
Advances in Silicon Anode Morphology for Enhanced Lithium-Ion
Battery Performance: Current Status and Future Perspectives
Siyuan Zhou
School of Physics & Electronics, Hunan University, Hunan, 410082, China
Keywords: Lithium Battery, Silicon Anode, Morphology.
Abstract: Lithium-ion batteries with high energy density are regarded as highly promising electrochemical energy
storage systems. However, traditional liquid electrolytes present significant safety risks due to their propensity
for leakage and flammability. In contrast, solid-state electrolytes have garnered extensive attention for their
enhanced safety performance, high energy density, and superior stability with lithium anodes. Consequently,
research on solid-state electrolytes has become increasingly prominent. Despite this, the development of solid-
state electrolytes remains in an exploratory phase, primarily hindered by issues such as high solid-state
impedance and side reactions with electrodes. Moreover, the challenge lies in integrating the performance
advantages of various solid-state electrolytes. The primary obstacle is the poor compatibility between solid-
state electrolytes and electrodes. Current strategies to address these issues include electrode modification,
electrolyte recombination, and the introduction of interface layers. Nevertheless, solid-state electrolytes have
not yet achieved the level of development necessary to fully replace liquid electrolytes. This article provides
a comprehensive review of the status of organic polymer and inorganic solid-state electrolytes and discusses
the future development trends of these materials.
1 INTRODUCTION
Today, the problems of energy supply and the
environment are becoming increasingly prominent
and have become the focus of global attention. With
the development of industrialisation and urbanisation
and the gradual depletion of traditional energy
resources, the energy crisis has become an urgent
problem. In order to meet this challenge, people have
turned to electrification technology and are constantly
exploring innovations in the field of new energy
sources. As an important energy storage and release
device, lithium-ion batteries are gradually becoming
the mainstream choice for electric vehicles,
renewable energy systems and other fields. In the
research field of automotive batteries, lithium-ion
batteries are favoured for their high energy density,
long cycle life and low self-discharge rate. However,
conventional carbon anode materials are restricted in
terms of capacity and energy density, making it
difficult to meet the growing market demand for
electric vehicles. Therefore, the search for new anode
materials has become one of the hotspots of current
research (Deng et al., 2020 & Manzetti and Mariasiu,
2015).
Silicon, as a potential high-capacity anode
material with advantages such as abundant resources,
high theoretical specific capacity, and good electrical
conductivity, is widely regarded as an ideal
alternative to traditional carbon anode materials.
Silicon materials are characterised by their ability to
hold more lithium ions and therefore have a higher
specific capacity, which is expected to significantly
increase the energy density and range of batteries.
However, there are some challenges associated with
silicon anode materials. Firstly, silicon materials
undergo volume expansion and contraction during
charging and discharging, leading to problems such
as electrode particle fatigue and electrode structure
damage, affecting the cycle life and stability of the
battery. Second, the low conductivity and high
surface area of silicon materials also increase
electrode polarisation and charge transport resistance,
reducing the charge and discharge rate and power
performance of the battery (Speirs et al., 2014).
Recently, silicon electrodes have garnered
significant research interest. Strategies like
nanostructure engineering, composite development,
electrolyte additives, and novel polymer binders have
been extensively developed, leading to notable
improvements in the electrochemical performance of
Zhou, S.
Advances in Silicon Anode Morphology for Enhanced Lithium-Ion Battery Performance: Current Status and Future Perspectives.
DOI: 10.5220/0013932900004914
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 2nd International Conference on Renewable Energy and Ecosystem (ICREE 2024), pages 297-302
ISBN: 978-989-758-776-4
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
297
silicon materials. Nanostructure engineering, in
particular, has demonstrated significant potential for
enhancing cycling stability and rate capability.
Various silicon nanostructures, including
nanoparticles, nanowires, thin films, and porous
structures, have been extensively studied. These
nanostructures significantly improve the
electrochemical performance of silicon materials by
efficiently accommodating volume expansion,
increasing active surface area, shortening lithium-ion
diffusion paths, and reducing electron and ion
transport distances. These advances provide new
ideas to address the practical applications of silicon
anode materials (Li et al., 2022).
This paper reviews and analyses the research
progress on the morphology of silicon anode
materials for lithium-ion batteries, and summarizes
the current research progress of silicon anode
morphology regulation in the field of lithium batteries
based on the existing research background and status
quo, pointing out its potential application prospects in
improving the energy storage performance of the
batteries, prolonging the battery life and improving
the safety. In view of the problems and shortcomings
of the current research, the possible future research
directions and development trends are proposed, and
the future research prospects of silicon anode
materials are envisioned.
2 RESEARCH PROGRESS OF
SILICON ANODE FOR
LITHIUM-ION ELECTRODE
2.1 Block Silicon
Lumpy silicon refers to large-size silicon particles or
lumpy silicon negative electrode materials, as shown
in Figure 1. Under the consideration of the influence
of morphology on energy storage performance,
lumpy silicon is used as a common silicon negative
electrode material morphology, which has its unique
superior high specific capacity and can achieve higher
energy density. The volume expansion is relatively
small, which is conducive to maintaining the stability
of the electrode structure. The preparation method is
relatively simple and can be mass-produced.
The high specific capacity of bulk silicon is
mainly due to its large volume and relatively small
surface area. This allows bulk silicon to store more
lithium ions, thereby increasing the energy density of
the battery. However, the volume expansion of bulk
silicon during charging and discharging may lead to
the destruction of the electrode structure. This is
because the silicon undergoes volume expansion as
lithium ions react with the silicon during charging and
discharging. Although the volume expansion of bulk
silicon is relatively small, it may still lead to structural
damage and performance degradation during long-
term cycling. Therefore, improving the cycling
stability and suppressing the volume expansion of
bulk silicon is the focus of current research.
Figure 1: TEM images of P-Si (Rajaeifar et al., 2022)
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
298
2.2 Silicon Nanoparticles
In nanostructure engineering, nanoparticles are one of
the most common silicon nanostructure.
Nanoparticles have the advantages of high specific
surface area and short ion diffusion paths, which are
beneficial to improve the cycling stability and rate
performance of batteries. In addition, nanoparticles
can be modified with suitable surface modifications
to improve the interfacial properties between them
and the electrolyte, thereby inhibiting SEI formation
and improving the cycle life of the battery.
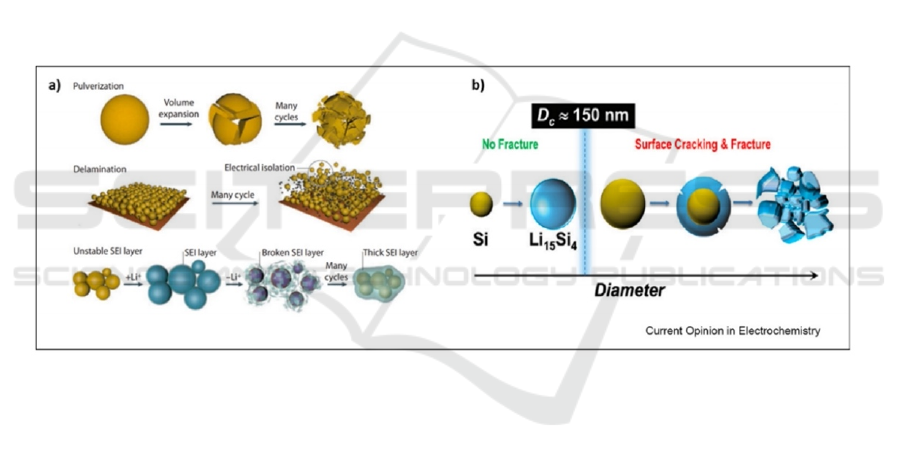
Particle-forming silicon materials, with sizes
spanning the micron to nanometer scale, are of great
interest in commercial mass production and are
considered as one of the most promising candidates
for next-generation lithium-ion battery (LIB) anode
materials. The particle size of silicon materials has a
substantial influence on the battery's lifespan and
stability of the electrodes. In particular, when the
particle size is reduced to the nanoscale, mechanical
stresses can be released quickly, thus exhibiting
greater resistance to structural fracture compared to
larger particle sizes. The results of Kim et al. showed
that the particles do not continue to grow when the
particle diameter is below 10 nm, which also applies
to lithium metal. Similar results were found in tin-
based electrodes, where further fracture of the
particles does not occur when the particle diameter is
below a certain critical size.
The technology for engineering silicon
nanoparticles is fairly well established, with the main
methods including chemical vapour deposition
(CVD), ball milling, molten salt electrolysis and
ferrothermal (carbothermal) reduction. Silicon
nanoparticles synthesised by CVD usually have a
uniform size distribution, however, the method
suffers from the high cost of precursors and
complexes, harmful silane gases, and low yields,
which limit its application in large-scale production.
In contrast, the ball milling method has the
advantages of high production capacity, low cost, and
easy doping with other elements, so it is widely used
in the preparation of silica nanoparticles. Wang et al.
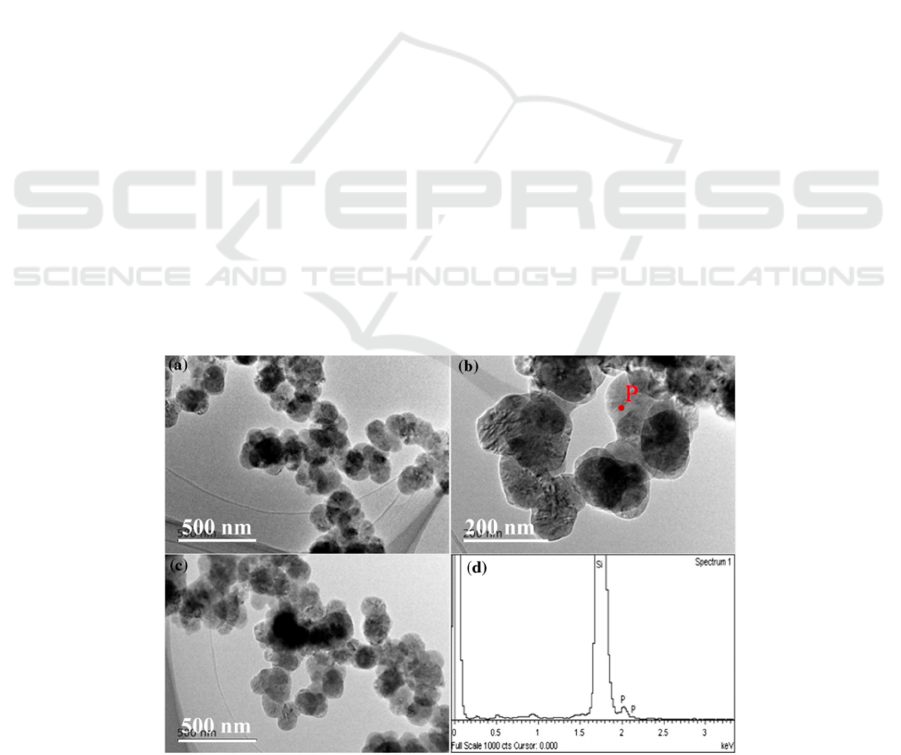
The morphology of silica nanoparticles as a raw
material is shown in Figure 2. From this figure, it can
be seen that there is an aggregate of spherical particles
with diameters between 20 and 70 nm, and its average
particle size is about 30 nm. In addition, the FESEM
images of the three graphites used in this study are
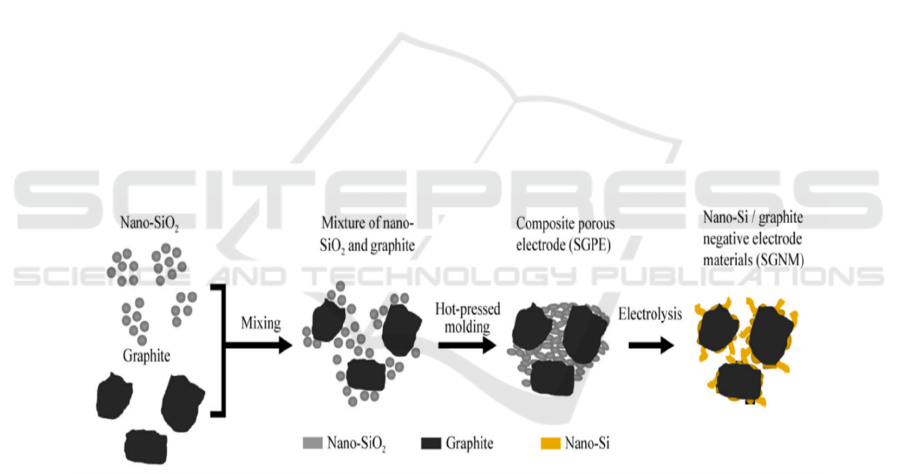
shown in Figure 2b-d.
Figure 2: The SGNM preparation process (Wang et al., 2022)
Recently, Zhu et al. proposed a simple and
scalable method to prepare silicon nanomaterials by
high-energy mechanical milling. This method
produces silicon nanoparticles of about 100 nm size,
which exhibit an exceptionally stable and reversible
capacity when coated with carbon, with a capacity
drop of less than 3% after 100 cycles. Although this
method is commercially available, there are still
many challenges in the process, such as large amounts
of impurities, surface oxidation, and size variation
(Tang et al., 2022).
In addition to ball milling and CVD, molten salt
electrolysis and ferrothermal (carbothermal)
reduction are also commonly used methods to obtain
silicon nanoparticles. Although these methods are
carried out at high temperatures, recently Lin et al.
proposed a strategy for the synthesis of silicon
nanocrystals at lower temperatures, which was
achieved in aluminium chloride molten salt.
However, silicon nanoparticles are seldom chosen as
anode materials because they tend to fragment during
repeated charge/discharge cycles, resulting in a loss
of electrical contact with the collector and a rapid
decay in capacity. To address these issues, various
additives such as carbonaceous materials, graphene,
conductive polymers, and metallic materials have
Advances in Silicon Anode Morphology for Enhanced Lithium-Ion Battery Performance: Current Status and Future Perspectives
299
been incorporated into silicon nanoparticles.
(Palomares et al., 2022).
2.3 Silicon Nanoparticles
Silicon nanotubes have a larger surface area and
smaller volume due to their hollow tubular structure,
so they can effectively slow down the volume
expansion of silicon when it alloys with lithium, thus
improving the stability and cycle life of the electrode.
As shown in Figure 3, the walls of the silicon
nanotubes can provide more electron transport
channels and reduce the transport distance of
electrons inside the material, and thus have higher
electron conductivity, which helps to improve the
conductivity and power performance of the electrodes
(Moyassari et al., 2022). The hollow structure and
nanoscale size of the silicon nanotubes facilitates the
rapid diffusion of lithium ions inside the tubes, and
therefore improves the lithium-ion diffusion rate of
the electrodes, which in turn improves the charging
and discharging rate and cycling performance of the
batteries. Silicon nanotubes have better deformability
due to their flexible tubular structure, which can
better adapt to the volume change during the alloying
reaction between silicon and lithium and reduce the
structural damage of the electrode material (Roland et
al., 2022; Sanad and Shenouda, 2023 & Li et al.,
2023). Overall, silicon nanoparticles and silicon
nanotubes, as an important form of silicon anode
materials, have unique advantages in enhancing the
efficiency and reliability of batteries, and controlling
the parameters of silicon nanotubes, such as tube
diameter and wall thickness, can further regulate their
electrochemical properties. However, the preparation
of silicon nanotubes usually requires more complex
processes, and thus there may be some challenges in
terms of cost and process control (Patel et al., 2023 &
Ishii et al., 2024).
Figure 3: The mechanisms of Si electrodes (Patel et al.., 2023)
2.4 Thin Film Silicon
The main feature of thin-film silicon as a battery
anode material is to form a thinner silicon layer by
precisely controlling the thickness of silicon to cope
with the problems caused by the volume expansion of
the traditional bulk silicon material during the
charging and discharging process. As shown in
Figure 4, thin-film silicon can mitigate the volume
expansion problem more effectively than bulk silicon.
Due to its smaller thickness, the volume expansion
will be more uniform, resulting in less damage to the
electrode structure (Ishii et al., 2024). Thin-film
silicon usually has a large surface area, which is
conducive to increasing the contact area between the
electrodes and the electrolyte, thus improving the
charge transfer rate and electrochemical capability of
the cell. By controlling the preparation process of thin
films, precise modulation of their microstructure can
be achieved to further optimise their electrochemical
performance (Bonkile et al., 2024). Thin-film silicon
also has some relative technical limitations.
Compared to bulk silicon, the preparation of thin-film
silicon is typically more complex and requires more
sophisticated processes and equipment, which can
increase costs and reduce production efficiency.
Although thin-film silicon can mitigate volume
expansion, it may still face poor cycling stability
during long-term cycling, which requires further
improvement and optimisation (Li et al., 2023 & Yao,
2023).
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
300

Figure 4: SEM images of Si electrode (Lukin et al., 2023)
The mechanism of slowing down the volume
expansion of thin-film silicon mainly stems from its
smaller thickness, which makes the volume change
more uniform and thus reduces the stress
concentration in the electrode structure and prolongs
the life of the cell. The large surface area, on the other
hand, helps to improve the contact between the
electrode and the electrolyte, which facilitates the
rapid transport of electrons and ions, and thus
improves the performance of the cell (ElKhamisy et
al., 2024). However, the complexity of the
preparation process and the challenge of cycling
stability still need to be addressed through process
optimisation and material design to drive further
application and development of thin-film silicon in
batteries. These advantages and challenges make
thin-film silicon a promising area in the study of
battery materials and provide important directions
and challenges for future research (Ette et al., 2020).
3 OUTLOOK OF SILICON
ANODE
Currently, for the technological innovation aspect of
silicon negative electrode, researchers should further
explore the properties of silicon negative electrode
materials with different morphologies and optimise
their electrochemical properties, especially the rod-
shaped nano-silicon materials which have great
potential to increase the capacity and energy density,
improve the cycling performance, and increase the
charge transfer rate. At the same time, the challenges
of complex and costly preparation processes need to
be focused on, and efforts should be made to develop
low-cost and large-scale preparation technologies for
silicon anode materials, and to use renewable
materials or waste resources to reduce the preparation
cost (Haneke et al., 2023).
In terms of the development trend, the mechanism
of the effect of morphology modulation on the energy
storage performance needs to be explored in depth
through experimental and theoretical analyses to
reveal the mechanism of its action at both the macro-
and microscopic levels. The experimental results and
discussion should focus on the performance
differences of different morphology silicon anode
materials in lithium batteries, and explore the
influence of morphology modulation on energy
storage performance and the possible direction of
improvement. Meanwhile, in terms of application
prospects, this paper predicts the application
prospects of silicon anode materials in electric
vehicles and energy storage systems, and explores the
research and application of multifunctional silicon
anode materials, such as silicon nanowire anode
materials with self-repairing ability or porous
structure.
4 CONCLUSION
In this paper, the impact of silicon anode morphology
on the energy storage performance of Li-ion batteries
is reviewed, and several ideas are put forward in
terms of technological innovation, cost reduction and
development trends. In addition, comprehensive
analyses have been conducted to consider how to
overcome the volume expansion and contraction of Si
anode materials during charge/discharge cycles, how
to improve the cycling stability and capacity retention
of Si anode materials, how to increase the
conductivity of Si anode materials to enhance the
charge/discharge performance of batteries, and how
to reduce the interfacial problems between the Si
material and the electrolyte to enhance the battery's
durability and safety.
Advances in Silicon Anode Morphology for Enhanced Lithium-Ion Battery Performance: Current Status and Future Perspectives
301

REFERENCES
Deng, J., Bae, C., Denlinger, A., & Miller, T.
2020 Joule 4(3) 511-515
Manzetti, S., & Mariasiu, F. 2015 Ren. Sust. Energ.
Rev. 51 1004-1012
Speirs, J., Contestabile, M., Houari, Y., & Gross, R.
2014 Ren. Sust. Energ. Rev., 35, 183-193.
Li, P., Xia, X., & Guo, J. 2022 Sep. Purif. Technol. 296
121389
Rajaeifar, M. A., Ghadimi, P., Raugei, M., Wu, Y., &
Heidrich, O. 2022 Res. Conserv. Recy. 180,106144
Wang, N., Liu, Y. Y., Shi, Z. X., Yu, Z. L., Duan, H. Y.,
Fang, S., 2022 Rare. Metals. 41(2) 438-447
Tang, F., Tan, Y., Jiang, T., & Zhou, Y. 2022 J. Mater.
Sci. 57(4) 2803-2812
Palomares, V., Nieto, N., & Rojo, T. 2022 Curr. Opin.
Electroche. 31 100840
Moyassari, E., Roth, T., Kücher, S., Chang, C. C., Hou, S.
C., Spingler, F. B., & Jossen, A. 2022 J. Electrochem.
Soc. 169(1) 010504
Roland, A., Fullenwarth, J., Ledeuil, J. B., Martinez, H.,
Louvain, N., & Monconduit, L. 2022 Battery.
Energy 1(1) 20210009
Sanad, M. M., & Shenouda, A. Y. 2023 J. Mater. Sci-
Mater. el. 34(14) 1146
Li, X., Zhang, Z., Gong, L., Fu, K., Yang, H., Wang, C., &
Tan, P. 2023 Chem. Eng. J. 470 144188
Patel, Y., Vanpariya, A., & Mukhopadhyay, I. 2023 J.
Solid. State. Electr. 27(2) 501-510
Ishii, N., Kakinuma, N., & Morimoto, H.
2024 Electrochemistry 92(1) 017006-017006
Bonkile, M. P., Jiang, Y., Kirkaldy, N., Sulzer, V., Timms,
R., Wang, H., ... & Wu, B. 2024 J. Power. Source. 606
234256
Li, X., Zhang, Z., Gong, L., Fu, K., Yang, H., Wang, C., &
Tan, P. 2023 Chem. Eng. J. 470 144188
Yao, Y. 2023 In MATEC Web of Conferences (Vol. 382,
p. 01017) EDP Sciences
Lukin, D. M., Guidry, M. A., Yang, J., Ghezellou, M., Deb
Mishra, S., Abe, H., ... & Vučković, J. 2023 Phys. Rev.
X. 13(1) 011005
ElKhamisy, K., Abdelhamid, H., El-Rabaie, E. S. M., &
Abdel-Salam, N. 2024 Plasmonics. 19(1) 1-20
Ette, P. M., Bhargav, P. B., Ahmed, N., Chandra, B.,
Rayarfrancis, A., & Ramesha, K. 2020 Electrochim.
Acta. 330 135318
Haneke, L., Pfeiffer, F., Bärmann, P., Wrogemann, J.,
Peschel, C., Neumann, J., ... & Placke, T. 2023
Small. 19(8) 2206092
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
302
