
Enhancing Catalyst Performance in Fuel Cells: Challenges and
Innovations
Nijie Fan
Inner Mongolia Normal University, Hohhot 010022, China
Keywords: Platinum-Based Catalysts, Proton Exchange Membrane Fuel Cells, Non-Metal Catalysts.
Abstract: The research of this study is to explore methods and innovations to improve the performance of fuel cell
catalysts. The oxygen reduction reaction (ORR) in the fuel cell is a critical step. Traditionally, platinum-based
catalysts were the best choice. However, their high cost limits the wide application. Therefore, this research
aims to find more cost-effective alternatives to improve the viability of fuel cells. This study divided
electrocatalytic materials into two parts which are platinum-based catalysts and non-noble catalysts for further
compare the different characteristics of various catalysts, including platinum-based catalysts, transition metals
oxides, transition metals nanomaterials, and single-atom catalysts. At the same time, the advantages and
disadvantages of electrocatalytic materials and non-metallic materials are compared, and the methods that
describe how to solve the research problems are proposed. This research also compares platinum-based
catalysts with transition metal catalysts and compares transition nanomaterials with traditional nanomaterials
which have different characteristics. In terms of environmental protection and sustainable development, non-
noble catalysts are more available and environmentally friendly than precious metal catalysts with complex
mining techniques. In the future, non-metal catalysts will have a wide range of commercial potential in various
applications and are expected to replace electrocatalytic materials.
1 INTRODUCTION
Proton Exchange Membrane Fuel Cells (PEMFCs)
are emission-free energy converters for various
applications, including stationary devices. They
convert chemical energy from hydrogen oxidation
and ORR into electrical power. These two reactions
also need the catalyst. However, hydrogen oxidation
and oxygen reduction need a catalyst, especially the
ORR which has naturally slow kinetics in PEMFCs
and requires electrocatalysts.
Platinum-based catalysts have been the preferred
choice owing to their superior efficiency in
facilitating ORR (Bai et al., 2022), with Pt-C catalysts
renowned for their exceptional electron and proton
mobility (Li et al., 2023). However, the exorbitant
cost associated with platinum has hindered its
widespread adoption, prompting a quest for more
cost-effective alternatives to enhance the feasibility
of PEMFCs
In response to this challenge, researchers have
turned their attention towards exploring alternatives
to platinum-group metals (PGMs) that offer stable
cell performance at a reduced cost (Snitkoff et al.,
2021). Among these alternatives, first-row transition
metals have emerged as promising candidates for
metal-based catalysts, primarily due to their
affordability relative to platinum.
In this study, we compare many styles of
catalysts and their characteristics (eg: platinum-based
catalyst, transition metals oxides catalyst, transition
metals nanomaterials, and Single atom catalyst.
Additionally, researchers have begun incorporating
heteroatoms into catalysts to enhance their
performance and transition from platinum to first-row
transition metals as cost-effective alternatives.
2 ELECTROCATALYTIC
MATERIAL
Due to the expensive cost of platinum-based
catalysts, the wide application of platinum-based
catalysts in fuel cells is limited, so many researchers
aim to find more cost-effective catalysts. Compare
the characteristics of platinum-based catalysts with
non-noble metals
The studies gradually add heteroatoms into the
electrocatalyst to improve the activities of the
Fan, N.
Enhancing Catalyst Performance in Fuel Cells: Challenges and Innovations.
DOI: 10.5220/0013906300004914
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 2nd International Conference on Renewable Energy and Ecosystem (ICREE 2024), pages 241-245
ISBN: 978-989-758-776-4
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
241

catalysts. At the same time, researchers switch the
catalysts from platinum-based catalysts to the less
costly catalysts transition metal catalysts.
2.1 Platinum-Based Catalyst
Recent studies revealed that Platinum-based catalysts
are becoming the most common catalyst in PEMFCs,
especially in oxygen reduction reactions. Based on
recent studies, Platinum-based catalysts have
different diameters. Platinum-based catalyst activity
became worse when the size of platinum
nanoparticles increased. When the diameter of the
platinum nanoparticles-based catalyst is 3nm, the
activity of the platinum nanoparticles-based catalyst
becomes maximum (Chen et al., 2022). The high
temperature also has a certain degree of influence on
the platinum-based catalyst. The interaction between
platinum and sulfur atoms doped in a carbon matrix
can inhibit the sintering of nanoparticles at 1000℃ so
that the average particle size of platinum-based
nanoparticles can be controlled within 5 nm at high
temperatures which can maintain the activity of
platinum-based catalyst (Han et al., 2022).
Firstly, silicon and lithium can form a variety of
alloys, such as Li
12
Si
7
, Li
13
Si
4
, Li
7
Si
3
, Li
15
Si
4
, Li
22
Si
5
,
etc., which not only have high capacity, but also have
de-embedded lithium potentials lower than 0.5 V vs.
Li/Li
+
, with Li
22
Si
5
having a capacity of up to 4200
mAh/g.
In addition to Si-Li alloys, there are other silicon-
based alloy materials. Mukanova et al. (2017)
achieved a facet capacity of 80 mAh/cm² by forming
a three-dimensional composite anode with silicon
thin films on graphene-coated nickel foam by
chemical vapour deposition and magnetron
sputtering. Ding et al. (2020) prepared a binder-free
anode with bilayer graphene-coated silicon
nanoparticles (SiNPs) embedded in the porous nickel
current collector, and although a respectable face
capacity was achieved, the performance of the
interfacial layer still needs to be improved. In view of
this, Tzeng et al. (2023) proposed thick and porous
silicon-based anodes, and constructed porous anodes
with electrical conductivity on nickel foam by mixing
SiNPs, phenolic resin binder, and the conductive
agent Super P, which exhibited excellent cycling
performance and charge storage capacity.
Experiments shown that the 80 nm nickel foam
nanoporous silicon-based anode maintained excellent
performance after a total of 50 cycles, where the
current density was 4 mA/cm
2
, retaining a retained
area capacity of up to 6.5 mAh/cm², which provided
a charge storage capacity of 23.4 C for an anode area
of 1 cm² at a current rate of 4 mA.
In particular, the eutectic reaction takes place
throughout the melting procedure because the melting
points of Cu and Si are slightly different. This makes
it simpler for Si to create the Cu
3
Si alloying phase and
evenly disperse it on the Si matrix resulting in greater
suppression of the volume growth. Zhang et al.
(2021) first prepared Si-Cu
3
Si composite with a
capacity of 1000 mAh/g after 300 cycles, while Li et
al. (2023) prepared P-doped Si-Cu alloys by the
vacuum melting method, which improve the electrical
conductivity and lower the Li
+
diffusion barriers to
significantly enhance the electrochemical
performance, Among these, the P
0.5%
Si-Cu alloy had
a significantly lower R
ct
value than the undoped Si-
Cu alloys, with a capacity of 1048 mAh/g after 60
cycles.
In addition, Si-Sn alloy anode has sparked much
interest because of its unique lithium embedding
ability and buffering effect on volume change. Tian
et al. (2021) successfully prepared a novel anode
material consisting of tin nanowires (SnNWs)
embedded with SiNPs by solid-gas reaction method,
which exhibited a high and stable capacity at both
room and low temperatures.
Although silicon alloy structure has significant
effect in solving the swelling problem of silicon-
based anode, its preparation process is complicated
and costly, which limits its mass production
application. Therefore, researchers have turned to the
strategy of compositing nanosilicon and carbon
materials to seek performance enhancement and cost
reduction.
2.2 Non-Noble Metal
Compared to platinum-based which belongs to
precious metals, non-noble metals are abundant on
Earth. These allow non-noble metal catalysts to have
a lower cost compared with platinum-based catalysts
wide appliance in the production process, which is
conducive to large-scale production and application.
In terms of environmental protection and sustainable
development, the process of precious metals may
cause pollution to the environment, while non-noble
metals are relatively environmentally friendly.
The activities of catalysts of non-noble metal
catalysts can be adjusted by changing their
composition and structure. This makes non-noble
metal catalysts have a wide range of applications and
prospects.
The researchers used the comparative study
method. The activities of catalysts of platinum-based
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
242
catalysts and transition metal catalysts were
compared. At the same time, transition metal
nanomaterials and traditional nanomaterials were
also compared. The studies divided the single-atom
catalyst into five categories based on the difference in
the supporting materials.
2.2.1 Transition Metals Oxides
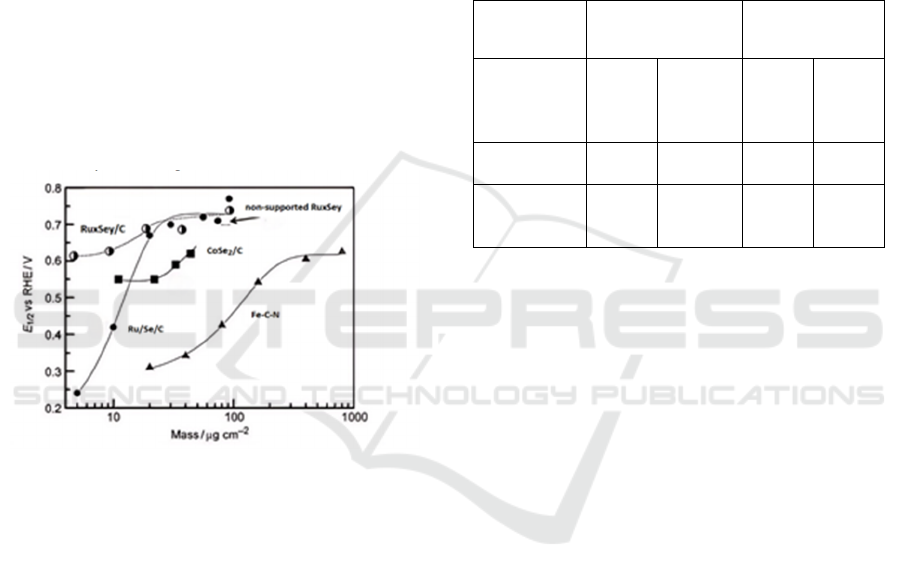
Chevrel-phase-type compounds unveil Ruthenium-
centered catalysts as viable non-noble metal
alternatives for ORR in acidic environments (Vante,
2010). Ruthenium-based catalysts employed in these
reactions yield H
2
O
2
, a trend observed to decrease
with increasing electrocatalyst loading. Studies
indicate that metal center activities, at equivalent
loadings, follow the sequence Fe < Co < Ru < Pt, as
depicted in Figure 1. Further analysis, correlating
oxygen-binding energies with RRDE experiments
measuring H
2
O
2
, underscores the pivotal role of
electrocatalyst loading (Vante, 2010).
Figure 1: The curve of E1/2 with Mass. Chalcogenides:
non-supported RuxSey (1), RuxSey/C (2), and Ru/Se/C
(3); CoSe2/C (4); and Fe-C-N (5) (Vante, 2010).
In alkaline environments, corrosion-resistant non-
noble metals such as Fe, Co, and Mn exhibit notable
activity in oxygen reduction reactions. Graphene
oxide catalysts based on non-noble metals feature
stable spatial crystal structures and high corrosion
resistance, contributing to their favorable activity in
these reactions (Tao et al., 2024). The catalytic
activity of Mn oxide catalysts varies with different
valence states. Zhang et al. (2020) observed enhanced
catalyst activity during the high-potential or
hypervalent state of Mn.
In addition to the advantages of transition metal
oxides in acidic and alkaline media, there are these
three characteristics. The first characteristic is the
hydroxyl groups inserted on the surface of transition
metal oxides can be further functionalized. The
second is to maintain crystal structure and prevent
metal particles from agglomeration. The last
characteristic is Transition metal oxides have better
alkali corrosion resistance than carbon-based
materials in precious metals. For instance, Meng Sun
and co-workers found graphene/graphene oxide
supported single transition metal oxides show good
oxygen reduction reaction performance and long-
term durability. The reason is the rich functional
hydroxyl groups, stable spatial crystal structure, and
good alkali corrosion resistance (Sun et al, 2015).
Table1: Compares the Pt/C catalyst with Fe3/NG-800 in a
different electrolyte by onset and half-wave potential.
0.1M KOH 0.1M KClO4
Onset
potent
ial
Half-
wave
p
otential
Onset
potent
ial
Half-
wave
potent
ial
Pt/C (Yan et
al., 2023
)
1.02V 0.85V 0.95V 0.82V
Fe3/NG-800
(Xiong et
al., 2022) 1.03V 0.86V 0.92V 0.77V
2.2.2 Transition Metals Nanomaterial
Compared to conventional nanomaterials, the carbon
material Vulcan XC-72 stands out as one of the most
popular choices (Vante, 2010). It's well-known that
Pt/C catalysts exhibit low activity and carbon
instability, resulting in slow oxygen reduction rates.
To enhance activity, scientists have turned to
transition metal nanomaterials, which are cost-
effective and readily available. Through pyrolysis and
etching techniques, researchers synthesized Fe3/NG
nanoparticles. Notably, the catalyst Fe3/NG-800
displays heightened activity at 800°C compared to
other temperatures. Figure 2 illustrates the onset
potential and half-wave potential of Fe3/NG-800
versus Pt/C (Xiong er al., 2022).
2.2.3 Single-Atom Catalysts
Unlike traditional catalysts and nanomaterial-based
catalysts, where metal particle activity is typically
concentrated at corners or edges, single-atom
catalysts (SACs), as outlined by Zhang Tao’s team,
significantly reduce the energy barrier and exhibit
more than an order of magnitude greater activity (Su
et al., 2021).
Single-atom catalysts have witnessed rapid
development in recent years, with a myriad of styles
Enhancing Catalyst Performance in Fuel Cells: Challenges and Innovations
243
emerging over the past decade. Researchers have
categorized them into five distinct types based on the
support material: single metal atoms anchored on
metals, metal compounds, non-metallic carbon-based
supports, MOFs, and zeolites.
The activities of single-atom catalysis can be
changed with the enhancement of coordination. In
order to increase the activities of single-atom
catalysis, the operator should carefully select the
appropriate support while performing appropriate
metal-carrier interactions (Li et al., 2019). For
instance, Hutching and co-workers found that there
are different activities of vinyl chloride monomers in
Au single-atom catalysts. The reason is that the ratio
of Au(I): Au(III) is caused by the different Au-Cl
coordination (Malta et al., 2017).
Recent studies have demonstrated that single-
atom catalysts can substantially enhance catalytic
activity by achieving maximal atomic utilization and
revealing ample active sites. Moreover, single-atom
catalysts exhibit high selectivity, as evidenced by the
comparison between desk current and ring current,
showcasing reduced desk current and increased ring
current. For instance, Yang and co-worker
successfully used a single platinum atom catalyst
which fixed on nanometer titanium nitride was
prepared by using chlorine ligands. According to
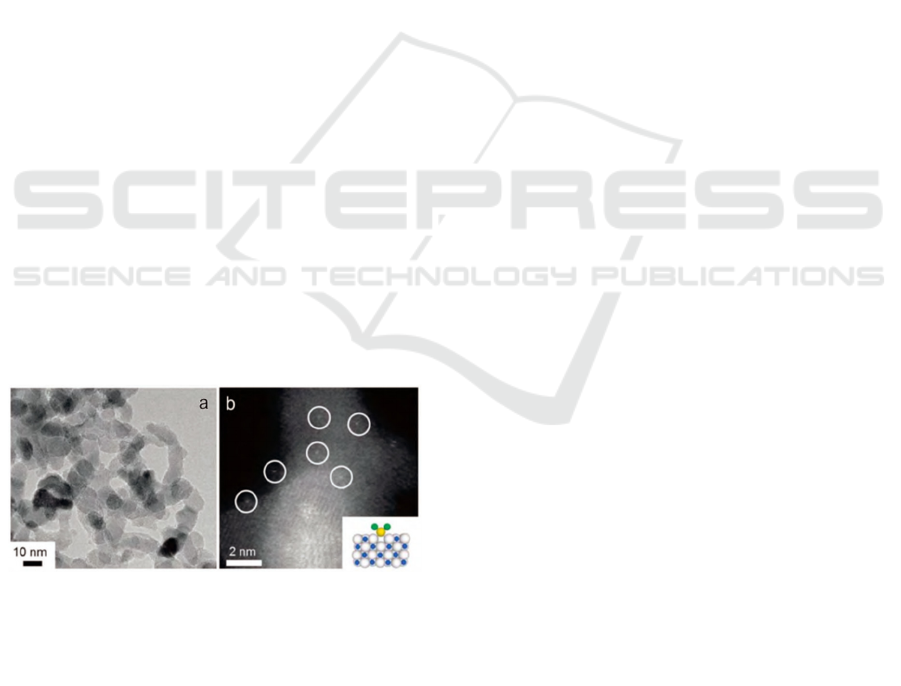
Figure 3. (a) which reveals high selectivity,
transmission electron microscopy images showed
that only TiN nanoparticles were present in 0.35wt%
of the platinum-TiN sample, and no platinum
nanoparticles were observed. However, Figure 3. (b)
is the HAPtDF-STEM image, where the white dots
are platinum nanoparticles.
Figure 2: (a) Transmission electron microscope image. (b)
HAPtDF-STEM image reveals 0.35 wt% Pt/TiN (Su et al.,
2021).
3 CONCLUSION
According to a comparison of electrocatalytic
material and non-metal material, they have different
advantages and disadvantages. As an electrocatalyst
material, platinum-based catalysts have high to be a
barrier to wide use.
In contrast, non-metal materials like Ru hold
promise for replacing Pt in oxygen reduction
reactions in fuel cells under acidic conditions.
Transition metal oxides exhibit high activity, ranking
second only to platinum-based catalysts. Notably, Mn
displays exceptional activity in alkaline environments
due to its unique structure and varying valence.
Transition metals nanomaterials, a subset of non-
metal materials, offer affordability and accessibility
compared to traditional electrocatalytic materials.
While traditional catalysts may lack sufficient
activity and durability in real-world applications,
single-atom catalysts show promise in overcoming
these limitations during oxygen reduction reactions.
However, their practical application remains limited.
Looking ahead, non-metal material catalysts hold
potential for widespread commercial use, potentially
replacing electrocatalytic materials in various
applications. Moreover, the incorporation of rare
earth elements into Proton Exchange Membrane Fuel
Cells can enhance their localized characteristics,
paving the way for further advancements in the field.
REFERENCES
Botao Qiao, Aiqin Wang, Xiaofeng Yang, Lawrence F.
Allard, Zheng Jiang, Yitiao Cui, Jingyue Liu, Jun Li
and Tao Zhang 2011 Nature Chemistry 17(2) 1441-49
Cui Chen, Liming Zhou, Ming Wen, Hao Cui, Manmen
Liu, Shaohong Liu 2022 Precious, Metals 43(4) 69-76
Grazia Malta, Simon A Kondrat, Simon J Freakley,
Catherine J Davies, Li Lu, Simon Dawson, Adam
Thetford, Emma K Gibson, David J Morgan, Wilm
Jones, Peter Wells, Peter Johnaton, C. Richard
A.Catlow, Christopher J. Kiely and Graham J.
Hutchings 2017 Science 355 1399
Hongliang Li, Mengling Wang, Laihao Luo and Jie Zeng
2019 Adv. Sci. 6 1801471
Jianan Su, Linzhou Zhuang, Shusheng Zhang, Qingju Liu,
Longzhou Zhang and Guangzhi Hu, 2021 Chin. Chem.
Lett. 32 2947-62
Jingsen Bai, Liting Yang, Zhao Jin, Junjie Ge and Wei Xing
2022 Chin. J. Catal. 43 6 1444-58
Liwei Xiong, Yukang Fu, Yongxin Luo, Youshan Wei, Ze
Zhang, Chaoguo Wu, Sicheng Luo, Gang Wang, David
Sawtell, Kefeng Xie, Tao Wu, Dong Ding and Liang
Huang 2022 J. Mater. Res. 37 2109–2123
Long Huang, Haichao XU, Bi Xing, Qiuxia Li, Wei Yi, and
Shigang Sun 2022 Electrochem, 28(1) 2108061
Meng Sun, Huijuan Liu, Yang Liu, Jiuhui Qu and Jinghong
Li 2015 Nanoscale 7(4) 1250-69
Min Li , Feng Liu , Supeng Pei , Zongshang Zhou , Kai Niu
, Jianbo Wu and Yongming Zhang, 2023
Nanomaterials 2023 13(3) 444
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
244

Nicolas Alonso-Vante 2010 CHEMPHYSCHEM 10.1002
Riflael Snitkoff ,Ariel Friedman ,Yan Yurko ,Piotr Zelenay
,Alan Bond and Lior Elbaz 2021, Reaearch Square
Rui Han, Qingchun Wang, Xiaona Zhou, Xiaolong Liang,
and Fenglong Zhang 2022, INDUSTRIAL CATALYSTS
10 9-15
Wei Yan, Xuan Wang, Mannan Liu, Kaiyue Ma, Liqi Wang,
Qicheng Liu, Caikang Wang, Xian Jiang, Hao Li,
Yawen Tang and Gentao Fu 2023 Adv. Funct. Mater.
34 2310487
Yong-Chao Zhang, Sana Ullah, Rongrong Zhang, Lun Pan,
Xiangwen Zhang and Ji-Jun Zou.2020 Appl. Catal., B
277 119247
Zeyu Tao, Hangcheng Liu, Jianfeng Liu and Zhengrong Shi
2024 BATTERY BIMONTHLY 54(1), 103-6
Enhancing Catalyst Performance in Fuel Cells: Challenges and Innovations
245
