
Research Progress on the Safety of Lithium Ion Battery Materials
Yuting Dong
1
, Junrui Li
2,*
and Yurui Lu
3
1
School of Chemical Engineering, Northeast Electric Power University, Jilin, 132011, China
2
School of Chemical Engineering, Zhejiang University of Technology, Hangzhou, 310014, China
3
School of Materials Science and Engineering, Hubei University, Wuhan, 430062, China
Keywords: Lithium-Ion Batteries, Battery Safety, Material Design.
Abstract: Lithium-ion batteries have received much attention as one of the key technologies in energy storage
technology. With the development of the new energy field, the energy density carried by lithium-ion batteries
is also increasing, and users are paying more and more attention to battery safety. At present, there have been
many accidents related to lithium-ion battery fire and explosion around the world, some of which have caused
serious threats to human normal life activities and human health. These safety issues are a message to us -
ensuring that the use of batteries is safe is critical, especially before considering high energy density battery
systems for future applications, must address their safety issues. The purpose of this review is to summarize
the root principles of lithium-ion battery safety problems from the three aspects of electrolyte, positive and
negative electrode, and focus on the latest progress in the field of material design, aiming to further enhance
people's understanding of battery safety and further develop the battery market in the future through this
article.
1 INTRODUCTION
In recent years, in response to the scientific concept
of green environmental protection, lithium-ion
batteries (LIB) have been applied on a large scale in
many industries because of their advantages such as
high unit energy, fast discharge efficiency and long
working life (Tarascon and Armand, 2001;
Goodenough and Kim, 2010 & Armand and Tarascon,
2008). Typically, a lithium-ion battery consists of a
positive electrode, an anode, and a diaphragm. The
positive electrode is usually made of lithium cobaltate
(LiCoO2), the anode is made of graphite, and the
diaphragm is made of polymer materials such as
polypropylene (PP) and polyethylene (PE). The
electrolyte consists of LiPF6, ethylene glycol
carbonate (EC), consists of at least one phosphate
acid salt and one or more flame retardant additives.
The safety of the battery is an important
consideration, and although it performs well under
normal conditions of use, safety hazards such as
overcharging, short circuiting, and high temperatures
may occur under conditions of abuse (Balakrishnan et
al., 2006; Wang et al., 2012; Wen et al., 2012;
Bandhauer et al., 2011 & Doughty and Roth, 2012).
In order to ensure that the battery can run safely
and smoothly, it can usually be protected by two ideas:
external physical detection and internal micro-
regulation. External protection mainly relies on small
physical electronic monitoring equipment such as gas
sensors and pressure detectors, although these devices
are designed and manufactured as small and fine as
possible, but most sensors still need to be equipped
with specific functional devices (such as heating disks,
etc.), and the demand for these components also
increases the space occupied by the matched battery
and its weight. The out-of-control environment of hot
pressing often increases the risk of battery safety
accidents. The internal protection scheme is mainly
through design or reaction modification to provide an
intrinsically safe and structurally reasonable material
for the manufacture of battery components, and this
scheme is also considered to be the "ultimate"
solution to ensure battery safety. The purpose of this
review is to summarize the existing safety issues of
lithium-ion batteries and to introduce the design of
relevant safety materials from three aspects: cathode,
anode and electrolyte, in order to improve the safety
of lithium-ion batteries and to promote the future
development and practical application of lithium-ion
battery material safety.
256
Dong, Y., Li, J. and Lu, Y.
Research Progress on the Safety of Lithium Ion Battery Materials.
DOI: 10.5220/0013910800004914
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 2nd International Conference on Renewable Energy and Ecosystem (ICREE 2024), pages 256-261
ISBN: 978-989-758-776-4
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

2 REASONS FOR SAFETY
PROBLEMS WITH LITHIUM-
ION BATTERIES
The organic liquid electrolyte in the lithium-ion
battery is inherently flammable, which also leads to
the easy loss of control of the battery under high heat
and pressure environment, which is one of the biggest
difficulties facing the safety of the lithium-ion battery
system. Therefore, fully understanding the causes and
processes of lithium-ion batteries in high
heat/pressure environment out of control, targeted
design of the functional materials of each part of the
battery, can better improve the safety and reliability
of lithium-ion batteries. For lithium-ion battery
thermal runaway inducement, can be roughly divided
into three categories: collision runaway, electrical
runaway, thermal runaway.
Collision runaway is due to the partial rupture of
the diaphragm caused by mechanical deformation of
the battery such as collision, extrusion, acupuncture,
etc. Electrical runaway is mainly due to the
penetration of the diaphragm during the battery
charging and discharging process, and thermal
runaway is due to the high-temperature environment
caused by overheating resulting in a large area
collapse of the diaphragm, and the result of the
destruction of the diaphragm caused by these three
inductions will eventually lead to an internal short
circuit inside the battery. A series of safety problems
caused by excessive local current. Usually different
incentives caused by the battery out of control, the
phenomenon will have a certain difference, but the
mechanism is similar.
3 ELECTROLYTE
The safety of lithium-ion batteries depends largely on
the characteristics of their electrolytes. The
electrolyte acts as a channel for the transport of
lithium ions within the battery and is usually
composed of organic solvents and lithium salts. The
safe and efficient operation of batteries often requires
electrolytes with high ionic conductivity, wide
electrochemical Windows, high safety and low cost.
However, the safety problem of electrolyte
flammability is a major difficulty faced by safety
performance.
At present, many researchers mainly use flame
retardant additives to reduce the problem of
electrolyte flammability. These additives are mainly
based on organophosphorus compounds or organic
halogenated compounds. However, most of these
additives have structural instability, low toxic
halogens in the ingredients may cause environmental
pollution and other problems, in order to avoid the
above problems, organophosphorus compounds
because of its efficient flame retardancy and
environmental friendliness and attention, become the
first choice of flame retardancy additives. The
mechanism of action of these phosphorous compound
flame retardants is usually to inhibit the transfer of
free radicals during the combustion process. At high
temperatures during combustion, phosphorous
compounds produce phosphorous free radicals due to
decomposition reactions, and such substances can
inhibit or even terminate the free radicals responsible
for continuous combustion generated during the
propagation of the chain reaction (Granzow, 1978).
However, phosphorus-containing flame retardants
can effectively reduce the flammability of the
electrolyte, but also have a certain impact on the
working performance of the battery. In view of the
adverse effects of such additives, some scientists have
proposed to modify the microscopic molecular
structure of phosphorous compounds as an idea and
successfully put into practice a variety of schemes:
fluorination of phosphoric acid compounds
containing alkyl with fluoride to obtain additives with
significantly improved stability and flame retardant
effect (Pires et al., 2015). Choose organic molecules
with excellent flame retardant properties and at the
same time have a protective shell (films), such as
dimethylallylphosphonate, where allyl polymerizes
on the graphite surface and forms a stable SEI film,
effectively preventing harmful side reactions (Jin et
al., 2013). The use of cyclic phosphazene instead of
organophosphorus additives (such as fluorinated
cyclophosphazene) enhances its electrochemical
compatibility (Xia et al., 2015). In addition,
researchers have proposed the preparation of a new
heat-triggered fiber protective diaphragm, flame
retardants can be polymerized as raw materials to
form a protective shell, and these shells are further
stacked to form a diaphragm through the polymer
processing process, so as to achieve the purpose of
effective flame retardant (Figure 1).
4 CATHODE MATERIAL
Cathode materials are a key component of lithium-ion
batteries and require a number of properties to ensure
battery performance and safety. These characteristics
include high capacity, stable structure, stable voltage,
good cycling performance and easy preparation.
Research Progress on the Safety of Lithium Ion Battery Materials
257
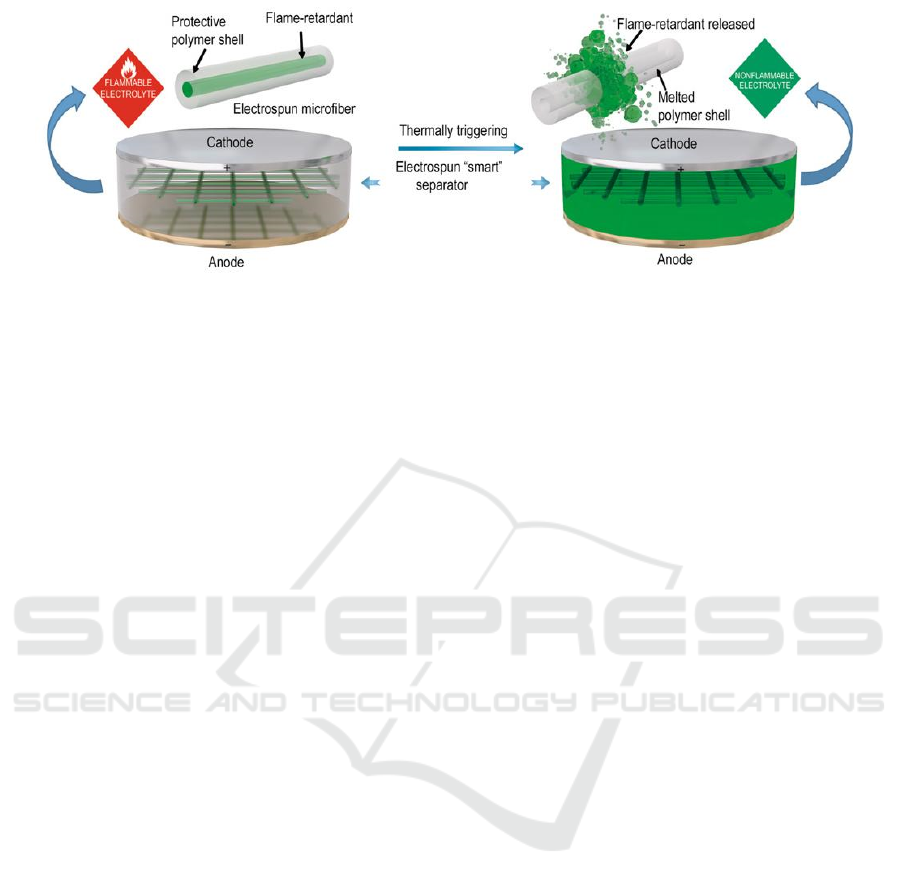
Figure 1: Schematic diagram of the "smart" electrospinning diaphragm of a lithium-ion battery with heat-triggered flame
retardant properties (Liu et al., 2017).
The operation of cathode materials directly affects the
safety performance of the entire battery. There are
very many choices of lithium-ion battery cathode
materials in the current market, among which lithium
cobalt oxide is the first choice for very many
electronic products, while lithium nickel cobalt
manganese oxide is the leading emerging material,
and lithium nickel cobalt aluminate and lithium iron
phosphate are also materials that can't be ignored
(Zhang et al., 2024). Among them, lithium nickel
cobalt manganese oxide ternary cathode materials
(LiNixCoyMn1-x-yO2, abbreviated as NMC) have
become the research hotspot of mainstream cathode
materials for lithium-ion batteries due to their high
specific capacity.
NMC cathode materials may face the following
problems during the cycling process: (1) lithium-
nickel mixing affects the normal de-embedding of
lithium ions, resulting in capacity loss and lower
diffusion coefficients of lithium ions, which affects
the performance of the batteries; (2) the
polycrystalline secondary particles of NMC are
susceptible to cracking and fragmentation during the
cycling process, especially at high temperatures or
high pressures; and (3) the Surface side reactions may
lead to cell blistering or explosion.
In response to the above problems, some existing
commonly used NMC material modification methods
include: (1) preparation of small-particle single
crystals to avoid inter-crystalline cracks and
fragmentation, and to improve structural stability and
cycling performance. For example, by preparing
agglomerated and single-crystal
LiNi0.8Co0.1Mn0.1O2, the capacity of 0.1C is
216.3mAh/g and 213.7mAh/g at 3.0-4.3V,
respectively. The thermal decomposition temperature
of agglomerated ternary cathode materials in the
charging state of 4.3V is 208℃, while the single-
crystal type is 216℃ and the amount of heat release
is lower, so the thermal stability is better. By
changing the crystal morphology of the material, the
monocrystalline structure can enhance the structural
stability while ensuring the capacity, improve the
cycling performance and increase the thermal
decomposition temperature, thus enhancing the safety
of the battery (Zhao, 2020). (2) Elemental doping, the
introduction of metallic or non-metallic elements to
reduce cation mixing, enhance the structural stability
and lithium ion diffusion efficiency, such as Wang et
al. synthesized two-dimensional porous B, N co-
doped carbon/titanium nitride (BNC-TN) composites
by hydrogel and ionothermal methods (Wang, 2023),
which can be clearly seen that its elemental doping
has been improved. Using this method can effectively
improve the reduction of diffusion coefficient due to
lithium-nickel mixing, and ensure that its surface side
reactions are reduced to improve its safety. (3)
Surface coating, utilizing stable materials to isolate
cathode materials from electrolyte contact and
improve cycle stability and thermal safety. By
wrapping other highly stable materials, such as
phosphate, fluoride, solid oxide, etc., outside the
ternary cathode materials, the ternary cathode
materials are isolated from direct contact with the
electrolyte due to the good thermal and structural
stability of the wrapped materials (Tian et al., 2023).
From the experiments of Li et al. who used poly-3-
octylthiophene as a wrapping material for surface
modification of the cathode material, it is evident that
this approach has significantly improved its cycling
stability and thermal safety (Li, 2023).
In general, in order to solve the safety problems of
mixed rows, fragmentation and side reactions in
NMC cathode materials, small-particle single crystals
are generally used to avoid electrode fragmentation,
and elemental doping and surface coating methods
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
258
are used to reduce the generation of lithium-nickel ion
mixed rows and side reactions, so as to improve the
electrochemical performance and service life of the
overall battery, as well as safety and stability in
multiple cycles.
5 ANODE MATERIAL
Early lithium-ion batteries were made directly from
lithium metal, but dendrite lithium was often present
in the charge and discharge process (Figure 3),
causing extremely serious safety hazards. The advent
of rocking chair batteries solved this challenge by
successfully bypassing dendrite lithium, and I use a
layered approach here to solve the lithium-ion storage
problem (Wang et al., 2018). At the same time, when
the electrolyte comes into contact with the carbon
material, it becomes a very tight and connected layer,
called the passivation layer or the solid electrolyte
interface, which allows the lithium ions to move in a
certain space and prevents the movement of electrons
in this space. Although the formation of passivation
film will lead to irreversible loss of stored energy
during the first charge and discharge process, it is
caused by the improvement of battery charging
efficiency (Nitta et al., 2015). Therefore, layered
materials and passivation films become an important
part of battery anode safety. At the same time, adverse
reactions such as the formation of lithium dendrites
are the main safety hazards. In order to improve the
safety performance, the negative electrode material
needs to have the characteristics of high efficiency,
small volume effect, large reversible capacity, high
electrolyte sensitivity, low thermal stability and low
impurity content.
The crystallization of the anode material will lead
to an increase in the sensitivity to the electrolyte,
resulting in a significant decline in performance and
cycle stability, and a potential safety hazard. Our
purpose is obviously to improve the anode material,
so we should discuss the changes of lithium dendrites
and solid-liquid films.
The change of solid-liquid film (SEI) thickness is
closely related to the attenuation of battery capacity
and battery safety, and its decomposition is also one
of the important factors affecting battery safety. The
main reasons leading to the generation of lithium
dendrites are low temperature (Wang, 2017 &
Hossain et al., 2006), overcharge (Ohsaki et al., 2005;
Dietz et al., 2018 & Li et al., 2001) and high rate
charge (Marcicki et al., 2014 & Li et al., 2019). The
formation of lithium dendrites may also be caused by
an energy imbalance (Gallagher et al., 2015). For all
anode materials, high performance generally means
lower risk. For example, compared with graphite, the
soft carbon material formed by synthesis has better
Figure 2: Formation process of lithium ion dendrites (Dhanya et al., 2021).
Research Progress on the Safety of Lithium Ion Battery Materials
259

conductivity at low temperatures and amplification
performance under normal use (Broussely et al.,
2005). This is mainly due to the low degree of
graphitization of soft carbon, which is usually
composed of extremely small graphite nanocrystals,
thus shortening the diffusion path of lithium (Persson
et al., 2010). Under the same overcharge conditions,
it was found that less lithium metal was deposited on
the carbon surface than on the graphite surface. This
may be related to the complete disordered structure of
the carbon and the low efficiency of the first cycle of
carbon when only a small amount of graphite is
deposited. Therefore, mixing hard carbon with
graphite has been proposed by some scholars as a way
to slow down the formation of lithium ion dendrites.
However, this approach is often unsuccessful for
several reasons (Liu et al., 2017).
In general, since we want to solve some problems
of lithium-ion batteries and increase the safety of
lithium-ion batteries, the following methods can be
used. First of all, the layered structure of the rocking
chair battery can effectively prevent the generation of
lithium dendrites, but in practical applications, the
layered structure is difficult to achieve. It is also
possible to reduce the formation of lithium dendrites
by changing the anode material to prevent the
formation of lithium dendrites, thereby improving
safety.
6 CONCLUSION
Safety is the key criterion to evaluate the commercial
application of lithium-ion batteries, which directly
affects the future development and application range
of lithium-ion batteries. With the increasing demand
for battery energy density and rapid charge and
discharge, the design requirements for battery
materials are also more stringent. To date, researchers
have proposed a variety of modified material
strategies to solve the dilemma faced by battery
safety. This review summarizes the design of related
safety materials from the positive electrode, anode
and electrolyte. Through research efforts to reduce
the flammability of electrolytes, such as the use of
environmentally friendly phosphorus-containing
flame retardants or structural adjustments to improve
electrochemical performance; The cathode material
of the battery was improved by preparing small
particle single crystal, element doping and surface
coating to improve the safety performance and cycle
stability of the battery. By studying the layer structure
of rocking chair battery to store lithium ions to avoid
the safety problems caused by negative dendrite
lithium, improve the anode material, control the
change of solid-liquid film thickness and
decomposition to enhance the overall battery
performance.
AUTHORS CONTRIBUTION
All the authors contributed equally and their names
were listed in alphabetical order.
REFERENCES
Tarascon J M and Armand M 2001 Nature 414 359-67
Goodenough J and Kim Y 2010 Chem. Mater. 22 587-603
Armand M and Tarascon J M 2008 Nature 451 652-7
Balakrishnan P G, Ramesh R and Kumar T P 2006 J. Power
Sources 155 401-14
Wang Q, Ping P, Zhao X, Chu G, Sun J and Chen C 2012
J. Power Sources 208 210-24.
Wen J, Yu Y andn Chen C 2012 Mater. Express 2 197-212
Bandhauer T M, Garimella S and Fuller T F 2011 J.
Electrochem. Soc. 158 1-25
Doughty D H and Roth E P 2012 Electrochem. Soc.
Interface 21 37-44
Granzow A 1978 Acc. Chem. Res. 11 77-183
Pires J et al. 2015 J. Power Sources 296 413-25
Jin Z, Gao H, Kong C, Zhan H and Li Z 2013 ECS
Electrochem. Lett. 2 66-8
Xia L, Xia Y and Liu Z 2015 J. Power Sources 278 190–6
Liu K, Liu W, Qiu Y C, Kong B, Sun Y, Chen Z, Zhuo D,
Lin D and Cui Y 2017 Sci. Adv. 3
Zhang Z Y, Bao X J and Xie S Z 2024 J. Industrial
Minerals & Processing 1 16
Zhao M T 2020 J. Shandong Chemical Industry 49(22)
138-44
Wang C C 2023 (Wuhan: Wuhan Institute of Technology)
Tian G L, Li J and Wu Y K 2023 J. Battery Bimonthly 53(3)
347-51
Li T F 2023 (Changsha: Central South University)
Wang A, Kadam S and Li H 2018 NPJ. Comput Mater 4(1)
15
Nitta N, Wu F X, Lee J T and Yushin G 2015 Mater Today
18(5) 252-64
Wang T, Liu X, Zhao D and Jiang Z 2004 Chem. Lett. Phys.
389(4–6) 327-31
Wang K 2017 J. Open Access Library 4(11) 1-12
Hossain S, Kim Y, Saleh Y and Loutfy R 2006 J. Power
Sources 161(1) 640-7
Ohsaki T, Kishi T and Kuboki T et al 2005 J. Power
Sources 146(1–2) 97-100
Dietz R, Bare O and Li J 2018 J. Power Sources 385 148-
55
Li J, Murphy E, Winnick J and Kohl P A 2001 J. Power
Sources 102(1) 302-9
Marcicki J, Conlisk A T and Rizzoni G 2014 J. Power
Sources 251 157-69
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
260

Li H, Zhang W and Yang X 2019 Electrochim Acta 326
134966
Gallagher K, Trask S, Bauer C 2015 J. Electrochem Soc.
163(2) A138-49
Broussely M, Biensan P and Bonhomme F 2005 J. Power
Sources 146(1–2) 90-6
Persson K, Sethuraman V and Hardwick L 2010 J. Chem.
Lett. Phys. 1(8) 1176-80
Liu H, Liu X and Li W 2017 Adv. Energy Mater 7(24) 24
Dhanya P, Malik W and Satishchandra O 2021 Energy &
Fuels 35
Research Progress on the Safety of Lithium Ion Battery Materials
261
