. After harvest, the
plants were stored in a cold storage facility at 8°C
with 60-70% humidity for 14 days. At the end of the
storage period, various parameters were measured,
including weight loss, chlorophyll content, phenol
and flavonoid concentrations, nitrate levels, and
mineral nutrient content. This approach enabled a
comprehensive evaluation of the effects of different
lighting conditions and storage durations on the post-
harvest quality of lettuce.
Measurements and Analyses Conducted in the
Experiment
Weight Loss: The fresh weight of the lettuce plants
was measured on days 1, 7, and 14 using a precision
scale. Based on these measurements, the percentage
of weight loss was calculated to assess moisture loss
over time.
Dry Matter (%): Dry matter content was determined
by measuring both the fresh and dry weights of the
plants. This analysis evaluated how much dry matter
was produced per 100 g of fresh lettuce under
different treatments.
Total Phenolic Content (mg GAE/100 g FW): The
total phenolic content in lettuce leaves was measured
using a modified version of the spectrophotometric
method described by Spanos and Wrolstad (1990).
Absorbance was recorded at 765 nm using a
spectrophotometer (Perkin Elmer Lambda EZ201
UV/VIS). Phenolic content was calculated from a
calibration curve prepared with gallic acid (Dasgan et
al., 2022; Ikiz et al., 2024).
Figure 1: Lettuce plants grown in a plant factory using a
hydroponic system under three different LED light spectra.
I-CRAFT 2024 - 4th International Conference on Research of Agricultural and Food Technologies
160

Total Flavonol Content (mg RUT/g FW): Flavonol
content in the lettuce leaves was measured according
to the method developed by Quettier-Deleu et al.
(2000). Absorbance readings were taken at 415 nm
using a spectrophotometer (Perkin Elmer Lambda
EZ201 UV/VIS), with flavonol content calculated
based on a calibration curve using rutin (Ikiz et al.,
2024).
Nitrate Content (ppm): A quarter of each lettuce
plant was used to determine nitrate levels using a
colorimetric method based on the salicylic acid
nitration procedure (Cataldo et al., 1975; Dasgan et
al., 2023a; Balik et al., 2025).
Total Soluble Solids (TSS) (%): The lettuce plants
were divided into four sections, and juice was
extracted from one-quarter of each plant using a
juicer. The soluble solid content (SSC) was measured
using a digital refractometer (Keskin et al., 2025).
EC and pH Measurements:To measure electrical
conductivity (EC) and pH, 100 ml of lettuce leaf juice
was extracted. The EC and pH values were recorded
using a combined pH and EC meter to evaluate the
effects of treatments on leaf nutrient balance (Keskin
et al., 2025).
Titratable Acidity (%): Titratable acidity was
measured by adding 50 ml of distilled water to 1 ml
of lettuce juice and titrating the mixture with 0.1 N
NaOH until the pH reached 8.1. The amount of NaOH
used was recorded to quantify acidity.
L, a, b Color Measurement: Lettuce color was
assessed using a digital colorimeter to measure
Hunter color parameters (L*, a*, b*). The colorimeter
was calibrated with a white ceramic plate (L = 96.96,
a = 0.08, b = 1.83) before each measurement. L*
represents brightness, a* measures red/green balance
(+a* for red, -a* for green), and b* indicates
yellow/blue balance (+b* for yellow, -b* for blue)
(Gould, 1977).
Nutrient Element Analysis: Macro- and
microelement content in lettuce leaves was analyzed
to determine the impact of different treatments on
plant nutrition (Dasgan et al., 2023b). Leaves were
washed with 0.1% detergent and rinsed three times
with distilled water to avoid contamination. The
cleaned leaves were dried at 65°C for 48 hours and
then ground. Samples were combusted at 550°C for 8
hours, and the resulting ash was dissolved in 3.3%
HCl. Potassium (K), calcium (Ca), magnesium (Mg),
and sodium (Na) were measured using emission
mode, while iron (Fe), manganese (Mn), zinc (Zn),
and copper (Cu) were analyzed using absorption
mode with an atomic absorption spectrometer.
Phosphorus (P) content was determined
spectrophotometrically using the Barton method.
Statistical Analysis
All experiments were conducted with three replicates.
Variance analysis (ANOVA) was performed using
JMP statistical software (Version 7.0, 2007).
Differences between treatments were assessed using
the Least Significant Difference (LSD) test, with
significance set at p < 0.05.
3 RESULTS AND DISCUSSION
The results of the analyses, including weight loss,
soluble solid content (SSC), dry matter content, pH,
titratable acidity, total chlorophyll, total flavonoid,
total phenolic content, and macro- and microelement
concentrations in the lettuce samples, measured at the
beginning and on day 14 of storage, are summarized
in Tables 1, 2, 3, 4, and 5.
Effects of Biofertilizers on Leaf Nutritional and
Antioxidant Compounds
At the start of storage, SSC values ranged from 2.29
to 3.01 °Brix. The highest SSC value (3.01 °Brix) was
recorded in the 1st LED treatment on the first day,
while the lowest value (2.29 °Brix) was observed in
the 2nd LED treatment on day 14 (Table 1). A
significant decline in SSC values occurred over the
14-day storage period, likely due to the respiration
process, during which sugars and organic acids
degrade.
The pH values of lettuce samples, recorded both
post-harvest and during storage, are also presented in
Table 1. The pH ranged between 5.75 and 6.10, with
the highest value (6.10) observed in the 1st LED
treatment on day 14, and the lowest (5.75) recorded
in the 3rd LED treatment on day 1. A significant
increase in pH occurred by the end of the storage
period, consistent with previous studies. Hassenberg
and Idler (2005) reported that lettuce washed with tap
water showed a pH increase from 6.11 to 6.39 within
six days. King et al. (1991) observed a similar rise in
pH in lettuce stored at 5°C. Additionally, Allende et
al. (2004) and Martin-Diana et al. (2006) suggested
that microbial activity and production methods
contribute to pH increases during storage.
Electrical conductivity (EC) values, which reflect
changes in the mineral balance during storage, ranged
from 23.42 to 27.56 dS/m. On the first day, the lowest
EC value (8.60 dS/m) was measured under the 3rd
LED treatment, decreasing further to 6.56 dS/m by
day 14. This suggests a deterioration in the water and
Effects of Different LED Light Spectra on the Shelf Life and Nutritional Quality of Hydroponically Grown Lettuce in a Plant Factory
161

mineral balance of the lettuce during storage. In
contrast, the 1st and 2nd LED treatments exhibited
higher EC values at the end of the storage period, with
the highest EC (27.56 dS/m) recorded under the 1st
LED treatment on day 14. These results indicate that
the 1st LED treatment was more effective in
preserving the mineral content and ion balance of the
lettuce plants during storage.
3.1 Implications for Post-Harvest
Quality Management
These findings underscore the importance of storage
conditions and light sources in maintaining the
mineral balance and overall quality of lettuce during
storage (Table 1). The ability of specific LED
treatments to manage EC fluctuations suggests that
targeted lighting strategies could play a crucial role in
maintaining post-harvest quality. The 1
st
LED
treatment, in particular, demonstrated potential for
preserving mineral content, highlighting the value of
optimized light conditions during storage. Research
like this contributes to improving storage practices,
enhancing quality parameters, and minimizing post-
harvest losses, thereby supporting more sustainable
agricultural practices.
Table 1: The changes in pH, EC and TSS values of the lettuce samples during the storage period.
Treatment
pH EC TSS
Day 1 Day14 Loss % Day 1 Day 14 Loss % Day 1 Day 14 Loss %
LED
1
5,92 6,10 -3,03 8,43b 6,10b 27,56a 3,01a 2,83a 5,66bc
LED
2
5,88 6,13 -4,24 8,40b 6,14b 26,92a 2,90a 2,50b 13,79a
LED
3
5,75 6,01 -4,51 8,60a 6,58a 23,42b 2,50b 2,29b 8,41b
LDS
0,05
n.s n.s
4,138 0,095 0,107 0,811 0,244 0,263 0,252
P 0,1193 0,2220 0,6971 0,0043* <0.0001* <0.000* 0,0055* 0,0074* 0,0061
TSS: Total soluble solids. There is no significant difference between means with the same letter in the same column; LSD:
the least significant difference.
3.2 Titratable Acidity and Dry Matter
in Lettuce During Storage
The titratable acidity of lettuce samples during
storage ranged from 0.35 to 1.61 g/100g, with the
highest value (1.61 g/100g) recorded in the 1st LED
treatment on day 1 (Table 2). The lowest value (0.35
g/100g) was observed in the 2nd LED treatment on
day 14. By the end of the 14-day storage period, the
reduction in titratable acidity varied between 62.67%
and 77.01%, with the highest loss (77.01%) occurring
in the 1st LED treatment on day 1, while the lowest
reduction (62.67%) was recorded in the 3rd LED
treatment on day 14. These results suggest that the 3rd
LED treatment may be more effective in preserving
titratable acidity during storage. Scuderi et al. (2011)
reported similar findings for Duende lettuce, where
titratable acidity decreased from 1.01 g/L on day 1 to
0.42 g/L by day 9 during storage at 4°C.
At the start of storage, the total dry matter content
of the lettuce samples ranged from 3.02 to 4.56
g/100g (Table 2), with the highest value (4.56 g/100g)
recorded in the 1st LED treatment on day 1 and the
lowest value (3.02 g/100g) observed in the 2nd LED
treatment on day 14. Over the 14-day period, the dry
matter content ranged between 12.85 and 27.20
g/100g, with the highest reduction (27.20 g/100g)
observed in the 1st LED treatment and the lowest
reduction (12.85 g/100g) in the 3rd LED treatment.
These results align with those reported by Scuderi et
al. (2011), who found that the dry matter content of
Duende lettuce decreased from 3.78% on day 4 to
3.59% on day 9 during storage at 4°C. Similarly,
Wagstaff et al. (2007) reported an increase in dry
matter content from 3.2% to 4.3% in Cos lettuce and
from 2.6% to 3.7% in Lolo Rossa lettuce over 10 days
of storage.
The findings from this study indicate that while
the reduction in dry matter content during storage was
statistically significant, the extent of the reduction
was moderate (p < 0.0001). These results highlight
the importance of selecting appropriate lighting and
storage conditions to minimize the degradation of key
quality parameters, such as titratable acidity and dry
matter content, during post-harvest storage.
I-CRAFT 2024 - 4th International Conference on Research of Agricultural and Food Technologies
162
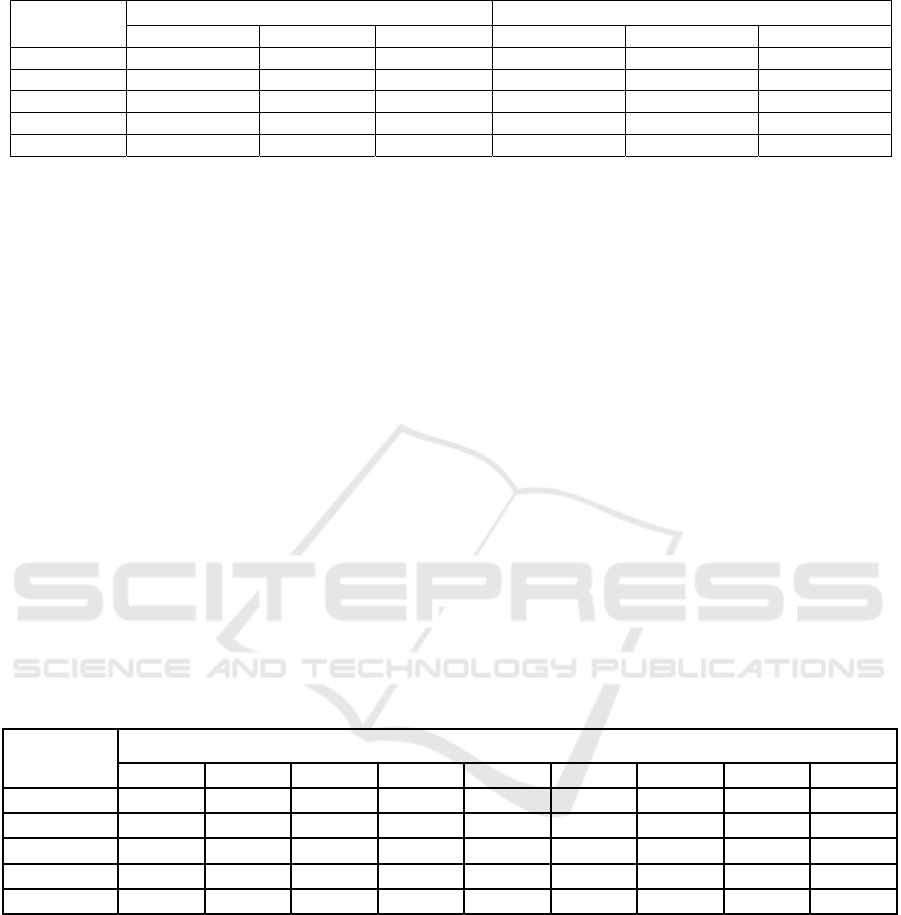
Table 2. Changes in dry matter and titratable acidity in lettuce during storage.
Practice
Acidity Dry Matter
Da
y
1 Da
y
14 Loss % Da
y
1 Da
y
14 Loss %
LED1 1.61a 0.37b 77.01a 4.56a 3.32a 27.20a
LED2 1.35b 0.35b 73.34a 3.94b 3.02b 15.43b
LED3 1.31b 0.50a 62.67b 3.45c 3.35a 12.85c
LDS
0.05
0.068 0.053 4.660 0.043 0.036 0.854
P <0.0001* 0.0009* 0.0007* <0.0001* <0.0001* <0.0001*
3.3 Effects of LED Lighting on Lettuce
Leaf Color Attributes
The statistical analysis revealed that the color
attributes of lettuce leaves, specifically L* (lightness)
and B* (yellow), were not significantly influenced by
the different LED treatments. However, a significant
difference was observed in the A* (red) parameter.
These findings suggest that LED lighting affects the
color characteristics of lettuce, as the lowest color
loss (5.8) was recorded under the 1st LED treatment
(Table 3).
For L* (lightness and brightness), the smallest
loss occurred in the 3rd LED treatment, while the
highest loss was observed in the 1st LED treatment.
Regarding the green color (-a*), the lowest loss was
also found in the 1st LED treatment, whereas the
greatest loss of yellow color (+b*) was recorded
under both the 2nd and 3rd LED treatments.
Kowalczyk et al. (2016) conducted a similar study
comparing different growing media, including rock
wool and cocopeat, within hydroponic systems
(Nutrient Film Technique, NFT), to cultivate two
types of head lettuce and one type of curly lettuce. In
their study, the color parameters of the Aficion
variety (Rijk Zwaan seed company) were measured.
For lettuce grown in rock wool, the values were 55.4
for L*, -14.8 for a*, and 39.1 for b*. In cocopeat, the
values were 63.2 for L*, -15.1 for a*, and 36.0 for b*.
In the NFT system, the measurements were 57.2 for
L*, -13.7 for a*, and 37.6 for b*.
In comparison, the L* values observed in this
study were lower than those reported by Kowalczyk
et al. (2016) in all three environments, indicating
reduced brightness and lightness. However, the a*
values recorded in this study were higher, while the
b* values were similar. Additionally, in Kowalczyk
et al.’s study, the tones of green in the Aficion variety
were assessed using a Minolta SPAD chlorophyll
meter, with values of 19.6 in rock wool, 24.2 in
cocopeat, and 19.6 in NFT.
Table 3. Effects of LED lighting on leaf color characteristics of curly lettuce cultivated in a hydroponic system.
Treatment
L, a, b color measurements
L1 L14 Loss % a1 a14 Loss % b1 b14 Loss %
LED
1
50,7 45,3 15,0 -11,6b -10,9b 5,8b 29,4 24,7 6,4
LED
2
49,9 44,6 10,8 -10,5ab -8,8a 14,8a 26,4 25,9 11,2
LED
3
49,5 41,8 10,5 -9,5a -8,2a 13,9ab 25,6 21,6 14,9
LDS
0,05
n.s n.s n.s
1,022 1,405 8,795
n.s n.s n.s
P 0,8723 0,4324 0,5529 0,0812 0,0026 0,0849 0,2690 0,1454 0,3514
L: Lightness (ranges from 0 [black] to 100 [white], a: Red/Green value (positive values indicate red, and negative values
indicate green), b: Yellow/Blue value (positive values indicate yellow, and negative values indicate blue). There is no
significant difference between means with the same letter in the same column; LSD: the least significant difference.
3.4 Changes in Phenolic, Flavonoid
Compounds, and Nitrate Levels in
Lettuce During Storage
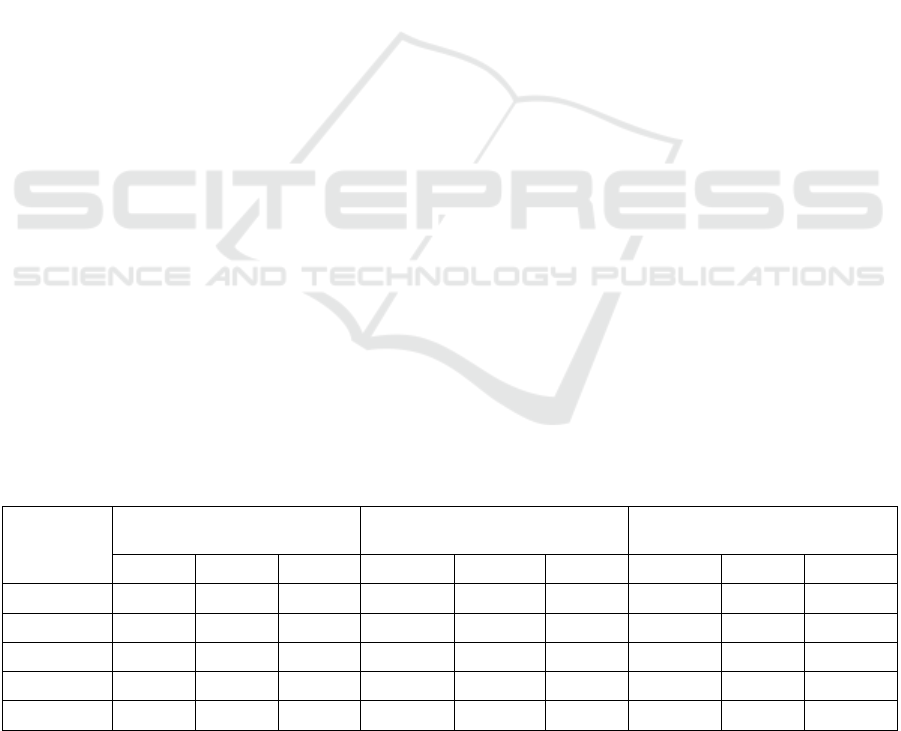
At the beginning of storage, the total phenolic
compound values in lettuce samples ranged from
61.70 to 63.96 μg GAE/g, showing no statistically
significant differences. On the 14th day of storage,
these values ranged from 49.11 to 49.28 μg GAE/g,
with no significant changes observed (Table 4). The
lowest loss of phenolic compounds (20.24 μg GAE/g)
occurred under the 3rd LED treatment, while the
highest loss (23.20 μg GAE/g) was observed under
the 2nd LED treatment. Ke and Saltveit (1988) found
similar results in their study on iceberg lettuce,
attributing changes in phenolic content to
physiological responses related to infections and
tissue damage.
Effects of Different LED Light Spectra on the Shelf Life and Nutritional Quality of Hydroponically Grown Lettuce in a Plant Factory
163
In this study, no significant differences in total
phenolic content were detected in the early days of
storage. It is likely that pre-treatment procedures
caused physiological responses and biochemical
reactions within the lettuce cells, contributing to
variations in phenolic content. Additionally,
enzymatic activity during storage may have
contributed to further reductions in phenolic levels.
Yamaguchi et al. (2003) observed that heat-treated
lettuces maintained stable phenolic content, while
untreated samples showed significant decreases over
time. Similarly, Altunkaya et al. (2009) reported a
decline in total phenolic content in lettuce during
storage.
Flavonoid content also varied based on the LED
treatments. On the first day of storage, the highest
flavonoid content (28.63%) was recorded under the
1st LED treatment, while the lowest (23.28%)
occurred under the 3rd LED treatment. After 14 days,
flavonoid content declined, with the 1st LED
treatment maintaining the highest level (22.24%) and
the 3rd LED treatment showing a reduction to
20.57%. The lowest flavonoid loss (11.67%) was
recorded under the 3rd LED treatment, while the
highest loss (22.61%) also occurred under the same
treatment. These findings highlight the role of
different LED light sources in influencing flavonoid
content, with the 1st LED treatment being particularly
effective in preserving flavonoids during storage. The
slower decline in flavonoid content under the 3rd
LED treatment also underscores the importance of
optimizing post-harvest storage conditions to retain
beneficial phytochemicals.
Nitrate content in the lettuce leaves showed
significant variation at the beginning of storage. The
lowest nitrate level (684 mg/kg) was observed under
the 2nd LED treatment, while the highest (978 mg/kg)
was recorded under the 3rd LED treatment. On the
14th day, nitrate levels again showed a similar
pattern, with the lowest value under the 1st LED
treatment and the highest under the 3rd LED
treatment (509 mg/kg). The most significant nitrate
losses occurred under the 3rd LED treatment (47
mg/kg) and the 1st LED treatment (42 mg/kg), while
the 2nd LED treatment showed the lowest nitrate loss
(26 mg/kg).
According to the Turkish Food Codex (2008), the
maximum allowable nitrate levels for lettuce vary
depending on the growing season and production
method. For lettuce harvested between October 1 and
March 31, the maximum nitrate levels are 4500
mg/kg for indoor-grown lettuce and 4000 mg/kg for
outdoor-grown lettuce. For the period between April
1 and September 30, the limits are 3500 mg/kg for
indoor-grown and 2500 mg/kg for outdoor-grown
lettuce. The nitrate levels recorded in this study
remained well below these thresholds, posing no
health risks to consumers. Zhang et al. (2018)
explored nitrate levels in hydroponically grown
lettuce using two lighting systems (fluorescent and
LED) with varying light intensities (150, 200, 250,
and 300 µmol/m²/s), red-to-blue light ratios (1:1 and
1:2), and lighting durations (12 and 16 hours). They
found that increasing light intensity from 150 to 300
µmol/m²/s and extending lighting duration to 16
hours reduced nitrate levels from 783 mg/kg to 359
mg/kg. In their LED treatments, nitrate levels
decreased as lighting duration increased, with nitrate
concentrations of 667 and 506 mg/kg for the 1:1 ratio
and 810 and 456 mg/kg for the 1:2 ratio.
In summary, the results from this study showed
higher nitrate levels at the beginning of storage,
which declined by the end of the 14-day period. These
findings align with those of Konstantopoulou et al.
(2010), who reported no significant changes in nitrate
levels after 10 days of storage.
Table 4. Changes in phenolic compound levels during the storage of lettuce.
Treatment
Total phenols
(mg GA 100g FW
−1
)
Total flavonoids
(mg RU 100g FW
−1
)
Nitrate
(mg kg FW
−1
)
Day 1 Day14 Loss % Day 1 Day 14 Loss % Day 1 Day 14 Loss %
LED
1
63,13 49,28 21,95 28,63a 22,24a 22,34a 800ab 454 42a
LED
2
63,96 49,11 23,20 27,16b 21,02ab 22,61a 684b 502 26b
LED
3
61,70 49,18 20,24 23,28c 20,57b 11,67b 978a 509 47a
LDS
0,05
n.s n.s n.s 1,41 1,56 3,05 224 n.s 13,34
P 0,1744 0,9077 0,2338 0,0002* 0,0964 0,0002 0,0491* 0,2021 0,0185*
FW: Fresh weigh, GA: Gallic acid, RU: Rutin There is no significant difference between means with the same letter in the
same column; LSD: the least significant difference.
I-CRAFT 2024 - 4th International Conference on Research of Agricultural and Food Technologies
164
3.5 Weight Loss Ratio in Lettuce
During Storage
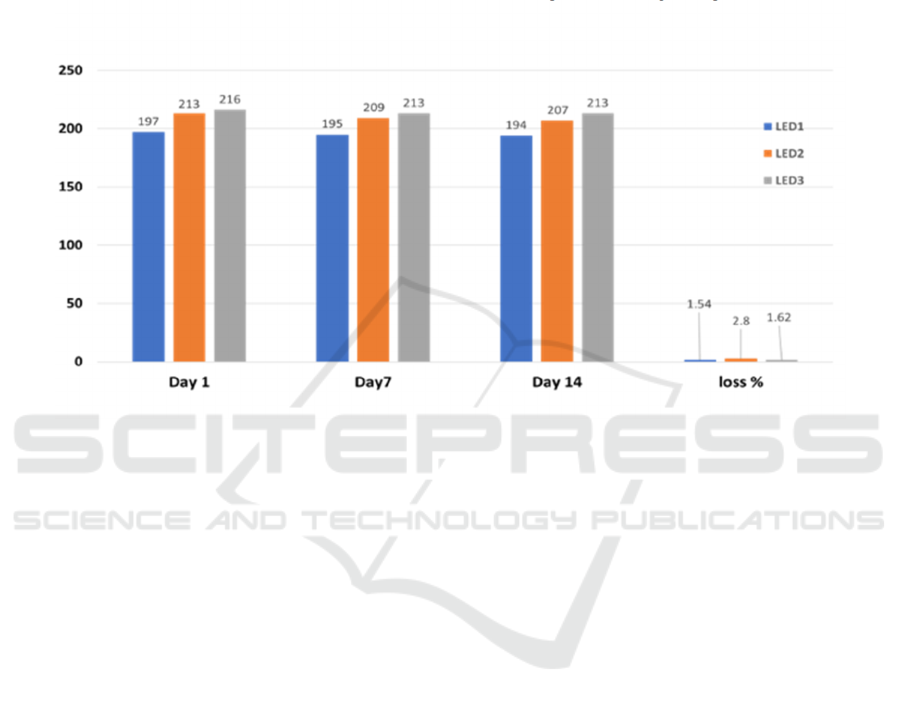
In this study, the initial fresh weight of lettuce
samples ranged from 197 g to 216 g, with no
statistically significant differences observed among
the treatments. By the 14th day of storage, the fresh
weight values ranged between 194 g and 213 g, again
showing no significant differences (Figure 2). The
lowest weight loss (1.54%) was recorded in the
samples stored under the 1st LED light treatment,
while the highest loss (2.8%) occurred in the 2nd
LED light treatment. These results indicate that
different LED light treatments had a limited effect on
fresh weight loss during storage.
Figure 2. The effect of using different leds on the fresh weight loss of lettuce during storage.
From a scientific perspective, these findings
suggest that LED light treatments did not
significantly influence water loss, cellular respiration,
or metabolic rates during storage. This highlights the
idea that LED lights are more effective during the
plant growth phase, rather than post-harvest. In
storage, environmental factors such as temperature
and humidity play a more critical role in preserving
fresh weight. Although LEDs can provide various
wavelengths to enhance plant growth, they do not
appear to significantly impact the metabolic activities
of lettuce during storage.
The absence of statistically significant differences
in fresh weight between the beginning and end of the
14-day storage period supports the conclusion that
LED treatments do not directly affect water loss.
Most of the water content in lettuce is stored
within leaf tissues, and water loss is more closely
related to environmental conditions, such as
temperature and humidity, rather than the type of
lighting used during storage. The fresh weight losses
observed, ranging from 1.54% to 2.8%, further
highlight the limited effect of different LED light
treatments on storage performance. These low
percentages suggest that LED lighting has a
negligible impact on metabolic processes during
storage. Instead, the findings emphasize the
importance of optimizing storage conditions to
maintain product quality.
These results align with the findings of Charles et
al. (2018), who reported that lettuce stored under low
light intensity (or in darkness) experienced less than
5% fresh weight loss, whereas high light intensity led
to weight losses of up to 30%. Their study
underscores the role of light intensity in influencing
moisture loss and spoilage. Low light or dark
conditions can effectively reduce water loss, while
exposure to high light intensity accelerates
dehydration and compromises product quality. In
summary, while LED lighting may offer advantages
for plant growth, its influence on post-harvest weight
loss is minimal. This study demonstrates that
environmental conditions, particularly temperature
and humidity, are the primary determinants of lettuce
quality during storage. Therefore, optimizing storage
practices remains essential for extending the shelf life
and maintaining the quality of lettuce.
Effects of Different LED Light Spectra on the Shelf Life and Nutritional Quality of Hydroponically Grown Lettuce in a Plant Factory
165
3.6 Nutrient Loss in Lettuce Under
Different LED Treatments
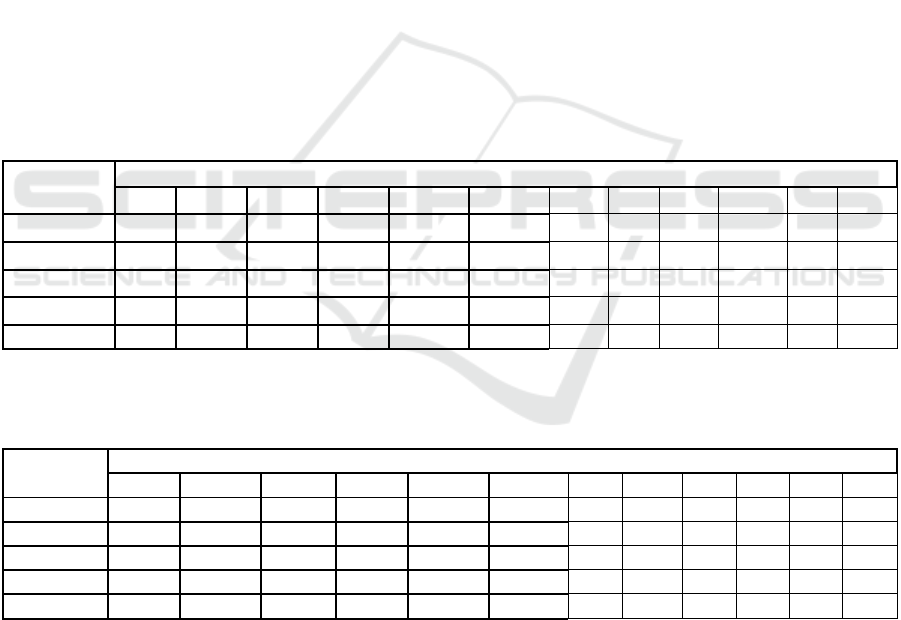
At the end of the 14-day storage period, nitrogen,
calcium, and magnesium levels showed lower loss
rates in the 3rd LED group compared to the 2nd LED
group (Table 5). Specifically, the loss rates for the 3rd
LED group were 1.72% for nitrogen, 12% for
calcium, and 11% for magnesium, whereas the 2nd
LED group exhibited higher losses of 8.22%, 14%,
and 17%, respectively. Excluding potassium from the
analysis, the lowest overall loss rate (2.17%) was
recorded in the 2nd LED group. However, potassium
loss rates varied, with the 1st and 3rd LED groups
showing 13% and 3.51% loss, respectively. In terms
of microelements, plants exposed to the 2nd LED
treatment exhibited the highest nutrient loss rates
after 14 days of storage compared to those under LED
1 and LED 3. However, when copper was excluded,
the nutrient loss associated with the 2nd LED
treatment remained significant. The copper loss rate
for LED 2 was 15.48%, compared to 12.67% in LED
1 and LED 3.
For manganese, the highest loss rate (59.95%)
was observed in the 2nd LED group, while the 1st and
3rd LED groups exhibited lower losses of 47.38% and
40.76%, respectively (Table 6). Similarly, iron losses
were greatest in the 2nd LED group (59%), with LED
1 and LED 3 showing losses of 52% and 51%,
respectively. In terms of zinc, the 2nd LED treatment
resulted in the highest loss rate (9.27%), while LED 1
and LED 3 had slightly lower losses of 7.29% and
7.55%, respectively. These findings demonstrate that
the type of LED treatment significantly influences the
retention of essential nutrients in lettuce during
storage, with the 3rd LED treatment generally
yielding lower loss rates for most nutrients. This
aligns with previous research highlighting the impact
of light quality on the stability of nutrients in post-
harvest produce (Zhang et al., 2018; Charles et al.,
2018). Further research could focus on optimizing
LED wavelengths and intensities to minimize nutrient
loss during storage, thereby improving the quality and
extending the shelf life of lettuce and similar crops.
Table 5. Changes in macroelement levels in lettuce during storage.
Treatment
Macro Elements
N1 N 14 Los% K 1 K 14 Loss % Ca1 Ca 14 loss % Mg 1 Mg 14 Loss %
LED
1
5.42 5.33 1.59b 8.48a 7.38b 13a 3.72 2.63 29a 0.46c 0.46 -0.43b
LED
2
5.60 5.15 8.22a 8.49ab 8.30a 2.17b 3.18 2.72 14b 0.57a 0.47 17a
LED
3
5.06 4.98 1.72b 8.84a 8.53a 3.51b 3.02 2.65 12b 0.54b 0.47 11a
LDS
0.05
n.s n.s
8.14 0.34 0.64 7.34 0.21 0.46 12.54 0.011 0.029 6.05
P
0.518 0.7618 0.0066 0.0776 0.0107* 0.0093* 0.0006* 0.8307 0.0313* <0.0001* 0.5493 0.0009*
There is no significant difference between means with the same letter in the same column; LSD: the least significant
difference.
Table 6. Changes in microelement levels in lettuce during storage.
Treatment
Micro Elements
Cu1 Cu 14 loss % Mn 1 Mn 14 loss % Fe 1 Fe 14 loss % Zn 1 Zn 14 loss %
LED1 25.3 21.33a 15.48 86.33 45.33ab 47.38ab 95a 44a 52 68 62 7.29
LED2 22.6 22.0a 2.84 95.00 38.00b 59.95a 86ab 39b 59 65 60 9.27
LED3 20.6 17.3b 12.67 90.66 53.33a 40.76b 79b 34c 51 63 58 7.55
LDS0.05 n.s 2.732 n.s 9.126 10.09 14.99 12.32 3.36 n.s 6.39 n.s n.s
P 0.4197 0.0120* 0.3679 0.1460 0.0278* 0.0519 0.0617 0.0011* 0.1310 0.2383 0.1985 0.5042
There is no significant difference between means with the same letter in the same column; LSD: the least significant
difference.
4 CONCLUSIONS
This study demonstrated that different LED
treatments had limited effects on parameters such as
total phenolic content, L (lightness) and B
(yellowness) color values, and weight loss during
storage (p > 0.05). However, LED treatments
significantly influenced key quality indicators,
including pH, electrical conductivity, acidity, dry
I-CRAFT 2024 - 4th International Conference on Research of Agricultural and Food Technologies
166

matter content, a (redness) color value, total soluble
solids, flavonoid concentration, and nitrate levels.
These results highlight the selective impact of
LED lighting on the biochemical and physical
properties of lettuce during storage. The findings
underscore the potential of specific LED treatments
in preserving nutritional and sensory qualities,
contributing to extended shelf life. This is
particularly relevant for fresh-cut and pre-packaged
lettuce products, which are increasingly favored by
urban consumers seeking convenience. In conclusion,
this study provides valuable insights into how LED
lighting can be leveraged to improve post-harvest
management strategies for lettuce. Future research
should focus on optimizing LED wavelengths and
integrating them with other preservation techniques
to maximize both quality and shelf life, meeting the
growing market demand for fresh, ready-to-eat
produce.
ACKNOWLEDGMENTS
We gratefully acknowledge the support of the
TAGEM/21/AR-GE/03 project, which enabled the
cultivation of lettuce in the plant factory system.
REFERENCES
Allende, A., Aguayo, E., & Artés, F. (2004). Microbial and
sensory quality of commercial fresh processed red
lettuce throughout the production chain and shelf life.
International Journal of Food Microbiology.
Altunkaya, A., Becker, E. M., Gökmen, V., & Skibsted, L.
H. (2009). Antioxidant activity of lettuce extract
(Lactuca sativa) and synergism with added phenolic
antioxidants. Food Chemistry, 115(1), 163–168.
Balik, S., Elgudayem, F., Dasgan, H. Y., & others. (2025).
Nutritional quality profiles of six microgreens.
Scientific Reports, 15, 6213.
https://doi.org/10.1038/s41598-025-85860-z
Charles, F., Nilprapruck, P., Roux, D., & Sallanon, H.
(2018). Visible light as a new tool to maintain fresh-cut
lettuce post-harvest quality. Postharvest Biology and
Technology, 135, 51–56.
Dasgan, H. Y., Aldiyab, A., Elgudayem, F., & others.
(2022). Effect of biofertilizers on leaf yield, nitrate
amount, mineral content and antioxidants of basil
(Ocimum basilicum L.) in a floating culture. Scientific
Reports, 12, 20917. https://doi.org/10.1038/s41598-
022-24799-x
Dasgan, H. Y., Kacmaz, S., Arpaci, B. B., İkiz, B., &
Gruda, N. S. (2023a). Biofertilizers improve the leaf
quality of hydroponically grown baby spinach
(Spinacia oleracea L.). Agronomy, 13(2), 575.
https://doi.org/10.3390/agronomy13020575
Dasgan, H. Y., Yilmaz, D., Zikaria, K., Ikiz, B., Gruda, N.
S. (2023b). Enhancing the yield, quality and antioxidant
content of lettuce through innovative and eco-friendly
biofertilizer practices in
hydroponics. Horticulturae, 9(12), 1274.
Degl’Innocenti, E., Guidi, L., Pardossi, A., & Tognoni, F.
(2005). Biochemical study of leaf browning in
minimally processed leaves of lettuce (Lactuca sativa
L. var. Acephala). Journal of Agricultural and Food
Chemistry, 53(26), 9980–9984.
Degl’Innocenti, E., Pardossi, A., Tognoni, F., & Guidi, L.
(2007). Physiological basis of sensitivity to enzymatic
browning in lettuce, escarole, and rocket salad when
stored as fresh-cut products. Food Chemistry, 104(1),
209–215.
Dharni, K., & Sharma, R. K. (2015). Supply chain
management in the food processing sector: Experience
from India. International Journal of Logistics Systems
and Management, 21(1), 115–132.
DuPont, M. S., Mondin, Z., Williamson, G., & Price, K. R.
(2000). Effect of variety, processing, and storage on the
flavonoid glycoside content and composition of lettuce
and endive. Journal of Agricultural and Food
Chemistry, 48(9), 3957–3964.
Fan, X., & Mattheis, J. P. (2000). Reduction of ethylene-
induced physiological disorders of carrots and iceberg
lettuce by 1-methylcyclopropene. Postharvest Biology
and Technology, 35(2), 151–160.
Fanourakis, D., Bouranis, D., Giday, H., Carvalho, D. R.,
Nejad, A. R., & Ottosen, C. O. (2016). Improving
stomatal functioning at elevated growth air humidity: A
review. Journal of Plant Physiology, 207, 51–60.
Gao, E., Cui, Q., Jing, H., Zhang, Z., & Zhang, X. (2021).
A review of application status and replacement progress
of refrigerants in the Chinese cold chain industry.
International Journal of Refrigeration, 128, 104–117.
Hassenberg, K., & Idler, C. (2005). Influence of washing
method on the quality of prepacked iceberg lettuce.
Agricultural Engineering International: The CIGR
Ejournal, Manuscript FP 05 003, Vol VII, November.
Hogewoning, S. W., Wientjes, E., Douwstra, P.,
Trouwborst, G., Van Ieperen, W., Croce, R., &
Harbinson, J. (2012). Photosynthetic quantum yield
dynamics: From photosystems to leaves. The Plant
Cell, 24(5), 1921–1935.
Iakimova, E. T., & Woltering, E. J. (2015). Nitric oxide
prevents wound-induced browning and delays
senescence through inhibition of hydrogen peroxide
accumulation in fresh-cut lettuce. Innovative Food
Science & Emerging Technologies, 30, 157–169.
İkiz, B., Dasgan, H. Y., & Gruda, N. S. (2024). Utilizing
the power of plant growth promoting rhizobacteria on
reducing mineral fertilizer, improved yield, and
nutritional quality of Batavia lettuce in a floating
culture. Scientific Reports, 14, 1616.
https://doi.org/10.1038/s41598-024-51818-w
Ke, D., & Saltveit, M. E. (1988). Plant hormone interaction
and phenolic metabolism in the regulation of russet
Effects of Different LED Light Spectra on the Shelf Life and Nutritional Quality of Hydroponically Grown Lettuce in a Plant Factory
167

spotting in iceberg lettuce. Plant Physiology, 88(4),
1136–1140.
Keskin, B., Akhoundnejad, Y., Dasgan, H. Y., & Gruda, N.
S. (2025). Fulvic acid, amino acids, and vermicompost
enhanced yield and improved nutrient profile of soilless
iceberg lettuce. Plants, 14(4), 609.
https://doi.org/10.3390/plants14040609
King, A. D., Jr., Magnuson, J. A., Török, T., & Goodman,
N. (1991). Microbial flora and storage quality of
partially processed lettuce. Journal of Food Science,
56(2), 459–462.
Konstantopoulou, E., Kapotis, G., Salachas, G.,
Petropoulos, S. A., Karapanos, I. C., & Passam, H. C.
(2010). Nutritional quality of greenhouse lettuce at
harvest and after storage in relation to N application and
cultivation season. Scientia Horticulturae, 125(2), 93–
101.
Kowalczyk, K., Mirgos, M., Bączek, K., Niedzińska, M., &
Gajewski, M. (2016). Effect of different growing media
in hydroponic culture on the yield and biological
quality of lettuce (Lactuca sativa var. capitata). Acta
Horticulturae, 1142, 105–110.
Likar, K., & Jevšnik, M. (2006). Cold chain maintenance in
food trade. Food Control, 17(2), 108–113.
Martinez, I., Ares, G., & Lema, P. (2008). Influence of cut
and packaging film on sensory quality of fresh-cut
butterhead lettuce. Journal of Food Quality, 31(1), 48–
66.
Mercier, S., Villeneuve, S., Mondor, M., & Uysal, I. (2017).
Time–temperature management along the food cold
chain: A review of recent developments.
Comprehensive Reviews in Food Science and Food
Safety, 16(4), 647–667.
Özgen, M., & Scheerens, J. C. (2006). Bazı kırmızı ve siyah
ahududu çeşitlerinin antioksidan kapasitelerinin
modifiye edilmiş TEAC yöntemi ile saptanması ve
antikanser özelliklerinin tartışılması. II. Üzümsü
Meyveler Sempozyumu, Tokat.
Özgen, M., & Tokbaş, H. (2007). The effect of illumination
on the antioxidant capacity of fruit tissues in Amasya
and Fuji apples. Journal of the Faculty of Agriculture,
Giresun University, 24(2), 1–5.
Saglam, S. (2007). The effect of jam production processes
on phenolics and antioxidant capacity in anthocyanin-
rich mulberry, cherry, and cornelian cherry fruits
(Master’s thesis, Selçuk University, Institute of
Science, Department of Food Engineering, Konya).
Scuderi, D., Restuccia, C., Chisari, M., Barbagallo, R. N.,
Caggia, C., & Giuffrida, F. (2011). Salinity of nutrient
solution influences the shelf-life of fresh-cut lettuce
grown in floating system. Postharvest Biology and
Technology, 59(2), 132–137.
Tay, S. L., & Perera, C. O. (2004). Effect of 1-
methylcyclopropene treatment and edible coatings on
the quality of minimally processed lettuce. Journal of
Food Science, 69(2), fct131–fct135.
Tian, W., Lv, Y., Cao, J., & Jiang, W. (2014). Retention of
iceberg lettuce quality by low-temperature storage and
postharvest application of 1-methylcyclopropene or
gibberellic acid. Journal of Food Science and
Technology, 51, 943–949.
Tsankilidis, G., Delis, C., Nikoloudakis, N., Katinakis, P.,
& Aivalakis, G. (2014). Low-temperature storage
affects the ascorbic acid metabolism of cherry tomato
fruits. Plant Physiology and Biochemistry, 84, 149–
157.
Wagstaff, C., Clarkson, G. J. J., Rothwell, S. D., Page, A.,
Taylor, G., & Dixon, M. S. (2007). Characterization of
cell death in bagged baby salad leaves. Postharvest
Biology and Technology, 46, 150–159.
Zhang, W., & Jiang, W. (2019). UV treatment improved the
quality of postharvest fruits and vegetables by inducing
resistance. Trends in Food Science & Technology, 92,
71–80.
Zhang, Y., Kaiser, E., Zhang, Y., Zou, J., Bian, Z., Yang,
Q., & Li, T. (2020). UVA radiation promotes tomato
growth through morphological adaptation leading to
increased light interception. Environmental and
Experimental Botany, 176, 104073.
I-CRAFT 2024 - 4th International Conference on Research of Agricultural and Food Technologies
168
