
Research Progress on Solid-State Electrolytes for Lithium-Ion
Batteries
Hanning Ma
Leicester International Institute, Dalian University of Technology, Panjin, 124000, China
Keywords: Lithium Battery, Solid-State Battery, Solid-State Electrolyte.
Abstract: Lithium-ion batteries with high energy density are regarded as highly promising electrochemical energy
storage systems. However, traditional liquid electrolytes present significant safety risks due to their propensity
for leakage and flammability. In contrast, solid-state electrolytes have garnered extensive attention for their
enhanced safety property, high energy density, and superior stability with lithium anodes. Consequently,
research on solid-state electrolytes has become increasingly prominent. Despite this, the development of solid-
state electrolytes remains in an exploratory phase, primarily hindered by issues such as high solid-state
impedance and side reactions with electrodes. Moreover, the challenge lies in integrating the property
advantages of various solid-state electrolytes. The primary obstacle is the poor compatibility between solid-
state electrolytes and electrodes. Current strategies to address these issues include electrode modification,
electrolyte recombination, and the introduction of interface layers. Nevertheless, solid-state electrolytes have
not yet achieved the level of development necessary to fully replace liquid electrolytes. This article provides
a comprehensive review of the status of organic polymer and inorganic solid-state electrolytes and discusses
the future development trends of these materials.
1 INTRODUCTION
Human productivity increased significantly after the
Industrial Revolution, and energy demand reached an
unprecedented height. Industrial production mainly
relies on fossil fuels such as coal, natural gas, and oil,
which are non-renewable and lead to serious
environmental pollution, such as global warming
caused by greenhouse gas emissions. Therefore, the
search for renewable new energy has become a huge
challenge for human development at this stage.
Currently, known renewable energy sources include
wind, solar, geothermal, tidal, etc. However, these
renewable energy sources are unstable and
intermittent, and how to integrate these intermittent
energy sources into a stable and efficient large-scale
electric energy storage system is the key to solving
the energy crisis today (Tao et al, 2022). Lithium-ion
batteries stand out in many electrochemical energy
storage systems because of their advantages of high
working voltage, high energy density, low self-
discharge rate, long cycle life, and no memory effect.
However, the widely used traditional lithium-ion
batteries have gradually exposed several problems
due to using liquid electrolytes. The organic
components in liquid electrolytes are prone to
decomposition, combustion, and even explosion
under high temperatures and currents. In addition,
these organic components may decompose at high
voltages, making liquid lithium-ion batteries used
now difficult to match cathode materials with high
electrode potentials. Therefore, one of the alternatives
with the highest expectations is all-solid-state
lithium-ion batteries prepared with solid-state
electrolytes.
It is worth noting that the solid-state electrolyte
currently is the main innovation which replaces the
diaphragm and electrolyte in the liquid electrolyte. In
this way, unnecessary chemical reactions between
electrodes to dissolve active substances can be
effectively inhibited, and the safety of batteries can be
also improved (Janek and Zeier, 2023). Meanwhile,
the solid electrolyte can be matched to the anode of
lithium metal with a high theoretical specific
capacity. Even better, this kind of anode can match
the cathode material with a high specific capacity,
thus comprehensively improving the energy density
(400Wh/kg&1000Wh/L) (Aspinall et al., 2024). To
pursue high energy density, the solid-state electrolyte
Ma, H.
Research Progress on Solid-State Electrolytes for Lithium-Ion Batteries.
DOI: 10.5220/0013915200004914
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 2nd International Conference on Renewable Energy and Ecosystem (ICREE 2024), pages 273-279
ISBN: 978-989-758-776-4
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
273

layer needs to be thin enough, and the material needs
to be thin and flexible, further broadening its
application prospects. In addition, batteries with
solid-state electrolytes also have the advantages of
strong endurance, wide temperature range and fast
charging speed.
Currently, the solid-state electrolyte is mainly
divided into organic polymer solid-state electrolytes
and inorganic solid-state electrolytes two categories.
The inorganic solid-state electrolyte can be divided
into oxide, halide and sulfide solid-state electrolyte.
In this article, the current research progress of
mainstream solid-state electrolytes is reviewed, and
the future development direction of solid-state
batteries is prospected.
2 SOLID-STATE ELECTROLYTE
2.1 Organic Polymer Solid-State
Electrolyte
Organic polymer solid-state electrolyte is mainly
composed of lithium salt with low dissociation energy
and a polymer matrix with a high dielectric constant
(Wang et al., 2023). According to the types of
conductive particles, the organic polymer solid-state
electrolyte can be divided into two categories: single-
ion polymer solid-state electrolyte and double-ion
polymer solid-state electrolyte. Single-ion polymer
solid-state electrolyte is mainly composed of single-
ion lithium salt and polymer matrix, in which anions
are usually fixed on the polymeric main chain or
provided to the polymer as an acceptor, so only cation
migration in this kind of solid-state electrolyte.
However, this causes anions to accumulate near the
anode, which increases the resistance and affects the
overall property of the battery. Double-ion polymer
solid-state electrolyte is mainly composed of double-
ion lithium salt and polymer matrix. The anions
usually move faster than the cations. Therefore,
double-ionic polymer solid-state electrolytes
generally have higher ionic conductivity, while
single-ionic polymer solid-state electrolytes have
higher cation migration numbers.
The organic polymer electrolyte matrix material is
usually a polymer with polar functional groups to
promote the dissociation and ion transfer of lithium
salts so that it can have a relatively lower interface
impedance (Wang et al., 2023). Simultaneously,
polymer solid-state electrolytes are more suitable for
large-scale production because of their good
flexibility and elasticity, easy processing and low
cost. However, its organic composition results in
limited thermal stability and a low electrochemical
window (<4V). At the cathode interface, there are
many factors contributing to the instability of the
interface, such as the oxidation decomposition of the
polymer, the side reaction between the polymer and
the cathode, and the ageing of the battery interface in
the long cycle. At the anode interface, because of the
high reducibility of lithium metal, the polymer is
reduced, and structural changes occur, thereby
reducing the overall property of the solid-state
battery.
To solve the above problems of organic polymer
solid-state electrolyte, the researchers proposed the
following solutions: 1) Improve the cathode material:
By combining the cathode material with the organic
polymer, the interface impedance can be effectively
reduced, the ion transfers much faster, and the
interface instability of the cathode can be solved
effectively. 2) Introduction of the interface layer:
reduce the physical contact between the polymer
solid-state electrolyte and the electrode, reduce the
interface impedance from the source and restrain the
side reaction between the cathode and the electrolyte.
The interfacial layer between the electrolyte and the
anode can also restrain the reduction reaction and the
growth of lithium dendrites.
2.2 Inorganic Solid-State Electrolyte
2.2.1 Oxide Solid-State Electrolyte
Oxide solid-state electrolytes are composed of metal
oxides containing lithium, which are mainly divided
into calcareous, garnet and NASICON types
according to their different structural forms (Yao et
al., 2023). The calcareous type has the structural
formula ABO
3
, where A is the larger cation (usually
rare earth ions), and B is the smaller cation (usually
transition metal ions). However, the calcareous type
of oxide solid-state electrolyte has large grain
boundary impedance and small ionic conductivity,
especially at low potential, thereby reducing the
conductivity of lithium ions (Luo et al., 2024).
Garnet-type (LLZO) oxide solid-state electrolytes
(such as Li
5
La
3
M
2
O
12
, M = Ta and Nb) are composed
of lithium, lanthanum, and transition metal elements,
as shown in Figure 1. This kind of oxide solid-state
electrolyte has high electrochemical stability to the
metal lithium anode and has a higher electrochemical
window, which can match the high voltage cathodes.
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
274

However, the main challenge is the interface
compatibility between the electrolyte and cathodes
and anodes.
NASICON-type oxide solid-state electrolyte, also
known as sodium superionic conductor, its structural
formula is AM
2
(PO
4
)
3
. Where A represents alkali
metal ions (Li
+
\Na
+
), M represents Ti, Ge, Zr and
other elements. By substituting lithium for sodium,
lithium-ion conductors with higher ionic conductivity
can be obtained. The typical NASICON-type oxide
solid-state electrolyte is LiTi
2
(PO
4
)
3
(LTP). This kind
of electrolyte is stable in air and water and is not easy
to react to. However, its cost is high, and it is difficult
for large-scale production. In addition, due to the
presence of Ti4+ ions, like calcareous oxide solid-
state electrolytes, these electrolytes are also easy to be
reduced by lithium metal anode at low potential,
thereby reducing the overall property of the battery.
To solve the problem of an oxide-solid-state
electrolyte, the researchers proposed the following
solutions: 1) the introduction of a buffer layer: for the
interface impedance problem between the solid-state
electrolyte and the cathode, a layer of aluminium
oxide can be deposited to reduce the interface
impedance; for the reduction reaction between several
particular solid-state electrolytes and the lithium metal
anode, the lithium-ion conductor can be used as the
buffer layer between the solid-state electrolyte and the
electrode to inhibit the reduction reaction. 2) Improve
the electrode: on the one hand, lithium alloy electrodes
can be manufactured to reduce adverse reactions. On
the other hand, the electrolyte component can be
compounded with the cathode active component by
Figure 1: (a)Cubic Li7La3Zr2O12 crystal structure(b)
Coordination polyhedral around Li1 and Li2 sites (Yao et
al., 2023).
heating and other methods to achieve a good
electrolyte-electrode contact interface, thereby
reducing the interface impedance. 3) Ion doping: The
skeleton of sodium superionic conductors can be
modified by intercalating cations of different valence
states and ionic radii, thereby causing lattice distortion
and improving ionic conductivity (Luo et al., 2024).
2.2.2 Halide Solid-State Electrolyte
Halide solid-state electrolyte is mainly composed of
lithium, transition metal elements and halogen
elements, and its structure is determined by the
coordination number determined by the atomic radius
ratio of metal elements and halogen atoms. According
to the coordination number, halide solid-state
electrolytes can be divided into three categories: 1)
Li
a
-M-Cl
6
; 2) Li
a
-M-Cl
4
, 3) Li
a
-M-Cl
8
. Among them,
Li
a
-M-Cl
8
is unstable at room temperature, and its
conductivity is lower than Li
a
-M-Cl
6
and Li
a
-M-Cl
4
.
Therefore, Li
a
-M-Cl
6
electrolytes and some Li
a
-M-
Cl
4
electrolytes have been studied more at present
(Chen et al., 2023).
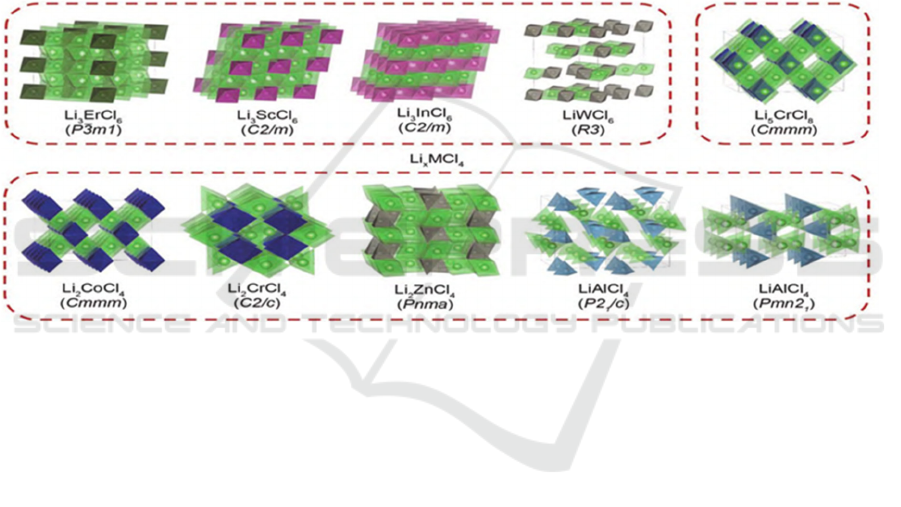
Li
a
-M-Cl
6
halide solid-state electrolytes are
mainly composed of group IIIB metal ions. The
common crystal structures of this kind of solid-state
electrolyte include the tripartite crystal system of the
P3m1 space group (hcp-T), the orthogonal crystal
system of the pnma space group (hcp-O) and the
cubic crystal system of C2/m space group (ccp).
Among them, the Li
a
-M-Cl
6
halide solid-state
electrolyte has better conductivity in the C2/m
structure. Lia-M-Cl
4
halide solid-state electrolytes are
mainly composed of trivalent and other valence metal
ions, as shown in Figure 2. The defect anti-spinel
structure has the highest electrical conductivity. Due
to the introduction of higher-priced cations, more
vacancies are created to maintain electrical neutrality,
and this structure is beneficial to the transport of
lithium ions.
Currently, halide solid-state electrolytes generally
have low phase transition temperatures, and a high
crystallinity phase can be obtained by the low
reaction temperature. In addition, fluoride and
chloride electrolytes have special advantages like
electrochemical windows (Yu et al., 2023). However,
halide-solid-state electrolytes have poor humidity and
air stability. This is because halogen salts are easy to
form into crystalline hydrates, and the more obvious
the polarization of the metal cation of halogen salts in
water, the easier it is to form a crystalline hydrate.
These crystalline hydrates will undergo a hydrolysis
reaction after heating, which makes it easy to form
metal oxide double salts that hinder the migration of
Research Progress on Solid-State Electrolytes for Lithium-Ion Batteries
275
lithium ions. However, some studies have shown that
halides show good stability in dry air, and after a
strictly controlled dehydration process, the hydrate
can be reversely converted to return to its structure
and electrochemical properties before water
absorption.
In addition, the instability of halide solid-state
electrolytes and lithium metal mainly depends on the
type of transition metal element in the halide
composition. For example, the Li
3
HoCl
6
electrolyte
can realize the stable cycle of assembled Lin-
symmetric batteries, but it is easy to short-circuit
when assembled with Li metal symmetric batteries.
In contrast, Li
3
HoCl
6
has better stability against Li
metal, which may be because the products formed by
fluoride contact with lithium metal can better fill the
vacancies and defects located in the solid-state
electrolyte and related interfaces, forming a denser
intermediate layer, thereby preventing the
development of further reactions.
In response to the above halide solid-state
electrolyte problems, the researchers proposed the
following improvement methods: 1) solid-phase
reaction synthesis: through ball milling to improve
the uniformity of raw material mixing and refine the
electrolyte particles, to improve the point contact
problem, reduce the interface impedance. 2) Liquid
phase synthesis: This method can save time and
improve efficiency, the halide is uniformly
distributed in the solid-state electrolyte, and it is
easier to obtain samples with uniform grain size after
crystallization.
Figure 2: Structure diagram of the third-class halide solid-state electrolyte (Chen et al., 2023)
2.2.3 Sulfide Solid-State Electrolyte
Sulfide solid-state electrolyte is mainly composed of
lithium, sulfur, phosphorus and other elements.
According to its crystal structure, it can be divided
into glass phase, glass ceramic phase and crystal
phase (Chen, 2021). Compared with the glass phase
and glass ceramic phase, crystalline sulfide solid-state
electrolyte has higher ion transport efficiency due to
its unique structure, and thus exhibits higher ionic
conductivity at indoor temperature (Qin and Gu,
2021). However, this kind of sulfide solid-state
electrolyte is prone to reduction and oxidation
reactions with lithium metal anode, which leads to the
instability of the interface between anode and
electrolytes. The sulfide solid-state electrolyte has
good mechanical strength, mechanical flexibility, and
high ionic conductivity, and its overall property is
greater than other types of solid-state electrolytes. For
example, the relatively low hardness of sulfide makes
it easy to deform during extrusion, thereby improving
the contact problem with the cathode through
deformation, which also makes it easier to process.
However, the material also has many shortcomings.
At the cathode interface, 1) insufficient ion and
electron penetration leads to a low utilization rate of
the cathode material; 2) There is a space charge effect.
Due to the large gap between the chemical potential
of lithium ions in the cathode and the electrolyte,
lithium ions tend to diffuse from the sulfide electrode
to the cathode, and an area with a low concentration
of lithium ions is easily formed on the sulfide side of
the interface, thereby reducing the ionic conductivity
and improving the interface impedance; 3) The
volume change of the cathode material leads to poor
contact and increased impedance. At the anode
interface, 1) the growth of lithium dendrites is a
problem, and 2) the interface is unstable, which
makes it easy to form a conductive phase layer mixed
with ions and electrons. In addition, due to the
sensitivity of sulfide to water vapour, it is easy to
react with water to produce toxic hydrogen sulfide
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
276

gas, resulting in a decrease in ionic conductivity (Yu
et al., 2023).
In response to the above sulfide solid-state
electrolyte problem, the researchers proposed the
following solutions: 1) Add a small amount of
electrolyte: Add a small amount of electrolyte
between the solid-state electrolyte and the electrode
or inside the solid-state electrolyte, so that it cannot
flow freely in the battery, choose the electrolyte that
does not react with the sulfide solid-state electrolyte
but can dissolve lithium salt. Alternatively, a glass
fibre diaphragm containing electrolyte can be
introduced into the cathode and the sulfide solid-state
electrolyte to improve the ionic conductivity and
solve the contact problem, thereby reducing the
interface impedance. 2) Composite with polymer
electrolyte: the polymer can act as a binder,
conductive network or skeleton material in the
composite to effectively inhibit the space charge
effect. 3) oxide-doped sulfide: can effectively absorb
H
2
S and inhibit the formation of H
2
S.
3 PROSPECTS FOR SOLID-
STATE ELECTROLYTES
The main factors contributing to the properties of
solid-state electrolytes are contact failure and
instability between electrodes and electrolytes. First,
the contact failure problem is that the electrolyte and
the electrode are both solid and the contact between
the two is point contact. The liquid electrolyte used
before like ethylene carbonate and diethyl carbonate
can fill all the pores caused by the charge and
discharge of the electrode. Additionally, the periodic
volume change of the electrode is unable to affect it.
Lithium tends to deposit in pores and rough edges of
grain boundaries, resulting in uneven deposition.
Therefore, the problem of the lithium dendrites
occurs. This non-uniformity of deposition will further
cause the contact between the solid-state electrolyte
and the cathode to decrease, thus the more lithium
depositing, the lithium dendrites will puncture the
electrolyte and connect the two electrodes, causing
the battery to short-circuit. Second, the presence of a
large solid interface impedance contributes to the
instability between the solid-state electrolyte and the
electrode. To improve the power density of solid-state
batteries, reducing the impedance can effectively
improve the transform efficiency of lithium ions.
Sufficient power density can achieve the
requirements of commercial mass-produced
production of power storage systems. The causes of
solid impedance at the interface include reduction
side reaction between electrolyte and electrode,
formation of space charge layer and periodic volume
change of electrode. When the solid-state electrolyte
matches the cathode, the mismatch of the
electrochemical windows results in the oxidation
decomposition of the solid-state electrolyte itself and
the anion replacement between the electrolyte and the
electrode. Those two kinds of reactions generally
occur simultaneously. When the solid-state
electrolyte matches the metal lithium anode, due to
the strong reduction of lithium itself, some cations
with high valency in the electrolyte are easy to have
reduction reactions, forming a new high-impedance
interface, which hinders the transmission of lithium
ions and makes the overall property of the battery
decline.
All kinds of solid-state electrolytes have their
property advantages, but there is no solid-state
electrolyte that can have all the property advantages
simultaneously and achieve inexpensive expenditure
and easy production, as shown in Figure 3. The real
problem facing solid-state electrolytes is that if it is to
replace traditional liquid lithium batteries in large-
scale commercial production, simply having high
ionic conductivity (such as the high cost of high-
property sulphide solid-state electrolyte) is not
enough to support its position in the industrial
application of all-solid-state batteries. Therefore,
researchers have developed composite solid-state
electrolytes, aiming to get a kind of comprehensive
one with various advantages to form an electrolyte
system with high ionic conductivity, good
processability, cycle stability and low cost (Zhan et
al., 2023). For example, in the oxide/sulphide
complex electrolyte, increasing the oxide content is
conducive to achieving uniform dispersion of the
oxide solid-state electrolyte, and improving the
interface bond between the oxide and the sulphide,
thereby increasing the migration rate of lithium ions.
In addition, organic polymers can also be combined
with sulphide solid-state electrolytes. The organic
polymer can be used as a binder to disperse the
sulphide in the composite film, to improve the
interface impedance. At the same time, the polymer
can also act as a skeleton, improve the flexibility of
the sulphide, and due to its low-cost characteristics,
large-scale preparation can be achieved. Through
these composite methods, the advantages of different
solid-state electrolytes can be effectively integrated,
and the limitations of a single material can be
overcome, to promote the industrial application of all-
solid-state batteries.
Research Progress on Solid-State Electrolytes for Lithium-Ion Batteries
277
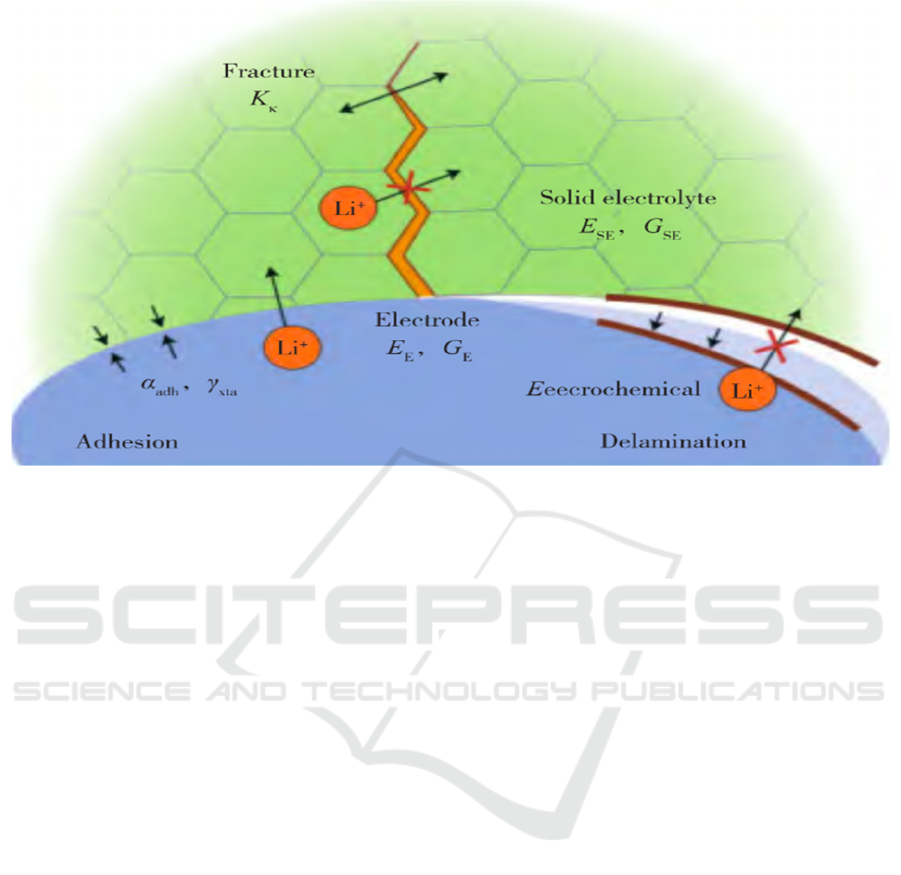
Figure 3: Contact diagram at the interface between the solid-state electrolyte and the electrode (FAMPRIKIST et al., 2019)
4 CONCLUSION
This study reviews the various types of solid-state
electrolytes, including oxide, halide, sulfide, and
organic polymer solid-state electrolytes, and analyses
their application potential and challenges. Each type
of solid-state electrolyte has its unique property
advantages, but there is no solid-state electrolyte that
can have comprehensive property advantages and
meet the requirements of low cost and easy
production. Although sulfide-solid-state electrolyte
has high ionic conductivity and good mechanical
properties, it is expensive and sensitive to water
vapour. Although oxide-solid-state electrolyte is
stable in air and water, it has the problem of large
interface impedance and insufficient ionic
conductivity. Halide-solid-state electrolytes have
advantages in terms of electrochemical windows, but
their humidity stability is poor and unstable with
lithium metal. Although the polymer solid-state
electrolyte has excellent machining properties and
low cost, its electrochemical window is narrow, and
its thermal stability is insufficient. The development
of a composite solid-state electrolyte has become an
effective solution to single limitations. Using
combination and doping, the advantages of various
solid-state electrolytes can be combined to form an
electrolyte system with high ionic conductivity, low
cost, good machining properties, and cycle stability.
For example, the oxide/sulfide composite electrolyte
can improve the interface bonding by increasing the
oxide content, thus increasing the migration rate of
lithium ions. The combination of organic polymer
and sulfide can improve the interface impedance and
flexibility by using the bonding and skeleton action
of the polymer and achieve low-cost, large-scale
preparation. In summary, the research on composite
solid-state electrolytes provides a new path for the
commercial application of all-solid lithium-ion
batteries. Future research should continue to explore
the combination strategy of different solid-state
electrolytes to optimize property, reduce production
costs, and promote the commercialization of all-solid-
state battery technology.
REFERENCES
Tao C, Ouyang B, Fan X, Zhou W, Liu W, Liu W 2022 J.
Carbon Energy 4(2) 170-199
Janek, J, Zeier W.G 2023 J .Nat Energy 8 230–240
Aspinall J, Sada K, Guo H. et al. 2024 J. Nat Commun 15
1-12
Wang L, Li C, Liu X.et al. 2023 J. Journal of Chemical
Engineering of Chinese Universities 37(1) 22-27
Yao Z, Sun Q, Gu Q. et al. 2023 J. Advances in New and
Renewable Energy 11(1) 76-84
Luo Q, Wu J and Li J 2024 J. New Chemical Materials 1-6
Chen S, Yu C, Luo Q. et al. 2023 J. Acta Phys-Chim Sin
39(08) 7-24
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
278

Yu T, Shao Z, Liu Y.et al. 2023 J. Mining and Metallurgy
32(4) 64-71
Chen M 2021 J. Information Recording Material 22(7) 29-
31
Qin Z, Gu Z 2021 J. Chinese Battery Industry 25(6) 329-
335
Zhang G, Zhang Z and Ma Y 2023 J. Acta Chim Sin 81(10)
1387-93.
FAMPRIKIST, CANEPAP, DAWSON JA, et al 2019 J.
Nat Materials 42(1) 163-175
Research Progress on Solid-State Electrolytes for Lithium-Ion Batteries
279
