
Modifying Electrodes to Enhance the Energy Density of Vanadium
Redox Flow Batteries
Kaixin Hu
1
, Zixiang Xia
2
and Kuangdi Zhu
3,*
1
No.2 High School of East China Normal University, Shanghai, 201203, China
2
Soochow University, Suzhou, 215006, China
3
University of British Columbia (Vancouver), Vancouver, V6T 1Z4, Canada
Keywords: Vanadium Redox Flow Battery, Electrocatalyst, Electrode Material, Energy Efficiency.
Abstract: Redox flow battery (RFB) as a promised way of large-scale energy storage has been getting more attention
recently, due to the gradually growth demand for electricity. Vanadium flow battery (VFB), an RFB
containing vanadium ions, has been proven to be a candidate of interest for industrial applications. They have
the advantages of relatively high safety, low costs and high durability against other RFBs. However, the high
costs and low energy density prohibit the application of VFBs. Promoting energy efficiency is crucial to
address those concerns since higher energy efficiency will lead to a decreased consumption of raw materials
and low energy dissipation. Electrodes, where the redox reactions occur, play a key role in improving energy
efficiency. There have existed a bunch of approaches to modify the performance of electrodes. In this paper,
recent research achievements on electrode modifications including surface modification, structural and
configuration modification, introducing electrocatalyst and electrode material modification will be discussed.
1 INTRODUCTION
To achieve the ambitious goals of reaching "peak
carbon" and attaining carbon neutrality, industries are
increasingly turning to alternative sources of
sustainable resources, including photovoltaics (PV)
and wind power, within the new energy sector (IEA,
2023). According to projections by the International
Energy Agency (IEA), onshore wind and PV are
anticipated to contribute up to one-third of global
electricity generation this year (IEA, 2023). Despite
their growing adoption, these sources of electricity
often encounter challenges related to intermittency
and instability, which can jeopardise the security and
reliability of the power grid.
Traditional batteries have several drawbacks in
these application scenarios. Firstly, they typically rely
on non-renewable energy sources such as fossil fuels
for charging, thus failing to achieve carbon neutrality
goals. Secondly, traditional batteries have relatively
low energy density, which is unable to support the
requirements of extensive energy repository.
Additionally, the lifespan of traditional batteries is
limited, requiring frequent replacement and
maintenance, thereby increasing costs and resource
consumption.
Redox flow batteries (RFBs), composed of two
electrodes, two current collectors, and a separator
(Emmett and Roberts, 2021), offer numerous
advantages including the ability to separate capacity
and power, deep charge and discharge capabilities,
rapid response times, long cycle life, high safety
standards, and adaptable designs. Consequently, they
hold immense potential for large-scale energy storage
solutions aimed at addressing the aforementioned
challenges. However, the widespread implementation
of existing RFB technology is impeded by various
drawbacks, including high operational and capital
costs, low energy density, and stability concerns
(Emmett and Roberts, 2021). Vanadium flow battery
(VFB) as a king of RFB has been obtaining more
interest recently. As the electrolytes in VFB only
contains vanadium compounds, the penetration
phenomena which is another common concern in
RFB, can be minimized.
Among these limitations, energy density and cost
efficiency are particularly crucial (Emmett and
Roberts, 2021). Obtaining a high energy efficiency is
an effective approach to address those problems
mentioned before (Emmett and Roberts, 2021). With
higher energy efficiency, the capital cost and
operation cost can be reduced by lower amount of
reactants and materials usage. Higher energy capacity
224
Hu, K., Xia, Z. and Zhu, K.
Modifying Electrodes to Enhance the Energy Density of Vanadium Redox Flow Batteries.
DOI: 10.5220/0013882300004914
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 2nd International Conference on Renewable Energy and Ecosystem (ICREE 2024), pages 224-228
ISBN: 978-989-758-776-4
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

is achieved by the reduced energy dissipation (Wu et
al., 2023). Electrode, as a major part of the VFB
system, provides the place for redox reaction to
happen and charge to transfer as well. By optimizing
electrode materials and selecting designs with high
activity and stability, the ohmic potential will be
reduced while the conductivity and redox reaction
rate can be enhanced. This can be achieved through
the use of novel nanomaterials or surface
modifications such as coatings or functionalization to
improve electrode catalytic activity, thereby
enhancing battery performance and energy
efficiency. Currently, the mainstream approach for
electrode modification involves improving electrode
materials and structures. However, this requires high
electrode stability, as high potential differences may
cause corrosion or oxidation of electrode materials.
This study provides a synthesized review of recent
improvements in electrode modifications for VFBs,
including modifications to electrocatalysts and
electrode materials and surfaces, aiming to address
challenges like scant energy concentration and
vanadium flow batteries' intrinsic high-expense
through improving power efficiency.
2 REACTION PRINCIPLES AND
STRUCTURES
A typical flow battery consists of a catholyte, an
anolyte, carbon felt electrodes, graphite flow fields, a
membrane, and current collectors, shown as Figure 1.
The catholyte and anolyte are injected into their
separate half-cells. The electrolyte flow is directed
over the carbon-felt electrodes by the graphite flow
fields (Emmett and Roberts, 2021). These electrodes
provide surface area for redox reactions (Emmett and
Roberts, 2021). The separator ensures zero species
crossover and uninterrupted transport of hydrated
hydrogen ions. In the course of the redox reactions,
the anolyte is oxidized by losing electrons. These
electrons flow to the cathode fluid through the
external circuit, where they are accepted and restored
the cathode solution. The figure below shows the
charging and discharge cycle of the pillar oxidation
and reduction fluid battery. Whenever a electron
(hydrogenated ion) moves from anode fluid to
cathode fluid, H
+
cross the membrane to keep the
charge neutral.
Figure 1: Charging and discharging process for a typical
Vanadium flow battery (Sankaralingam et al., 2021)
Electrode materials mainly affect the energy
density of the battery through these aspects:
Conductivity, Surface area, Electrochemical activity
and Corrosion resistance.
Highly conductive electrode materials can reduce
the ohmic loss inside the battery, thereby improving
the efficiency of the battery. The specific area of the
electrode material surface significantly influences the
electrochemical reaction rate. Therefore, as long as
the specific surface area becomes larger, the more
active sites used in electrochemical reactions, which
can accelerate the charge transfer process and
increase the battery's power density and energy
density. The rate and reversibility of electrochemical
reactions are determined by the electrochemical
activity exhibited by electrode materials. Highly
electroactive materials more efficiently catalyse
vanadium ion redox reactions, reduce overpotential,
and improve battery energy efficiency. The
electrolyte used in vanadium flow batteries is usually
highly acidic (such as sulfuric acid), which requires
the electrode material to have good corrosion
resistance to ensure long-term stable operation.
Otherwise, the electrode will corrode and react, and
the battery's energy density will be reduced.
3 IMPROVEMENT METHODS
As stated before, the modification of VFBs focuses
on electrodes and electrolytes. Meanwhile, the
electrode regulates the electrochemical activity of
oxidation and reduced coupling and provides active
sites for the oxidation reaction, this is of great
significance for improving battery performance,
making a critical contribution. Modifications on the
electrode itself and configuration can manipulate the
electrochemical polarizations, concentration and
ohmic which are key factors for VFB performance
evaluation (Wu et al., 2023).
Modifying Electrodes to Enhance the Energy Density of Vanadium Redox Flow Batteries
225

3.1 Surface Modification
There are three important parameters for the
performance evaluation of VFBs. Wettability
indicates the distribution of electrolyte on electrode.
Voltage efficiency (VE) indicates the ratio of input
voltage (charging) and output voltage (discharging).
Energy efficiency (EE) refers to the energy
dissipation during the charging and discharging
process. It is defined as the ratio between the actual
energy output and the theoretical energy output.
These factors determine the performance of VFB. EE
and VE are key to the improvement of VFB’ s
performance. Higher EE will lead to a lower energy
reduction, in other words, the output energy can be
promoted. Similar effect can be expected with an
increased VE. The goal of surface modification is to
maximize all three parameters - wettability, voltage
efficiency, and energy efficiency, in order to achieve
an increase in energy density. Carbon felt (CF) and
graphite felt (GF) are commonly used electrodes in
VFBs due to they are highly conductive and stable
under acidic conditions. However, modifications are
essential for CF and GF to overcome their drawbacks
on low catalytic activity and specific surface area.
Increasing the surface functional groups by thermal
treatment and doping is one of the most common
ways to address those shortcomings. Thermal
treatment and doping can introduce more oxygen-
containing functional groups (OCFs) to the electrode
surface resulting in enhancement on wettability and
electrochemical activity. Coating is another
alternative for surface modification. By introducing
electrocatalyst to the electrode surface, the
performance of electrode becomes highly tuneable
(Wu et al., 2023).
3.2 Structural and Configure
Modification
The modification of electrode structure can
effectively increase the active surface area, enhancing
the power and energy density of the flow battery by
supplying additional active sites for reactions. In
addition to the optimization of electrode structure and
configuration, the optimization of flow field design
can also contribute to improving the performance of
redox flow batteries, such as energy density. A recent
study (Yaji et al., 2018) employed topology
optimization to refine the flow field configuration in
vanadium redox flow batteries. The study formulated
the optimization problem with the objective of
maximizing the generation rate of vanadium species,
considering the porous nature of the carbon fibre
electrode and the mass transfer coefficient dependent
on local velocity. The results indicated that the
interdigitated flow field pattern is an effective design
for VRFBs, which can help improve the charge-
discharge efficiency of VRFBs (Yaji et al., 2018).
Therefore, the energy density of flow batteries can be
improved to a certain extent by optimizing the flow
field design.
3.3 Introducing Electrocatalyst
Electrocatalysts are typically heterogenous catalysts
promoting electrochemical reactions and are widely
used in electrode modification (Raveendran et al.,
2023). The catalytic mechanism of electrocatalysts is
sophisticated and varies among different materials.
Overall speaking, electrocatalysts function at the
surface of electrode altering the reaction pathway and
reducing the activation energy of electrochemical
reactions (Raveendran et al., 2023). They play crucial
roles in VFBs offering enhancement on the reactivity
of the system and more active sites for ion exchange
to take place. Adding an electrocatalyst into the VFB
system can increase energy efficiency and lowering
costs.
3.3.1 Carbon-Based Electrocatalyst
Carbon-based electrocatalysts are responsible for
increasing the surface area of electrodes (Liu et al.,
2018) represented by carbon nanotube (CNT), carbon
felt, carbon nanosheet, graphite oxide, etc. Compared
to the other two electrocatalysts, carbon-based
electrocatalysts are stable in acidic solutions with
high conductivity, cheap and easy to manufacture.
However, they experience limitations on low specific
surface area and low catalytic activity (Wu et al.,
2023).
Modifications on carbon-based electrocatalysts
are essential for their further application. Increasing
the number of OCFs is an applicable way of
reinforcing the catalytic activity and gathering more
attention recently (Molina-Serrano et al., 2024).
Although higher numbers of OCFs introduced to the
electrocatalyst by thermal treatment as mentioned in
3.1 can increase the catalytic activity in carbon-based
electrocatalysts by enhancing the wettability, mass
transfer rate and number of active sites, there exists a
trade-off between the activity and conductivity
(Molina-Serrano et al., 2024). Heteroatom doping is
another strategy to raise the conductivity and catalytic
activity (Wu et al., 2023 & Liu et al., 2018). The
active sites on carbon electrode are regulated by the
defects introduced by the doped heteroatom on lattice
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
226

resulting in a higher electron affinity. There exists
varies types of doping dominated by nitrogen, oxygen
and phosphorate, offering doped electrodes a high
tunability on properties including reversibility, EE,
power and durability to fit varies working conditions.
3.3.2 Metal-Based Electrocatalyst
Metal-based electrocatalyst are typically metal oxides
and metal halides. They are highly promised
materials with low cost and high conductivity. Metal-
based electrocatalysts are mainly in deposited form
and able to decrease the energy barrier of redox
reactions happens at the electrode which is one of the
major limitations of CF and GF electrodes restricting
the reactivity.
Bi deposition is an important method of
modification where the Bi ions reduced on the carbon
electrode by deposition can largely enhance the redox
reaction rate, VE and EE of VFB by implying a
greater charge exchange rate under high current
density (Liu et al., 2018). However, this
reinforcement is bounded by the amount of Bi loaded
onto the electrode, and this boundary value has not
been studied thoroughly for VFBs yet. In parallel with
ion deposition, transition metal oxide is also used for
meta-based electrocatalyst with lowest cost among
different improvement methods (Liu et al., 2018).
Due to the transition metals have various valency
states and are able to act as active sites for receiving
reactive species. Those evenly dispersed metal oxide
particles generate more surface-active OCFs and
active sites, making the electrode more hydrophilic
and permeable for electrolyte. They are also stable at
working conditions and easy to be prepared. Besides
of metal oxide, metal boride, carbide and nitride
which are covalent bonded electro-deficient
compounds, are materials of interest at present. They
can promote the electron transfer by accepting
unpaired electrons from the metal ions resulting in a
significantly higher electric conductivity than metal
oxides (Wu et al., 2023). Such high electric
conductivity enables a much higher electron transfer
rate in VFBs.
3.3.3 Composite-Based Electrocatalyst
Composite, by definition, includes a wide range of
different materials. Composite-based electrocatalysts
have a significantly higher electrocatalytic activity
than individual composition, due to the interactions
between the electrocatalyst and support. For instance,
Bi metal and carbon-based materials composite is one
of the composite-based electrocatalysts of interest
recently (Wu et al., 2023). By introducing Bi metals
into the complex and regulated carbon structure, the
diffusion pathways of electrochemical reactants can
be manipulated. With this, composite materials are
able to provide a better electrode performance than
each single materials included. As discussed in
previous, transition metal compounds act as active
sites for redox reaction to happens and carbon-based
materials acts as support being responsible for electric
conduction. Besides of metal-carbon composites,
polymer composite and metal-organic-frameworks
(MOFs) are other types of composites with huge
prospect on electrode modification (Liu et al., 2018).
MOFs are highly tuneable materials with high
porosity and specific surface area, in other words,
they are able to provide a significant number of active
sites for redox reactions and their tunability enables
them suitable for various type of operation
requirements incorporating with various type of
composited materials.
3.4 Electrode Material Modification
As discussed before, traditional electrode materials
such as GF and CF experience various of limitations
and electrode material modification is necessary to
VFBs. Similar to electrocatalysts, doping, coating,
surface functionalization and application of
composite materials are methodologies widely used
at present (Liu et al., 2018). Some examples can be
found in Table 1.
Qiao et al. (Qiao et al., 2022) successfully
prepared nitrogen-doped carbon felt using an
ammonium sulphate hydrothermal synthesis strategy,
significantly promoting the electrochemical property
of vanadium redox flow battery electrodes. This
paper indicates the importance of nitrogen doping in
strengthening the hydrophilicity and electrochemical
reactivity of electrode materials, achieving a 3.91%
increase in efficiency in energy use contrasted to the
original pure carbon felt under a charge flow density
of 80 mA ⋅ cm
. This innovative perspective
promotes progress in the field of flow batteries,
particularly in the realm of vanadium.
Deng et al. (Deng et al., 2022) fabricated a multi-
dimensional framework electrode material by the
composite of three different-dimensional carbon
materials structures (0D, 2D, and 3D), which offers
high electrocatalytic activity, rapid charge transfer,
and a large area for redox reactions. This MFC-GF
electrode, owing to the high electrocatalytic activity
of its edge carbon, exhibits excellent electrochemical
performance towards the redox pairs and suppresses
the hydrogen evolution reaction in the negative
electrolyte.
Modifying Electrodes to Enhance the Energy Density of Vanadium Redox Flow Batteries
227
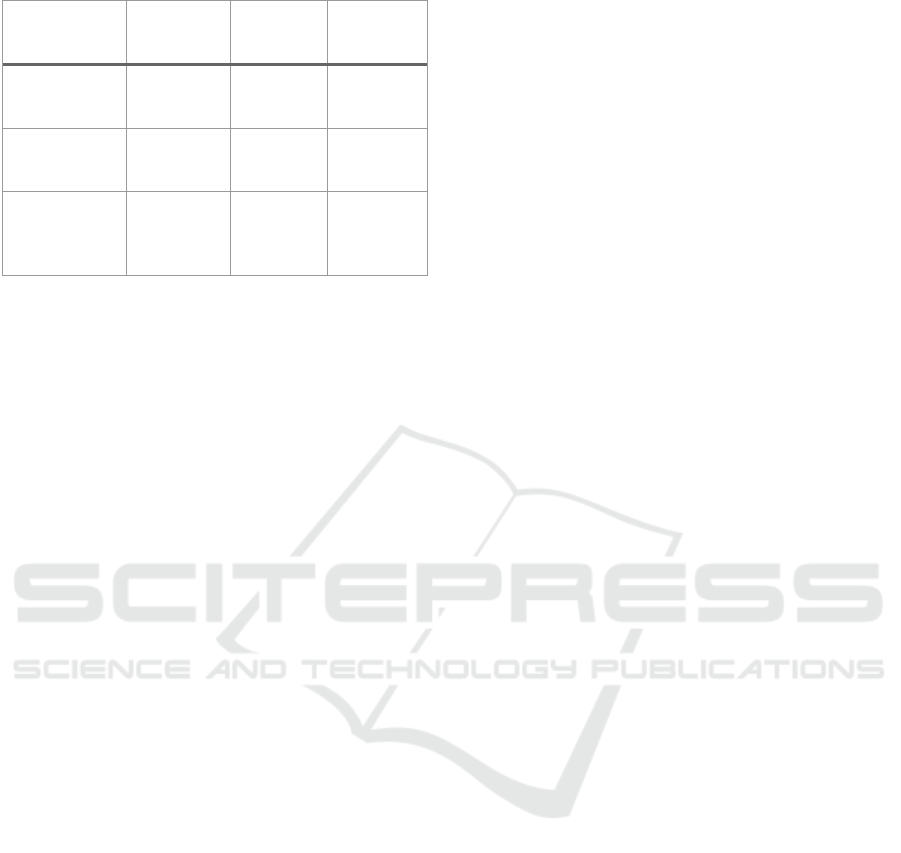
Table 1. Modification of electrode material.
Electrode
material
Increase in
EE
Reference
electrode
material
Reference
N doped CF 7.2% CF Qiao et al.
(Qiao et
al., 2022)
MnO@C/CF 8.5 % CF Chen et al.
(Chen et
al., 2023)
MFC-GF 18.3% GF Deng et
al. (Deng
et al.,
2022)
4 CONCLUSION
The activity of carbon-based electrocatalysts can be
enhanced by increasing the number of oxygen-
containing functional groups. Additionally,
introducing dopant atoms at active sites can
significantly increase electron affinity. The
deposition of Bi can effectively increase the charge
transfer rate of carbon electrodes, and covalently
bonded electro-deficient compounds combined with
unpaired electrons from metal ions can achieve higher
electrical conductivity. Composite-based
electrocatalysts incorporating metal-organic
frameworks (MOFs) can supply a wealth of active
centers for reactions, thereby significantly enhancing
the performance of batteries. The modified graphite
felt, MFC, significantly increases current density and
lifespan due to its high electrocatalytic activity and
hindrance of hydrogen evolution.
In the future, modifications in electrode materials,
such as thermal treatment, doping, and coating, will
become increasingly prevalent and play a more
significant role due to their significant enhancement
on vanadium redox flow battery performance. We
look forward to the development and application of
even better electrode materials to further enhance
flow battery performance.
AUTHORS CONTRIBUTION
All the authors contributed equally and their names
were listed in alphabetical order.
REFERENCES
IEA 2023 Renewable Energy Market Update – June 2023.
ed IEA (Paris: IEA)
Emmett R K and Roberts M E 2021 Journal of Power
Sources 506
Wu M, Fang M, Nan M, Chen X and Ma X 2023
Chemistry–An Asian Journal 18 e202201242
Sankaralingam R K, Seshadri S, Sunarso J, Bhatt A I and
Kapoor A 2021 Journal of Energy Storage 41 102857
Yaji K, Yamasaki S, Tsushima S, Suzuki T and Fujita K
2018 Structural and multidisciplinary optimization 57
535-46
Raveendran A, Chandran M and Dhanusuraman R 2023 13
3843-76
Liu T, Li X, Zhang H and Chen J 2018 Journal of energy
chemistry 27 1292-303
Molina-Serrano A J, Luque-Centeno J M, Sebastián D,
Arenas L F, Turek T, Vela I, Carrasco- Marín F,
Lázaro M J and Alegre C 2024 ACS Applied Energy
Materials 7 2779-90
Qiao L, Fang M, Guo J and Ma X 2022 ChemElectroChem
9
Deng Q, HuangYang X Y, Zhang X, Xiao Z H, Zhou W B,
Wang H R, Liu H Y, Zhang F, Li C Z, Wu X W and
Guo Y G 2022 Advanced Energy Materials 12
Qiao L, Fang M, Guo J and Ma X 2022 ChemElectroChem
9 e202200292
Chen F, Cheng X, Liu L, Han L, Liu J, Chen H, Zhang Q
and Yan C 2023 Journal of Power Sources 580
233421
Deng Q, HuangYang X Y, Zhang X, Xiao Z H, Zhou W B,
Wang H R, Liu H Y, Zhang F, Li C Z and Wu X W
2022 Advanced Energy Materials 12 2103186
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
228
