
A Review of the Application of Nitrogen Doping in LiFePO
4
Cathode
Materials for Lithium-Ion Batteries
Wanting Chang
C
ollege of Chemistry, Jilin University, Changchun 130000, China
Keywords:
Nitrogen Doping, Lithium Iron Phosphate, Synthesis Method, Nitrogen and Sulfur Dualdoped Carbon.
Abstract: Lithium phosphate is a commonly used material for lithium battery-positive electrode materials, with high
energy density, high stability, and long service life. Still, it also has disadvantages such as poor conductivity
and heavy weight. Discussion based on the structure and electrochemical properties of lithium iron phosphate,
this review focuses on the modification methods of carbon-coated, nitrogen doping, and nitrogen-sulfur co-
doping. It analyzes the electrochemical properties after doping through impedance tests, EIS, SEM, and other
methods. It can be found that these methods can effectively reduce the charge transfer resistance and increase
initial discharge capacity, making the battery have a higher and more stable capacity. This review also
discusses the synthesis method of doped lithium iron phosphate. Two methods are introduced: using sucrose
and melamine as carbon sources and using polydopamine as a precursor. Also shows the synthetic advantages
and improvement principles. Both methods can indicate good electrochemical performance of lithium iron
phosphate doped with nitrogen.
1 INTRODUCTION
Lithium batteries are the most popular type of
batteries used nowadays. It can be utilized for storing
energy using natural resources (such as integrated
lithium-ion battery power source, marginal website
lithium-ion battery power source), etc. Among them,
lithium iron phosphate which is used as a common
material has attracted much attention. Lithium iron
phosphate (LiFePO
4
) has an alternating layer
structure of octahedral-coordinated iron and
octahedral-coordinated lithium mixed with phosphate
tetrahedrons (Malik et al, 2013). Compared to
LiMO
2
, lithium cobalt oxide (LiCoO
2
, LCO) other
traditional lithium-ion batteries, LiFePO
4
has
advantages in energy density, working cycles,
stronger durability, and excellent safety and
environmental protection (Li et al, 2016).
Furthermore, LiFePO
4
has many excellent
electrochemical properties. Such as high cycle
stability, safety, platform potential, and good high-
temperature performance.
However, LiFePO
4
still has some disadvantages,
such as heavy weight, low energy density, poor
conductivity, and easy mixing between Li-Fe sites.
To overcome these problems, a common approach is
to nanosize LiFePO
4
to reduce the risk of blockage
(Yang et al, 2016). In addition, scientists have
proposed a series of improvement methods, including
doping, nitrogen-doped carbon layers, high
conductivity coatings, morphology optimization, and
nanosize (Li et al, 2016), (Cech et al, 2013, Chen et
al, 2023, Zhang et al, 2013). This article will review
nitrogen-doped carbon layers, focusing on
improvement strategies for nitrogen-doped carbon
layers and specific methods for the electrical
characteristics of the electrolyte LiFePO
4
.
2 THE CHARACTERISTICS AND
STRUCTURE OF LiFePO
4
2.1 The Structure of LiFePO
4
LiFePO
4
is commonly used in lithium-ion batteries.
Its crystal structure has a unique alternating layer of
octahedral-coordinated iron and octahedral-
coordinated lithium mixed with phosphate
tetrahedrons. This unique structure gives LiFePO
4
material stable chemical properties (Yang et al,
2016),(Eftekhari, 2017). During the electrochemical
reaction of LiFePO
4
materials, as lithium ions are
inserted and removed, their crystal structure will
undergo certain changes. The conversion from
314
Chang, W.
A Review of the Application of Nitrogen Doping in LiFePO4 Cathode Materials for Lithium-Ion Batteries.
DOI: 10.5220/0013935600004914
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 2nd International Conference on Renewable Energy and Ecosystem (ICREE 2024), pages 314-318
ISBN: 978-989-758-776-4
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

LiFePO
4
to FePO
4
is a minor phase transition that
corresponds to different lattice parameters. In the
phase transition of this decay process, we can see that
LiFePO
4
has limited electronic and ionic conductivity
relative to lithium ions.
A notable feature of LiFePO
4
is its low electronic
and ionic conductivity. This is mainly because the
mobile lithium ions are restricted to 1D channels
along the b-axis, and their movement is restricted by
phosphate ions (Gabrisch et al, 2003). In addition, the
strong covalent bond between oxygen and P5 ions
forms a stable (PO
4
)
3-
unit, and this structural stability
enhances the safety of the material. To improve the
conductivity of LiFePO
4
, a common method is to
enhance its electronic conductivity by introducing
nitrogen atoms into the carbon structure. This
modification measure can improve the properties of
LiFePO
4
in lithium-ion batteries while maintaining
their high stability and safety characteristics.
Figure 1. LiFePO4 crystal structure of (Bi et al, 2013)
.
2.2 Electrochemical Properties
LiFePO
4
has many excellent electrochemical
properties as the electrode, which provides a good
basis for improving the performance and expanding
the applications of lithium-ion batteries.
First, the platform potential is relatively flat. The
insertion/extraction potential platform of LiFePO
4
is
relatively flat, which gives it relatively stable
discharge voltage and charge voltage, which it is
advantageous to the battery’s energy density and
cycle performance. The long platform of the voltage
curve of charging and discharging LiFePO
4
is about
3.4 V (0.2 C). When the temperature reaches 5°C, the
LiFePO
4
discharge capacity decreases from 139.6
mAh/g to 77.6 mAh/g (Kim et al, 2008).
Second is the high cycle stability. Because the
oxygen atoms in the LiFePO
4
structure are tightly
bound to the Fe and P atoms, it performs better at
higher temperatures compared to LiCoO
2
and other
layered electrode materials. The high lattice stability
enables LiFePO
4
to maintain its structural integrity
during long-term cyclic charge and discharge, thereby
ensuring the cycle life of the battery.
Third is safety. Golubkov et al showed that,
under adiabatic conditions, a heating element placed
around an 18650 LFP cell with 100% SOC can
provide constant power, the maximum temperature of
the cell during TR was between 400° C-450° C
(Bugrynice et al, 2008). This shows that compared
with other lithium-ion battery-positive electrode
materials, LiFePO
4
has a lower risk of thermal
runaway and explosion, making lithium-ion batteries
safer and more reliable.
Fourth is the low self-discharge rate. By using in
situ Raman spectroscopy to study the performance of
LiFePO
4
particles of different sizes and morphologies
during charging and self-discharging, it can be found
that LiFePO
4
has a low self-discharge rate, and the
battery suffers less loss during storage and idleness,
so it can maintain relatively long battery life.
Finally, there is the good high-temperature
performance. LiFePO
4
has good stability and cycle
performance in high-temperature environments and
can remain stable even at 400°C. It makes it suitable
for various high-temperature environments, such as
electric vehicles.
3 MODIFICATION STRATEGY
Materials with a larger surface area to volume ratio
can provide shorter paths for ions to travel, thereby
achieving higher power and energy density.
However, the increased surface area may also have
adverse effects, because the increased electrolyte
exposure promotes electrolyte decomposition, the
formation of unstable interfaces, and the dissolution
of transition metals (Quilty, 2023). Poorly performing
binders can negatively impact the cycling
performance of lithium batteries. Therefore,
structures containing highly π-conjugated polymer
chains should be considered when designing and
synthesizing polymer binders.
3.1 Carbon-Coated
Carbon coating is a good way to increase the
conductivity of the electrode. The addition of N, S, B,
P, F and other heteroatoms into the carbon network
further enhances the electrochemical activity.
Heteroatoms can act as electron donors, increase
carbon electron transfer, break down graphite’s
ordered structure, and promote the diffusion of
lithium ions. Simultaneously, a lot of defects will be
A Review of the Application of Nitrogen Doping in LiFePO4 Cathode Materials for Lithium-Ion Batteries
315

generated to promote the transmission of lithium in
the carbon layer.
The reversible capacity of carbon-coated
LiFePO
4
at 0.2C is about 140 mAhg
−1
. After the
cycle, it can maintain its capacity. And the capacity is
much greater than naked LiFePO
4
. By studying the
capacity of carbon covering LiFePO
4
of different
materials, it can be found that compared with other
carbon sources, graphite-covered LiFePO
4
has better
power chemical performance. After the impedance
measurement for different materials, the LiFePO
4
covered with graphite has a lower lithium-ion
migration resistance, and it also has a lower charge
transfer resistance. From this, we can conclude that
the addition of carbon will significantly reduce the
size of LiFePO
4
particles. The reduction of grain size
of LiFePO
4
and the enhancement of carbon electrical
contact will significantly improve the
electrochemical performance (Shin et al, 2006).
Bai N et al. (2016) studied Hierarchical porous
LiFePO
4
/C. Not only is the synthetic vegetable
protein pollution-free and easy to extract, but it also
has a circulation capacity of up to 166.8 mAhg
−
1
.
LiFePO
4
/C has a high capacity at different rates. At
high rates, the discharge capacity of carbon-coated
LiFePO
4
is twice that of the original LiFePO
4
, and the
capacity retention rate also is high.
Xiao Q.C et al. (2014) studied Li
1·2
(Mn
0.54
Co
0.13
Ni
0.13
)O
2
@AlF
3
/C (LMSAC) which is
prepared by AlF
3
and the carbon hybrid layer of
LMSS. The LMSS coated with AlF
3
/C has an initial
coulombic efficiency of more than 85%. Therefore,
carbon-coated improves the rate performance and
cycle performance. Impedance experiments show that
the electrode has a lower R
ct
and better conductivity
after carbon-coated.
3.2 Nitrogen Doping
The particle size of LFP/C-N (LiFePO
4
/N doped C)
materials prepared by nitrogen doping technique is
about 143 nm, in which LiFePO4 contains N. The
specific capacity of the material is 160.7 mAhg
-1
(0.1
C). It was demonstrated that the capacity of the
LiFePO
4
/C cathode did not decay after a long period
of operation, while the core-shell structure improved
its cycling performance.
Through the SEM image of LFP/C-N, it can be
found that the N element is distributed in the LiFePO
4
particles and surface carbon membranes. In addition,
it can be found from the structure of N doped that the
N doped in the carbon coating can expand the gap,
effectively increase the density of the carrier, and
increase the conductivity. When measuring 0.1C, the
charging and discharge voltage of each material can
obtain the conclusion that the discharge capacity
gradually increases with the increasing carbon
covering and N doped. After performing impedance
experiments, it can be found that materials with a
nuclear shell structure have lower charge transfer
resistance, and the N doped can further reduce the
transfer resistance. This also illustrates that the
nuclear shell structure and N-doped have a synergy
effect (Zhang et al, 2022).
Wang p et al. (2016) uses polybenzoxazine to
make LiFePO
4
nitrogen-doped material. Nitrogen
doping can provide a fast transport path for Li
+
. The
insertion process of Li
+
has a flat platform potential,
indicating that the battery is stable. It can maintain
75.8% of the initial discharge capacity, and through
continuous charge and discharge tests, it can be found
that it has stable cycle performance and excellent
recyclability.
Figure 2. (a) SEM image of LFP/CN. (b) LiFePO4
granules (Bai et al, 2016)
3.3 Nitrogen and Sulfur Dualdoped
Carbon
Double-doped carbon materials are carbon-doped
with nitrogen and sulfur. Compared with single-
doped materials, double-doped carbon materials have
better electrochemical properties. The heteroatom is
an electron donor, which can enhance the electron
transfer, and destruction of C, and facilitate the
diffusion of lithium ions. Moreover, doping with NSC
can create more defects, which can be used as the
active site. The cathode discharge capacity of LFP
doping by NSC was 155 mAhg
-1
and showed no
significant decay with cycling, which was
significantly higher than that of LFP. Furthermore,
NSC@LFP has 126 mAh·g-1 in the first period of 1
C. Heteroatom-doped carbon layer is an effective
method to modify the electrode (Xiao et al, 2014).
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
316
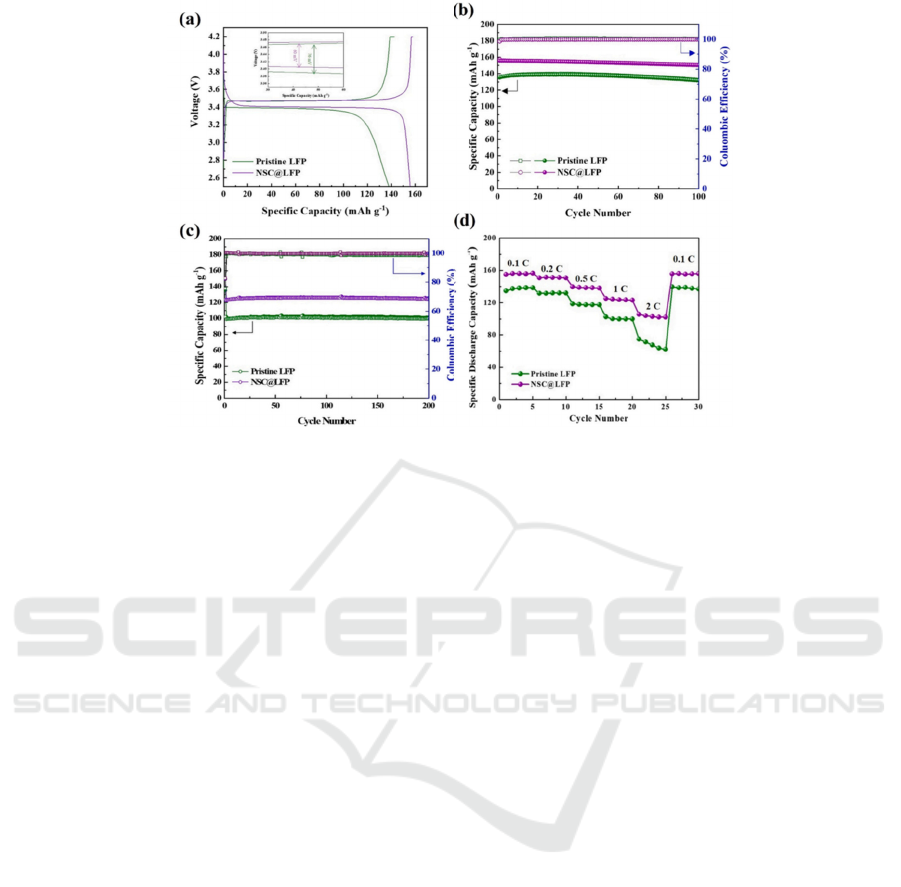
Figure 3. (a) the potential profile, (b) cycle efficiency, (c) cycle performance (d) the ability of pure LFP to NSC@LFP (Xiao
et al, 2014)
.
4 THE SYNTHESIS METHOD OF
LiFePO
4
4.1 Synthesis of Nitrogen-Doped
LFB-C with Sucrose and Melamine
as Carbon Sources
Nitrogen-doped LFB-C can be synthesized using
sucrose and melamine as the carbon source. The
addition of melamine helps in the appearance of
active surfaces. The effect of nitrogen content on
particle size was not significant due to the low
nitrogen content. The coating was formed uniformly
on the lithium iron phosphate surface. The nitrogen-
doped carbon layer and lithium iron phosphate
formed a "core-shell" conductive network, which
would greatly facilitate electron transfer and thus
improve electrochemical performance.
After measuring the EIS, the EIS is composed of
a straight line in the low-frequency area and a half in
the high-frequency area. The Warburg impedance is
used to measure the linear relationship in the low-
frequency range, and it can reflect the diffusion
ability of the lithium-ion in iron phosphate/lithium
iron phosphate. The half-circle diameter of the
modified sample was smaller than that of the
modified one. The reason for this is that nitrogen
provides a lot of conductive carriers for the carbon
layer particles in the LFP/C, so the LFP/C resistance
is reduced, and the slope of the inclined portion of the
nitrogen-modified sample is higher than that of the
untreated sample, which indicates that the Warburg
impedance is decreased, and the doping of LiFePO
4
is advantageous to the improvement of the
electrochemical property.
4.2 Synthesis of Nitrogen-Doped
LiFePO4 by Microwave Heating
with Polydopamine as Precursor
Carbon-Nitrogen
LiFePO4 and Tris buffer were mixed with dopamine
and stirred for 2 h. The weight ratio of
dopamine/LiFePO4 was 1:9, and then nitrogen-doped
lithium iron phosphate was synthesized by
microwave heating. Generally speaking, the particle
size of the electrode material has a great influence on
its electrochemical properties, but the microwave
heating method makes the product particles
homogeneous and small in size, so the influence is
small. The LiFePO4/CN synthesized by this method
has better cycling and electrochemical properties, as
shown in Figure 3 (Zhang et al, 2022).
A Review of the Application of Nitrogen Doping in LiFePO4 Cathode Materials for Lithium-Ion Batteries
317

Figure 4. (a) capacity and (b) cycling properties (Zhang et al, 2022).
5 CONCLUSIONS
In summary, doping with C, N, and S can effectively
improve the energy properties of the cathode.
Moreover, compared with single doping, nitrogen and
sulfur dual doping can make lithium iron phosphate
have better electrochemical properties. Double
doping materials can enhance the electron transfer of
carbon, damage the ordered structure of graphite,
increase the edge defect, form small pores, and
accelerate the diffusion of lithium ions.
In addition, this review also introduces two
methods for synthesizing nitrogen-doped LiFePO
4
:
mixing the LiFePO
4
sample with sucrose and
melamine and synthesizing nitrogen-doped LiFePO
4
was carried out by using polydopamine as a precursor
of carbon-nitrogen, and by microwave heating.
As a common lithium battery cathode material,
LiFePO4 has a longer service life and stronger
durability. However, LiFePO4 also has a lower
energy density, so how to improve its shortcomings
with cheaper materials is an important challenge.
REFERENCES
Bai N et al. 2016 Mater Technol 32 203
Bi Z, Zhang X, He W, Min D and Zhang W 2013 RSC Adv.
3 19744
Bugrynice P.J, Davidson J.N and Brown S.F 2018 Energy
Procedia 151 74
Cech O, Thomas J. E, Sedlarikova M, Fedorkova A,
Vondrak J, Moreno M. S and Visintin A 2013 Solid
State Sci. 20 110
Chen Q, Liu H, Hu J, Wang L, Li Y and Yao Y 2023 Ionics.
29 4537
Eftekhari A 2017 J. Power Sources. 343 395
Gabrisch H, Yazami R and Fultz B 2003 J. Power Sources.
119-121 674
Han B, Meng X, Ma L and Nan J 2016 Ceramics
International 42 2789
Kim H.-S, Kong M, Kim K, Kim I.-J and Gu H.-B 2008 J.
Electroceram. 23 219
Li S, Liu X, Liu G, Wan Y and Liu H 2016 Ionics. 23 19
Malik R, Abdellahi A and Ceder G 2013 J. Electrochem.
Soc. 160 A3179
Quilty C et al. 2023 Chem Rev 123 1327
Shaji N et al. 2023 J. Electrochem. Energy Convers.
Storage 72 108710
Shin H. C, Cho W. I and Jang H 2006 Electrochimica Acta
52 1472
Wang P et al. 2016 ACS Appl .Mater.Interfaces 40 26908
Xiao Q.C et al. 2014 Mater Technol 29 A70
Yang Z, Dai Y, Wang S and Yu J, 2016 J. Mater. Chem. A.
4 18210
Zhang B, Wang S, Liu L, Li Y and Yang J 2022 Materials
15 4738
Zhang H, Deng Q, Mou C, Huang Z, Wang Y, Zhou A and
Li J 2013 J. Power Sources. 239 538
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
318
