Biochemical Properties of Plant Polyphenols
Muhabbat Honkeldieva
a
, Komil Bukhorov
b
, Zokir Markaev
c
, Yusuf Yakubov
d
and Mekhriniso Sayfiyeva
e
Tashkent State Agrarian University, 100140, University str. 2, Tashkent, Uzbekistan
Keywords: Plant Polyphenols, Gossypol Clathrates, Thermal Analysis.
Abstract: Secondary metabolites are small organic molecules originated from primary metabolites during the embolism
of plant. Polyphenols are secondary metabolites that are common in plant kingdom. Gossypol is a specific
secondary metabolite in Gossypium species. The natural compound gossypol forms stable clathrates with
vapor of diethyl ether solvent. Experiments carried out at room temperature, +40⁰C and -5⁰C. This work
describes investigation of gossypol clathrates by X-Ray powder diffraction and TG-DSC analysis
.
1 INTRODUCTION
Polyphenols are natural compounds that are part of
daily consumed fruits, vegetables, cereals. They are
one of the secondary metabolites in plants, which
usually protect the plant from ultraviolet radiation and
various pathogenic diseases, and are involved in
increasing the plant's immunity (Claudine et al.,
2004). In addition to fruits, vegetables, and grain
products, secondary metabolites are formed in large
quantities in the cotton plant, which is considered a
technical crop. The cotton plant grown in Uzbekistan
is an annual plant belonging to the Malvaceae family,
Gossypium genus and Gossypium hirsutum species.
During the ontogenesis of the cotton plant, secondary
metabolites are formed in various organs, of which
the substance belonging to the polyphenol class is
gossypol. The cotton plant contains sesquiterpene-
forming gene GhTPS1 and monoterpene-forming
gene GhTPS2, which play an important role in the
biosynthesis of gossypol. The substance gossypol is
synthesized in the glands of the root, stem, leaf and
seed of the cotton plant, it exhibits the properties of a
phytoalexin and protects against the attack of insects
as well as external pathogenic effects (Tianlun et al.,
2020).
a
https://orcid.org/0009-0009-6764-4437
b
https://orcid.org/0009-0006-4409-0513
c
https://orcid.org/0009-0003-4053-2853
d
https://orcid.org/0000-0001-8729-0052
e
https://orcid.org/0009-0005-5423-654X
Gossypol - C
30
H
30
O
8
(1,1'6,6',7,7'-hexahydroxy-
5,5'-di-isopropyl-3,3'-dimethyl-(2,2'-binaphthalene)-
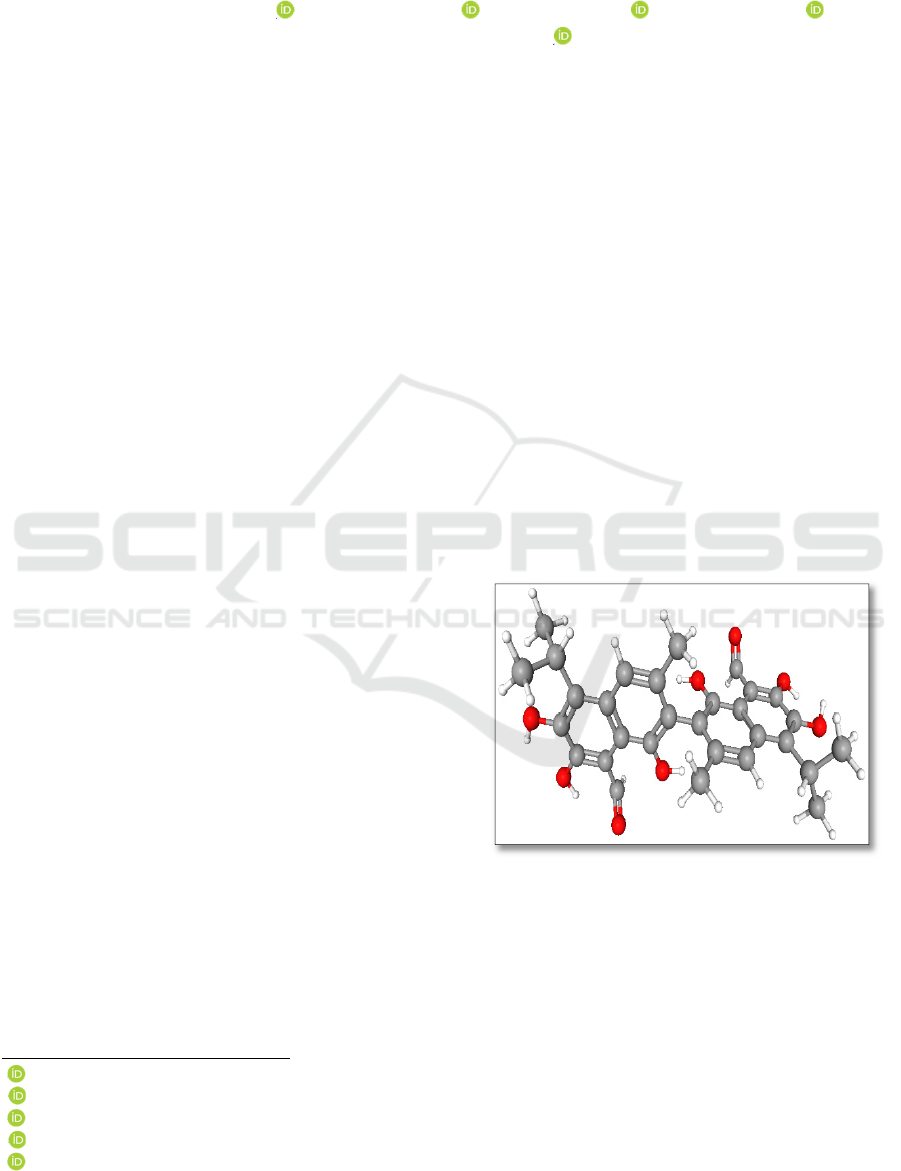
8,8'- dicarboxyaldehyde) (Fig. 1), a yellow pigment,
is an organic substance containing two naphthyl
groups, six hydroxyl groups, two aldehyde groups,
two methyl and two isopropyl groups.
Figure 1: Structural formula of gossypol.
Aldehyde and hydroxyl groups in gossypol
increase its biochemical activity. In the gossypol
molecule, two "+" and "-" gossypol form mutual
enantiomers, and this situation causes its biochemical
properties to differ. According to the functional
Honkeldieva, M., Bukhorov, K., Markaev, Z., Yakubov, Y. and Sayfiyeva, M.
Biochemical Properties of Plant Polyphenols.
DOI: 10.5220/0014223400004738
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 4th International Conference on Research of Agricultural and Food Technologies (I-CRAFT 2024), pages 137-143
ISBN: 978-989-758-773-3; ISSN: 3051-7710
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
137

groups in the gossypol molecule, various chemical
compounds are formed, in which the properties of
gossypol are formed by bonds such as covalent bonds,
ionic bonds, and hydrogen bonds. One of the unique
properties of the gossypol molecule is that it creates a
Van der Waals voltage due to the polarization of
electron shells as a result of the interaction of dipole
moments. Although these stresses are weak, they are
important in the formation of various combinations of
gossypol. In addition, there are several polymorphs of
gossypol, and in the crystal structure of these
polymorphs, gossypol molecules form an interlayer
and a tubular channel. These polymorphs are well
soluble in organic solvents with polar lower
molecular weight and form the corresponding
clathrates as a result of recrystallization (Honkeldieva
et al., 2023), (Honkeldieva et al., 2015).
In recent years, the production of various
medicinal preparations based on gossypol substance
has been launched. It is important that the chemical
composition of the gossypol substance does not
change during the storage period of the medicinal
product, and that additional intermediate products are
not formed under the influence of external factors.
The biochemical activity of gossypol polymorphs is
determined by its content of many functional groups
and the richness of hydrophilic and hydrophobic
bonds. This article describes the study of the
formation of appropriate clathrates by gossypol under
the influence of vapors of organic polar solvents.
Based on the results of the experiments, the stability
of the properties of gossypol and its changes under
the influence of heat were studied by the TG-DSC
method. The transformation of gossypol polymorph
substance in the initial crystal phases and formation
of corresponding clathrates was proved by X-Ray
Powder Diffraction X-ray phase method
(Honkeldieva et al., 2015).
2 MATERIALS AND METHODS
X-ray Analysis. The X-ray powder diffractions were
obtained using a Shimadzu X-ray diffractometer,
model LabX XRD-6100, using CuKa radiation
(λ=0.154 nm), current of 40 mA and operating
voltage of 40 kV. The instrument features a vertical
goniometer, and a linear scintillation detector with a
graphite diffraction monochromator. Aluminium
disks were used as sample supports, and the samples
were swept with incidence angles from 4 to 35, at 0.02
increments, 2 s per increment.
Thermal analysis. The thermal analysis system
was employed to acquire the simultaneous
Thermogravimetry and Differential Scanning
Calorimetry – TG-DSC curves (NETZSCH STA 409,
Germany). Dry air and nitrogen were used
individually as furnace atmosphere purge gases, with
a flow rate of 50 mL min-1 in both cases. The
temperature program consisted of heating the samples
from 25 to 350℃, at a heating rate of 10℃ min-1.
Samples weighing 5 mg samples were placed in
aluminum crucibles with a perforated cover. The
application possibilities comprised the whole
spectrum of TG and DSC analysis.
3 RESULTS AND DISCUSSION
A solid:gas phase was used to produce gossypol
clathrates by absorption method. In the case of P3
polymorph of gossypol, which has polycrystalline
properties in the solid phase (
Zhao et al., 2020), organic
polar solvent diethyl ether was chosen for the gas
phase. Diethyl ether is an aliphatic ether with the
chemical formula CH
3
-CH
2
-O-CH
2
-CH
3
. The boiling
temperature of diethyl ether is T=34.15℃, it is a
colorless liquid with a quick volatile and
characteristic smell. In medicine, diethyl ether is used
as a general pain reliever. A person who has worked
a lot with diethyl ether may develop an asthetic
tendency, as a result of which the disease "Ether
zombie" is observed, and this disease leads to a
decrease in memory and slowing down of physical
activity.
The conducted experiments are based on the
absorption of volatile diethyl ether vapors on the
surface of polycrystalline gossypol polymorph, as a
result of which the formation of the corresponding
clathrate was studied. A chemical hermetic vessel
was selected for the experiment, and a filter paper was
cut into a box shape, placed in a four-layer case, and
2 ml of diethyl ether solvent was poured into it. As a
result, a chamber saturated with diethyl ether vapor
was created and placed in this chamber after weighing
and determining the mass of two bulk scales. The first
sample was considered as a control and was placed in
a blank state without the gossypol polymorph 35 mg
(6,7∙10
-5
mol) of the P3 polymorph of gossypol was
taken into the second batch. Then, the hermetically
sealed container was closed and left for 24 hours to
allow absorption in the solid:gas phase. After one
day, the first and second samples were repeatedly
pulled, and it was observed that the mass of the empty
sample did not change, while the mass of the sample
containing the P3 polymorph of gossypol increased.
The ongoing experiment was continued until the mass
of the gossypol polymorph contained byuks reached
I-CRAFT 2024 - 4th International Conference on Research of Agricultural and Food Technologies
138
a constant. The corresponding experiments were
carried out at room temperature, +40℃ and -5℃. In
carrying out these experiments, we used an electronic
analytical balance of the Talent TE-64 Sartorius
model, made in Germany, Weighing Paper, size: 3x3
inches, and Filter papers, size: 110 mm Dia, made in
England for weighing substances.
A) X-ray Analysis. The formation of gossypol
clathrates using the absorption method in the
solid:gas phase was studied using X-ray phase
analysis. According to the results of X-ray phase
analysis, it was found that diethyl ether vapors were
completely absorbed by P3 polymorph of gossypol at
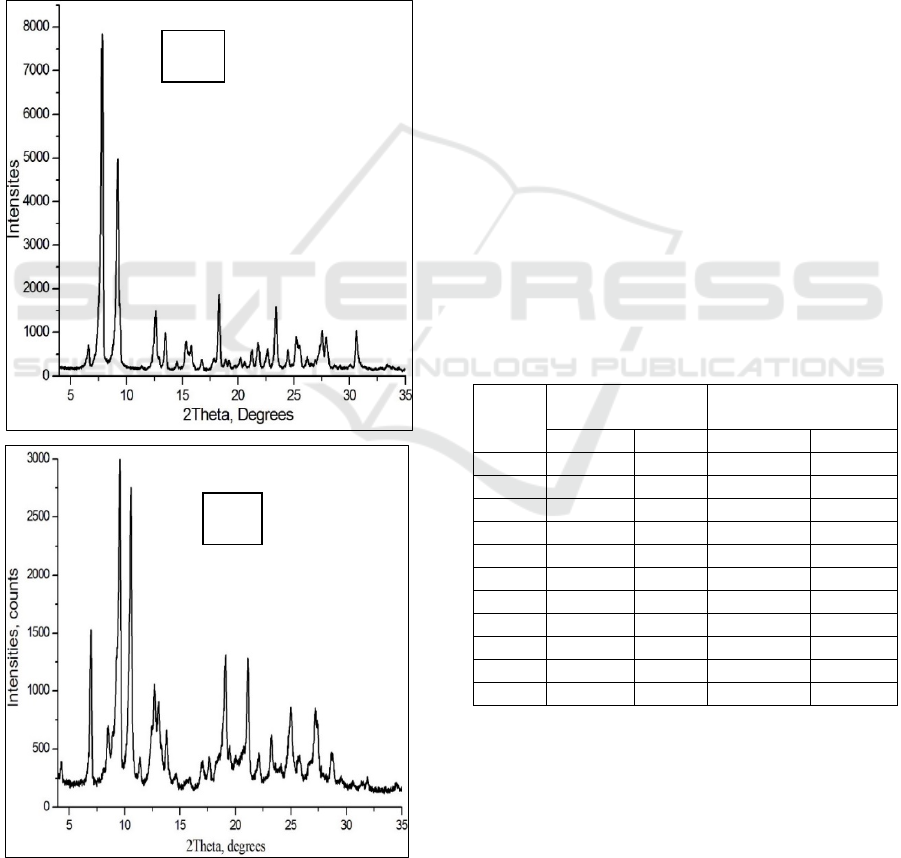
room temperature (Fig. 2).
Figure 2: A) X-Ray Powder diffraction pattern of
gossypol’s polymorph Р3 in room temperature;
B) X-Ray Powder Diffraction pattern formation of clathrate
gossypol:diethyl ether in room temperature.
It can be seen from the figure that A) X-Ray
Powder Diffraction pattern is an analysis of the P3
polymorph of gossypol in pure form, where
2Theta=7.5⁰; 9.0⁰; 12.5⁰; 13.0⁰; 18.0⁰; 23.0⁰; 27.5⁰; X-
rays falling at an angle of 31.0⁰ respectively I
0
=7997;
5000; 1500; 1040; 2001; 1700, 1100, 1200 showed
intensity.
B) X-Ray Powder Diffraction pattern is the
analysis of gossypol:diethyl ether clathrate at room
temperature, where 2Theta=6.9⁰; 9.0⁰; 10.5⁰; 13.0⁰;
13.2⁰; 19.0⁰; 21.0⁰; 23.0⁰; 25.0⁰; 27.5⁰; X-rays falling
at an angle of 28.0⁰ respectively I
0
=1500; 3000; 2750;
1000; 900; 1300; 1301; 600; 770; 800; 600 shows an
intensity of 2Theta=13.0⁰; Formation of paired peaks
was observed at 13.2⁰. This indicates the formation of
new phases in the initial P3 polymorph, and as a
result, it was determined that gossypol:diethyl ether
clathrate was formed.
The pure P3 polymorph is a polycrystalline
substance, the highest peak of its intensity peak was
equal to I
0
=8000. In the formation of gossypol:diethyl
ether clathrate, the peak intensity is equal to I
0
=3000,
and it was observed that the polycrystalline nature of
gossypol:diethyl ether clathrate decreased by 2.67
times compared to the polycrystalline nature of
polymorph P3 (Table 1).
Table 1: X-ray analysis of pure gossypol P3 polymorph and
gossypol:diethyl ether clathrate at room temperature.
Peaks P3 pol
y
morph
Goss
y
pol:dieth
y
l
ether clathrate
2Theta I/I
0
2Theta I/I
0
1 7,5⁰ 7997 6,9⁰ 1500
29,0⁰5000 9,0⁰ 3000
312,5⁰1500 10,5⁰ 2750
413,0⁰1040 13,0⁰ 1000
518,0⁰2001 13,2⁰ 900
623,0⁰1700 19,0⁰ 1300
7 27,5⁰ 1100 21,0⁰ 1301
8 31,0⁰ 1200 23,0⁰ 600
9 - - 25,0⁰ 770
10 - - 27,5⁰ 800
11 - - 28,0⁰ 600
The results of the experiment conducted at a
temperature of +40℃ also showed that there was an
absorption process between P3 polymorph of
gossypol and diethyl ether vapors (Fig. 3).
Analysis of gossypol:diethyl ether clathrate at
+40℃ X-rays incident at an angle of 2Theta=6,9⁰;
8,0⁰; 9,0⁰; 11,0⁰; 11,5⁰; 12,5⁰; 13,0⁰; 14,0⁰; 17,5⁰;
21,0⁰; 22,3⁰; 27.0⁰ respectively it was observed that
A
B
Biochemical Properties of Plant Polyphenols
139
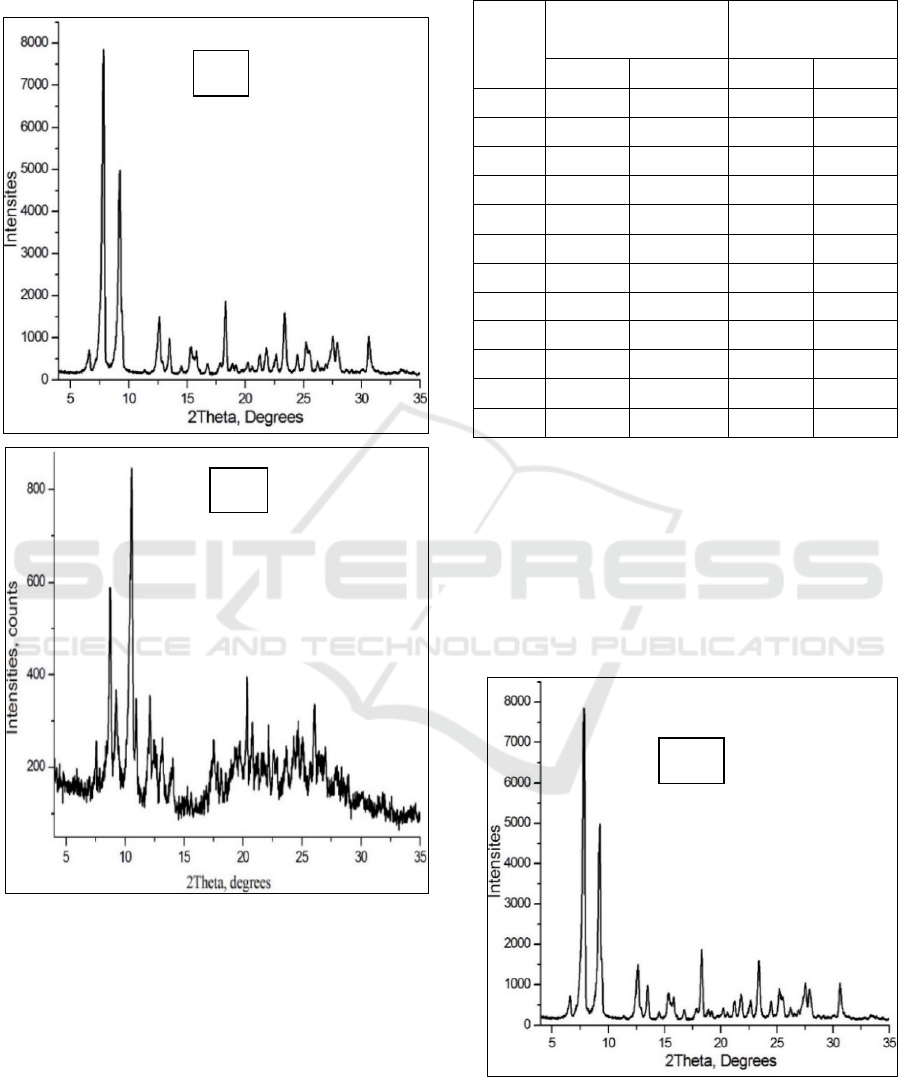
I
0
=250; 600; 350; 810; 330; 370; 280; 210; 220; 400;
300; 350 exhibits intensity.
Figure 3: A) X-Ray Powder diffraction of pure gossypol’s
polymorph Р3 in +40℃; B) X-Ray Powder Diffraction
pattern formation of clathrate gossypol:diethyl ether in
+40℃.
The pure P3 polymorph is a polycrystalline
substance, the highest peak of its intensity peak was
equal to I
0
=8000. In the formation of gossypol:diethyl
ether clathrate at a temperature of +40℃, the peak
intensity is equal to I
0
=3000, and it was observed that
the polycrystalline nature of gossypol:diethyl ether
clathrate decreased by 10 times compared to the
polycrystalline nature of polymorph P3 (Table 2).
Table 2: X-ray analysis of pure gossypol P3 polymorph and
gossypol:diethyl ether clathrate at +40℃.
Peaks P3 pol
y
morph Goss
y
pol:dieth
y
l
ether clathrate
2Theta I
0
2Theta I
0
1 7,5⁰ 7997 6,9⁰ 250
29,0⁰5000 8,0⁰ 600
312,5⁰1500 9,0⁰ 350
413,0⁰1040 11,0⁰ 810
518,0⁰2001 11,5⁰ 330
623,0⁰1700 12,5⁰ 370
7 27,5⁰ 1100 13,0⁰ 280
8 31,0⁰ 1200 14,0⁰ 210
9 - - 17,5⁰ 220
10 - - 21,0⁰ 400
11 - - 22,3⁰ 300
12 - - 27,0⁰ 350
This table 2 indicates that polymorph P3 lost its
crystallinity under the influence of diethyl ether
vapors and changed to an amorphous state.
Analysis of gossypol:diethyl ether clathrate at -
5℃ X-rays incident at an angle of 2Theta=5,0⁰; 13,0⁰;
14,2⁰; 17,5⁰; 21,0⁰; 21,7⁰; 22,0⁰; 22,5⁰; 24,0⁰; 25,3⁰;
28,0⁰ respectively it was observed that I
0
=1250; 1400;
1200; 410; 400; 500; 650; 410; 420; 490; 495 exhibits
intensity (Fig. 4).
Figure 4: A) X-Ray Powder diffraction of pure gossypol’s
polymorph Р3 in -5℃.
A
B
A
I-CRAFT 2024 - 4th International Conference on Research of Agricultural and Food Technologies
140
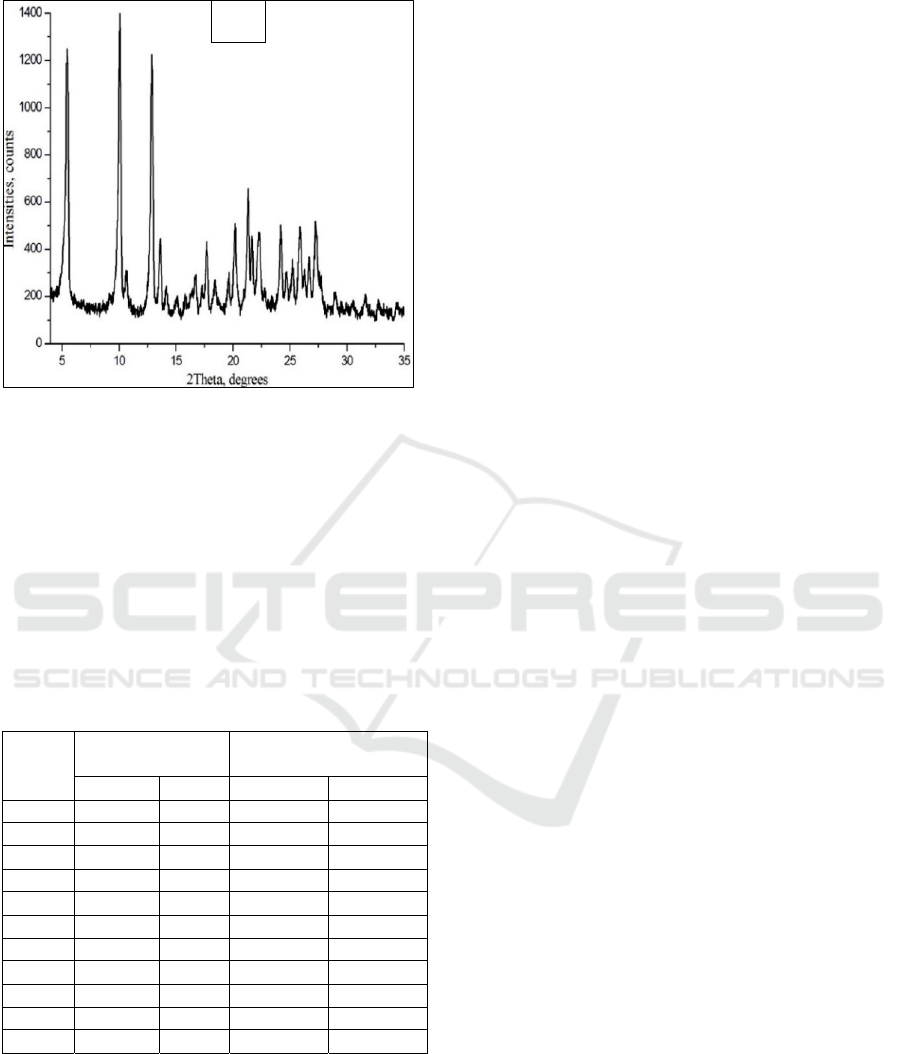
Figure 4: B) X-Ray Powder Diffraction pattern formation
of clathrate gossypol:diethyl ether in -5℃.
The pure P3 polymorph is a polycrystalline
substance, the highest peak of its intensity peak was
equal to I
0
=8000. In the formation of gossypol:diethyl
ether clathrate, the peak intensity is equal to I
0
=1400,
and it was observed that the polycrystalline nature of
gossypol:diethyl ether clathrate decreased by 5,72
times compared to the polycrystalline nature of
polymorph P3 (Table 3).
Table 3: X-ray analysis of pure gossypol P3 polymorph and
gossypol:diethyl ether clathrate at -5℃.
Peaks P3 pol
y
morph
Goss
y
pol:dieth
y
l
ether clathrate
2Theta I
0
2Theta I
0
1 7,5⁰ 7997 5,0⁰ 1250
2 9,0⁰ 5000 13,0⁰ 1400
3 12,5⁰ 1500 14,2⁰ 1200
4 13,0⁰ 1040 17,5⁰ 410
5 18,0⁰ 2001 21,0⁰ 400
6 23,0⁰ 1700 21,7⁰ 500
7 27,5⁰ 1100 22,0⁰ 650
8 31,0⁰ 1200 22,5⁰ 410
9 - - 24,0⁰ 420
10 - - 25,3⁰ 490
11 - - 28,0⁰ 495
Thermal analysis. This study uses combined
thermogravimetric analysis (TG)/differential thermal
calorimetry (DSC). The use of thermal techniques
such as thermogravimetry (TG) and differential
scanning calorimetry (DSC) has been proposed as a
reproducible, informative, rapid, low-cost and small-
sample consuming method to characterize the
complete quality continuum of organic materials.
TG is a thermogravimetric analysis, in which the
phenomenon of degradation in the studied substance
under the influence of temperature, the formation of
polymorphic modifications as a result of phase
changes, the processes of desolvation and
decomposition, as well as the thermal stability of the
substance, as well as the composition of the
substance, are determined.
TG is a method of thermal analysis in which
changes in physical and chemical properties of
materials are measured as a function of increasing
temperature (with constant heating rate), or as a
function of time (with constant temperature and/or
constant mass loss). TG can provide information
about physical phenomena, such as:
• second-order phase transitions (including
vaporization, sublimation, absorption, adsorption,
and desorption);
• chemisorption;
• desolation (especially dehydration);
• decomposition;
• solid-gas reactions.
DSC is a thermo-analytical technique in which the
difference in the amount of heat required to increase
the temperature of a sample and reference is
measured as a function of temperature. Both the
sample and reference are maintained at nearly the
same temperature throughout the experiment.
Generally, the temperature program for a DSC
analysis is designed such that the sample holder
temperature increases linearly as a function of time.
DSC can provide information about physical
phenomena, such as:
• Melting Point/Melting Range;
• Heat Capacity;
• Crystallization;
• Thermal Stability;
• Decomposition Temperature;
• Purity.
In the TG-DSC method, the studied substance is
compared with the control substance. For this
analysis, chemically pure gossypol P3 polymorph
was selected as a control. The result of thermal
analysis of Gossypol P3 polymorph is shown in
Figure 5. TG-DSC curve was performed in the
temperature range of 25-350℃. According to the TG-
B
Biochemical Properties of Plant Polyphenols
141
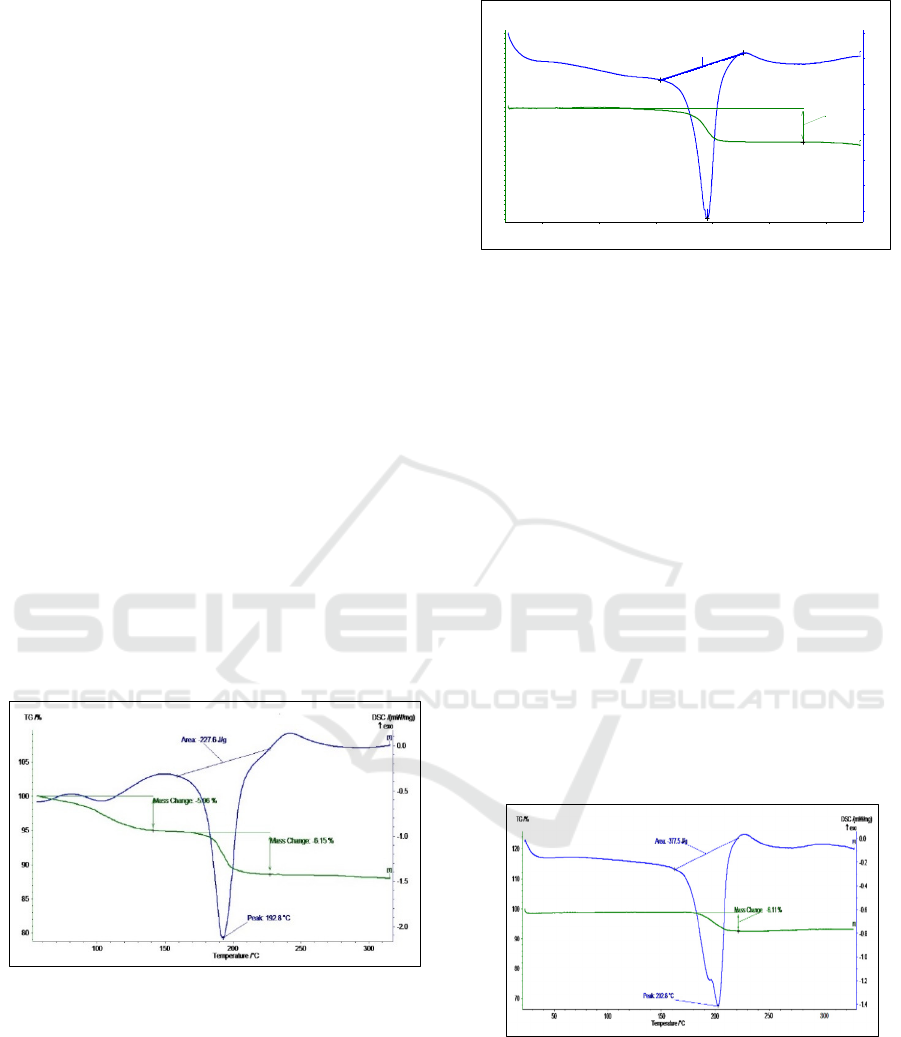
thermogravimetric results, it was observed that the
mass reduction of gossypol P3 polymorph proceeds
in 2 stages. The 1st stage of mass reduction was
observed in the temperature range of 100-140℃, and
the mass difference of P3 polymorph was reduced
from 100% to 94.94%, resulting in a mass difference
of 5.06%. The 2nd stage of mass reduction started
slowly at 175℃ and rapidly decreased in the
temperature range of 192.8-200℃. No mass change
was observed starting at 230℃. In the 2nd stage, the
mass difference was 6.15%.
According to DSC-differential scanning
calorimetry analysis, a two-step "phase transition"
process was observed when gossypol P3 polymorph
was exposed to heat. The first "phase transition"
process corresponds to 100-120℃, and in this
temperature range, one molecule of water was
released from the composition of the gossypol P3
polymorph. The second "phase transition" process
corresponds to 175-192.8℃, and in this temperature
range, another molecule of water is separated. The
strongest lower peak of the endothermic peak
corresponded to 192.8℃, where it was observed that
the P3 polymorph of gossypol was converted to
dianhydrogossypol, and the amount of heat required
for the endothermic reaction was 227.6 J/g. In the
DSC diagram, there was no peak at all from 230℃,
which indicated the complete decomposition of the
dianhydrogossypol molecule and the beginning of the
carbonization process.
Figure 5: TG-DSC curve of gossypol P3 polymorph.
The result of thermal analysis of gossypol:diethyl
ether clathrate produced at room temperature is
shown in Figure 6. The mass reduction of
gossypol:diethyl ether clathrate was observed to
proceed in one step.
Figure 6: TG-DSC curve formation of gossypol:diethyl
ether clathrate in room temperature.
Mass reduction was observed in the temperature
range of 155-200℃, and the mass difference was
6.91%. In gossypol:diethyl ether clathrate, the "phase
transition" process was not observed until 155℃, due
to which the hydroxyl groups of gossypol P3
polymorph form mutual hydrogen bonds with diethyl
ether. As a result, water and diethyl ether contained
in gossypol:diethyl ether clathrate were
simultaneously desolvated at 195.3℃, and
dianhydrogossypol molecule was observed to be
formed. The amount of heat required for the
endothermic reaction was 299.5 J/g. The
dianhydrogossypol molecule was completely
decomposed and the beginning of the carbonization
process coincided with the temperature of 280℃.
The result of thermal analysis of gossypol:diethyl
ether clathrate produced at +40℃ temperature is
shown in Figure 7. The mass reduction of
gossypol:diethyl ether clathrate was observed to
proceed in one step.
Figure 7: TG-DSC curve formation of gossypol:diethyl
ether clathrate in +40℃.
Mass reduction was observed in the temperature
range of 185-210℃, and the mass difference was
6.11%. In gossypol:diethyl ether clathrate, the "phase
transition" process was not observed up to 165℃.
This clathrate was desolvated with diethyl ether
solvent at 195.3℃ and water at 202.8℃, resulting in
50 100 150 200 250 300
Temperature /°C
-1.4
-1.2
-1.0
-0.8
-0.6
-0.4
-0.2
0.0
DSC /(mW/mg)
80
85
90
95
100
105
110
115
TG /%
Mass Change: -6.91 %
Area: -299.5 J/g
Peak: 195.3 °C
[1]
[1]
↑
ex
o
I-CRAFT 2024 - 4th International Conference on Research of Agricultural and Food Technologies
142
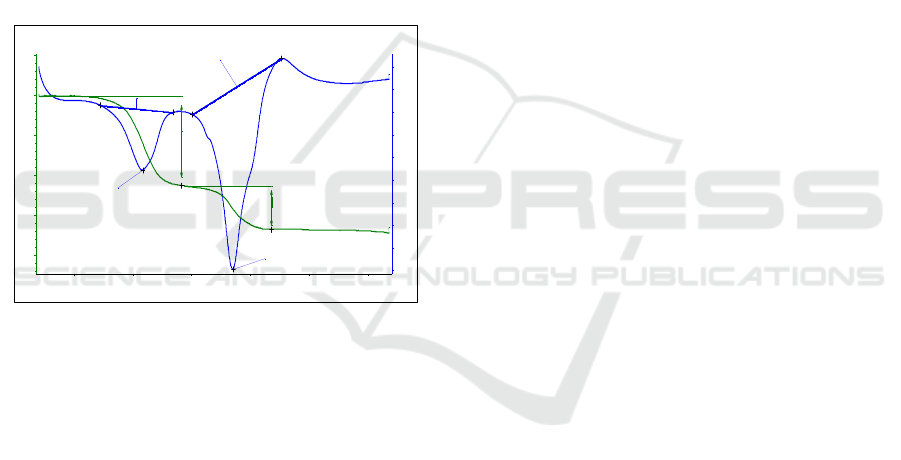
the formation of dianhydrogossypol molecule, and
the amount of heat required for the endothermic
reaction was -377.5 J/g. The dianhydrogossypol
molecule was completely decomposed and the
carbonization process started at a temperature of
225℃.
The result of thermal analysis of gossypol:diethyl
ether clathrate produced at -5℃ temperature is shown
in Figure 8. The mass reduction of gossypol:diethyl
ether clathrate was observed to proceed in two steps.
The 1st stage of mass reduction was observed in the
temperature range of 80-145℃, and as a result of
mass reduction of gossypol:diethyl ether clathrate
from 100% to 88.82%, the mass difference was
11.18%. The 2nd stage of mass reduction started
slowly at 160℃ and rapidly decreased in the
temperature range of 185.5-200℃. No mass change
was observed starting at 230℃. In the 2nd stage, the
mass difference was 5.42%.
Figure 8: TG-DSC curve formation of gossypol:diethyl
ether clathrate in -5℃.
Gossypol:diethyl ether clathrate formed at -5℃
was observed to produce two endothermic peaks
when affected by temperature. The first endothermic
peak corresponded to the interval of 80-145℃, where
diethyl ether was released, and the lowest
endothermic peak corresponded to 108.4℃. The
second endothermic peak was formed in the
temperature range of 175.5-200℃, and the lowest
peak of this endothermic peak corresponded to
185.5℃, where two molecules of water in the
clathrate were separated and turned into
dianhydrogossypol, and the amount of heat required
for the endothermic reaction was respectively in the
first stage it was -86.9 J/g, and in the second stage it
was -273.2 J/g. The dianhydrogossypol molecule was
completely decomposed and the beginning of the
carbonization process coincided with the temperature
of 220℃.
4 CONCLUSIONS
Based on the results of the analysis, it can be
concluded that:
1. The P3 polymorph of gossypol was observed
to form gossypol:diethyl ether clathrate in the
solid:gas phase with diethyl ether at room
temperature, +40℃ and -5℃, respectively;
2. P3 polymorph was found to be stable up to
100℃, desolvation of water molecules was observed
in the temperature range of 100-140℃;
3. The resulting gossypol:diethyl ether clathrate
was stable up to 155℃ at room temperature, and
water and diethyl ether were found to desolvate
simultaneously at 195.3℃;
4. Gossypol:diethyl ether clathrate formed at
+40℃ is stable up to 163℃, desolvation of diethyl
ether at 195.3℃ and water molecule at 202.8℃ was
observed;
5. Gossypol:diethyl ether clathrate formed at -
5℃ is stable up to 75℃, desolvation of diethyl ether
solvent at 108.4℃ and water molecule desolvation at
185.5℃ was observed;
6. The stability of the formed clathrates was
found to increase from left to right in the cross
section: gossypol:diethyl ether clathrate formed at -
5℃ → gossypol:diethyl ether clathrate formed at
room temperature → gossypol:diethyl ether clathrate
formed at +40℃.
REFERENCES
Honkeldieva, M., Bukhorov, K., Sayfiyeva, M., Choriyeva,
G., 2023. Study on the clathrates and polymorphes of
plant polyphenols. IOP Conference Series: Earth and
Environmental Science., 1142(1), 012068.
Honkeldieva, M., Kunafiev, R., Hamidov, H.I., 2015.
Redetermined structure of gossypol (P3 polymorph).
Acta Crystallographica Section E: Crystallographic
Communications, 71(7), o442–o443.
Honkeldieva, M.T., Talipov, S.A., Ibragimov, B.T., 2015.
Gossypol inclusion compound with pyrazine: Crystal
structure and thermal behavior. Journal of Inclusion
Phenomena and Macrocyclic Chemistry, 83(3-4), 369–
375.
Manach, C., et.al., 2004. Polyphenols: food sources and
bioavailability. The American Journal of Clinical
Nutrition, 79 (5), 727–747.
Zhao, T., et al., 2020. Cotton roots are the major source of
gossypol biosynthesis and accumulation. BMC Plant
Biol., 20(88). doi: 10.1186/s12870-020-2294-9.
50 100 150 200 250 300
Temperature /°C
-0.9
-0.8
-0.7
-0.6
-0.5
-0.4
-0.3
-0.2
-0.1
0.0
DSC /(mW/mg)
80
85
90
95
100
105
TG /%
Mass Change: -11.18 %
Mass Change: -5.42 %
Peak: 108.4 °C
Peak: 185.5 °C
Area: -86.9 J/g
Area: -273.2 J/g
[1]
[1]
↑
ex
o
Biochemical Properties of Plant Polyphenols
143
