
Effect of Cobalt-Platinum Nanoalloy Combination with Non-Metallic
Catalyst Fe-N-C on Anti-Poisoning Performance of Hydrogen Fuel
Cell Catalyst
Yue Feng
Zhejiang Wanli University, Ningbo, China
Keywords: Hydrogen Fuel Cell, Platinum Cobalt Nano Alloy Catalyst, Catalyst Poisoning, Catalyst Catalytic Effect.
Abstract: Due to the growing global demand for sustainable energy sources, the advent of hydrogen energy reduces
dependence on fossil fuels. Hydrogen possesses a high calorific value and emits non-polluting substances in
comparison to traditional fossil fuels. As a result, hydrogen energy exhibits significant potential for
development. Hydrogen fuel cells are a major technology for the realization of multi-directional hydrogen
energy applications. Changes in catalyst activity during battery use will affect battery life. Catalyst poisoning
is a major difficulty affecting battery life. Combining cobalt-platinum nanoprecious metals with non-metallic
catalysts Fe-N-C. This paper combined with a number of current research results explores the effect of
combining cobalt-platinum nanoprecious metals with non-metallic catalysts Fe-N-C on the catalyst's
antitoxicizing effect. The catalyst was investigated for battery performance studies. Cost of catalyst
manufacturing is evaluated into battery use. The feasibility of using batteries for manufacturing is analyzed.
Under a multi-faceted inspection, combining nanoprecious metals with non-precious metal catalysts plays an
important role in the anti-toxicity effect and performance enhancement of batteries. Under conditions of
catalyst poisoning, Exploiting the anomalous infrared absorption effect of cobalt-platinum on carbon
monoxide, Improved catalyst resistance to toxicity. Significantly extends battery life. For the distribution of
metals over spatial locations. The combination of precious metals and catalysts is particularly important. In
this paper, we mainly consider metal-nanometal bonding, graphene encapsulation and graphene tessellation.
Comparison of graphene mosaicing method for battery performance has a large improvement through the
study. The method also reduces the use of precious metal catalysts. In addition, compared with the
conventional nickel-cobalt alloy. The advantages of cobalt-platinum nano-alloys are not only reflected in their
antitoxicity effect. The performance of the catalyst is further enhanced.
1 INTRODUCTION
With utilization of fossil fuel increasingly
intensifying, the environment problem created behind
it cannot be ignored it becomes the centre of global
attention. The development of hydrogen fuel cells
meets the demand of non-pollution and zero-
emission. The advent of this technology favourale to
the continue advancement of global carbon emissions
and carbon neutrality. Nowadays, alkaline fuel cell
(AFC), acidic fuel cell (PAFC), proton exchange
membrane fuel cell (PEMFC) etc. are widely used.
Among the cells, PEMFC is the one most widely used
especially in the new energy electric vehicle with
good prospects for development (Xu, Zhu and Xu,
2023). The ability to convert chemical energy directly
into electrical energy with the advantage of no
pollution, and enables hydrogen fuel cells to generate
electricity with much greater efficiency than
generator (Xu, Zhu and Xu, 2023). Although the
hydrogen fuel cells have such superior performance,
the problem behind the battery is still non-negligible.
The issue arises from the trade-off between the
battery’s performance and construction cost. It is
not feasible to optimize both factors simultaneously.
The precious metal catalyst exhibits excellent
catalytic properties. However, its high price becomes
a limiting factor as demand for its application
increases and resources become increasingly scarce
(Zhang et al., 2023). Non-precious mental catalyst
has low price, but compared with precious one, the
catalytic properties are not outstanding. The other
problem is catalyst poisoning. Nowadays, the most
mature non-metallic catalyst is Fe-N-C, with the
increase in the temperature carbon contain in it.
Porous structures are characteristic of hydrogen
140
Feng, Y.
Effect of Cobalt-Platinum Nanoalloy Combination with Non-Metallic Catalyst Fe-N-C on Anti-Poisoning Performance of Hydrogen Fuel Cell Catalyst.
DOI: 10.5220/0013850900004914
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 2nd International Conference on Renewable Energy and Ecosystem (ICREE 2024), pages 140-144
ISBN: 978-989-758-776-4
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

energy fuel cells, where the oxidation of the carbon
reacts with oxygen, carbon monoxide will be
generated. The production of carbon monoxide by the
electrode, its attachment to the catalyst surface, and
occupation of the active site can lead to catalyst
poisoning, which is a primary factor in decreasing
battery life (Huang, Wang and Pei, 2023). Pt-Co-N-C
catalysts exhibit excellent stability and catalytic
effectiveness under both acidic and alkaline
conditions, thereby exerting a positive impact on
delaying the process of catalyst poisoning (Deng et
al., 2023).
By employing the co-reduction method to
produce nanopolyhedral-type metals of Pt and Co, the
catalyst reaction contact area is increased and its
stability is enhanced compared to using a single metal
(Cao et al., 2023). It exhibits superior catalytic
activity, particularly when using Pt and Co for
absorption compared to a single metal catalyst.
Additionally, the nickel-cobalt alloy shows an
anomalous infrared effect in the nanometer range.
The addition of cobalt strengthens the ability of
platinum to absorb carbon monoxide, in addition,
making use of co-reduction method increases the
degree of alloying of two metals. For the structural
performance, this method provides certain guarantee
for the stability of catalyst by reduction- deposition
method (Dey and Dhal, 2020 & Chen et al., 2023).
This method compared with other preparation method
simplifies the manufacturing process, furthermore,
applying this approach catalyst usually have high
catalystic activity within a relatively wide
temperature range. The proposal of such catalyst will
contribute to the mitigation of catalyst poisoning
resulting from carbon monoxide and improve battery
performance. The aim of this article is to investigate
the antitoxicity ability of catalysts in hydrogen energy
batteries, and discuss the impact of the use of the
catalyst on the performance of the battery and analyze
its feasibility.
2 PLATINUM-COBALT
INFRARED ANOMALOUS
ABSORPTION
Electrochemical deposition to manufacture the
catalyst and primary potentiometric infrared
spectroscopy is used to detected abnormal infrared
absorption effect of cobalt platinum alloy,
quantification of infrared anomalous absorption
intensity using infrared absorption factors at different
wavelengths of light (according to the formula), as
shown in figure1. According to the figure, compared
with the native platinum and cobalt electrodes, the
infrared absorption of the nano-platinum and cobalt
film electrode is significantly enhanced. Even if the
infrared absorption intensity of platinum-cobalt
monometallics is increased by a factor of five, the
value is still lower than that of platinum-cobalt alloys
(Chen, Guo and Sun, 2014). In a researcher conducted
in University of California a graphene-nanosphere
encapsulated platinum-cobalt nanocatalyst was
developed. The catalyst has excellent durability after
endurance testing mass activity remains 78% (Zhao
et al., 2022). Besides, the team UCLA embeds tiny
crystals of platinum cobalt in rice-resistant bags made
of graphene. The catalyst maintains the advantages of
high efficiency and high toxicity resistance. Reduced
the use of platinum 40% (Zhao et al., 2022).
Generally, Infrared anomalous absorption effects
in platinum-cobalt nano-alloys provide the new idea
in the study of battery antitoxicity. In catalyst
manufacture, different process methods lead to
various effect of resistance to poisoning. Among
these manufacture methods, the catalyst made by the
team UCLC is most reliable. Although
electrochemical deposition has the shortest
preparation cycle among several methods and the
mild manufacture conditions, the compactness of its
internal structure greatly reduces the effective
utilisation of the catalyst. This technology is an
extension of the use of thin metal films, and provided
a theoretical basis for further research that followed.
The research team in Los Angeles and UCLA used
the more precise graphene wrapping and embedding
methods. The difference between the two lies in the
different spatial position relationship between cobalt
platinum alloy and graphene, which leads to the
difference in the consumption of nano alloys and
effective contact area during reaction. Using cobalt
platinum alloys of the same quality, graphene
embedding method has a larger effective reaction area
compared to encapsulation method, and the catalyst
prepared by embedding method requires less
platinum cobalt material. Achieved the goal of
reducing resource consumption. Compared to
graphene encapsulated catalysis, embedded catalysts
have weaker resistance to poisoning. But using multi-
layer graphene to increase surface area can solve this
problem.
Effect of Cobalt-Platinum Nanoalloy Combination with Non-Metallic Catalyst Fe-N-C on Anti-Poisoning Performance of Hydrogen Fuel
Cell Catalyst
141
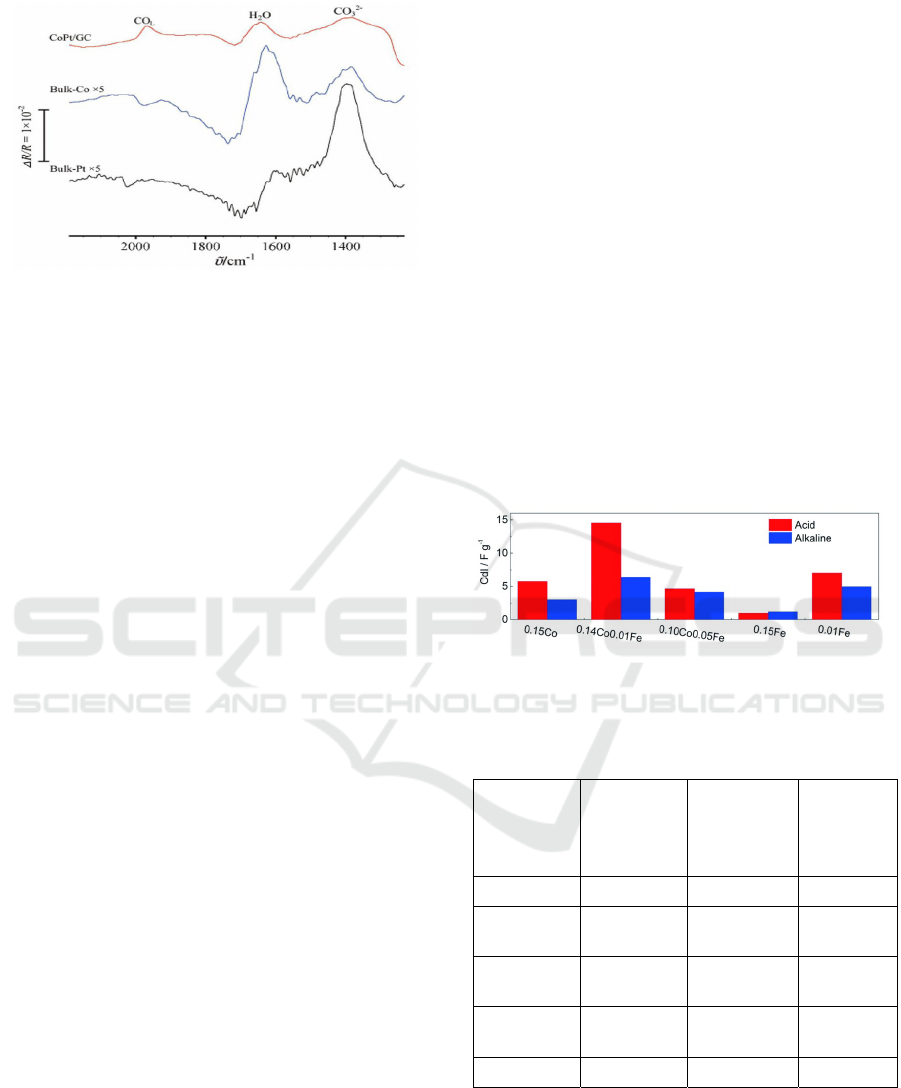
Figure 1: MSFTIR spectra of saturated adsorption of CO
on C and Co, Pt electrodes (Chen, Guo and Sun, 2014)
3 EFFECT OF IRON AND
COBALT IN CATALYSTS ON
BATTERY PERFORMANCE
The greatest impact of PtCo-Fe-N-C catalyst on
battery performance is the interaction of cobalt and
iron. The catalytic mechanism of iron and cobalt is
the reaction of both with oxygen and form
coordination bonds. The addition of water reacts to
form Fe(OH)
2
and Co(OH)
2
, afterwards OH-
disengage the catalyst (Jiang et al., 2021). Use of
cobalt and iron to enhance battery performance
provides new ideas. Bimetallic interdoping is used in
manufacture of bimetallic catalysts. Later, EIS was
utilized to detect catalyst double layer capacitor.
Utilizing different ratios of cobalt to iron to
quantitative cell performance figure 2. The larger
capacitance represents that in ORR process, the
catalyst has a greater catalytic surface area. And when
the ratio of cobalt to iron reaches 14:1, catalyst has
higher catalytic performance relatively. And the
durability of the cell's catalytic have better
performance under acidic conditions compared to the
alkaline environment (Lei et al., 2023). Based on the
chart half-wave potential and limiting current are the
basis for judging the catalytic oxidizing ability of the
catalyst. Platinum has a better catalytic oxidizing
ability than nickel and catalytic effects of iron and
cobalt oxidation much lower than the catalytic effect
of iron, cobalt and nickel ternary metal catalysts (Li,
2023). This demonstrates the limitations of bimetallic
catalysts. However, it is affirmed that ternary metals
have a significant role in enhancing catalyst
performance. And a ternary metal catalyst for
platinum instead of nickel Provides some basis.
Previous scientists have used DFT calculation. Based
on the binding energy of ORR activity to free radicals
on metals. The table1 was plotted. In Table 1 the ORR
catalytic performance of Pt is much better than that of
Ni in the most suitable potential energy. Based on the
trend line on the way, platinum has higher catalytic
activity than nickel (Guo, 2024). This is better
illustrated by the catalytic activity of the different
metals in figure 3, where platinum has a higher upper
limit of catalytic performance compared to nickel.
In generally, the performance enhancement of
catalysts is closely related to the use of multiple
metals. Compared to a single metal, the anti-
poisoning ability and catalytic efficiency are greatly
enhanced. Although in terms of manufacturing costs,
with the addition of multiple metals, the
manufacturing cost of catalysts will also increase.
The prolonged service life of the catalyst and the
increased reaction efficiency cannot be ignored. In
the balance between catalyst performance and
manufacturing cost. The iron cobalt platinum ternary
nano alloy catalysts have the highest cost-
effectiveness.
Figure 2: The comparison of double layer capacitance
(Cdl) values (Li, 2023)
Table 1: Summary of ORR performance of catalysts and
pt/c at 0.1M KOH (Guo, 2024)
Catalyst
name
Half-wave
potential(
V)
Limiting
current(
mA/cm
2
)
Tafel
slope(
mV/dec
)
Ni@NDC 0.793 4.98 109
Fe-Co-
Ni@NDC
+0.109 +0.38 -39
Co-
Ni@NDC
+0.051 +0.36 -2
Fe-
Co@NDC
+0.053 -2.23 +16
Pt/C +0.040 +0.33 -20
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
142
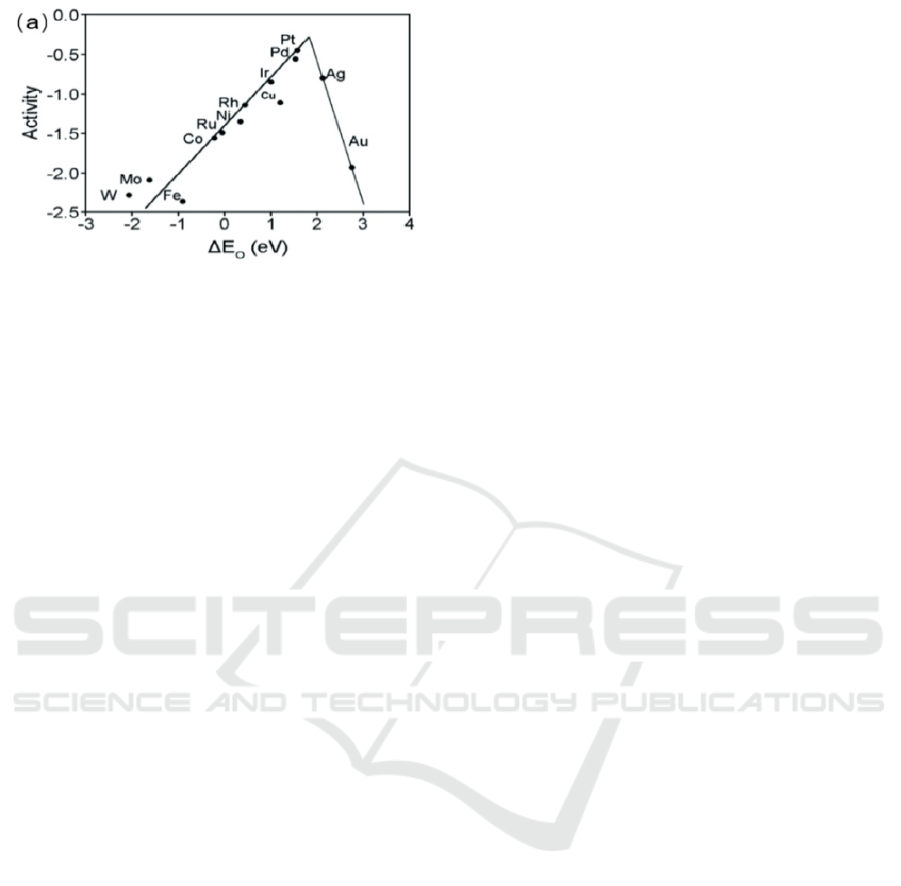
Figure 3: Transition metals and precious metals ORR
oxygen active adsorption energy △E0
4 CONCLUSION
With collation of the literature reveals that even
though the catalyst has good catalytic properties as
well as resistance to toxification. The scope of use of
this technology is limited. The use of ternary metal
catalysts has a tremendous amount of voltage and
energy within them. Mismatched original materials
may cause structural damage inside the battery.
Accelerates battery aging and can even be potentially
dangerous. Even if this technology incorporates
precious metals into non-metallic catalysts enhance
the performance of non-metallic catalysts. But
compared to catalysts made directly from precious
metals there may still be some gaps. For battery
manufacturing costs, this catalyst uses two types of
precious metal ctalysts will undoubtedly lead to an
increase in battery manufacturing prices. In order to
pursue the good anti-poisoning properties of
catalysts. Using Platinum to replace metals such as
Nickel would lead further increase the manufacturing
cost of catalysts. In addition, the manufacturing of
nanometals compared to the direct use of large-
diameter particle metals. The technical costs
contained within it cannot be ignored in catalyst
process manufacturing. Fabrication techniques for
nanometers have process drawbacks. When battery
life runs out battery aftercare is unavoidable. Leakage
of cobalt from the catalyst in this battery can cause
some ecological damage. In future research on
hydrogen energy cells, multi-component nanometal
catalyst technology could provide new idea and solve
the catalyst poisoning in the field of hydrogen fuel
cells, and in subsequent studies, it is not limited to the
effect of carbon monoxide on battery catalysts. It is
possible to advance the study of the catalyst's
antitoxicity to other gases. In future studies,
compared to mitigating cell catalyst poisoning
tackling catalyst poisoning at its source is even more
important. In catalyst material selection, it is hoped
that a catalyst will be created that will be able to
absorb and convert carbon monoxide. Formation of a
new energy source through internal conversion of
carbon monoxide. Achieving the goal of sustainable
recycling of hydrogen energy cells. Researching high
performance and anti-toxicity catalysts while
pursuing simpler manufacturing processes. Striving
for a better balance between performance and
manufacturing costs. In the field of responding to
hydrogen energy fuel cell catalyst poisoning still has
a bright future.
REFERENCES
Xu Zhihong, Zhu Xiaowen, Xu Caini 2023 Structural
characteristics of hydrogen fuel cells and development
overview of hydrogen fuel cell vehicles. Times
Automotive 13 88-90
Zhang Yanhong, Zhou Qi, Wang Caikang, etc. 2023
Development status of non-precious metal catalysts
used for catalyzing CO
2
hydrogenation reaction to
generate carbon containing fuels. Energy Research and
Utilization 5 2-9
Huang Xuelian, Wang Jun, Pei Danni. 2023 Simultaneous
measurement of inorganic gases and total hydrocarbon
impurities in hydrogen gas for fuel cell vehicles using a
gas chromatograph with one injection. Metrology and
Testing Technology 50 21-24
Deng Han, Sun Xiaohui, Lu Lu, etc. 2023 Preparation and
Performance Study of PtCo-N/C Low Platinum
Catalyst. Journal of Dalian Jiaotong University 44 99-
103+113
Cao Yunzhong, Zheng Junning, Wu Hui, etc. 2023
Research progress on Pt based catalysts for catalytic
hydrolysis of ammonia borane to produce hydrogen.
Rare Metals 47 1122-1131
S. Dey, G.C. Dhal 2020 Property and structure of various
platinum catalysts for low-temperature carbon
monoxide oxidations. Materials Today Chemistry 16
2468
Xiaojie Chen, Wenqiong Gou, Jiaqi Xu, etc. 2023 Low-
loading and ultrasmall Ir nanoparticles coupled with
Ni/nitrogen-doped carbon nanofibers with Pt-like
hydrogen evolution performance in both acidic and
alkaline media. Chemical Engineering Journal 471
144481
Chen Qingsong, Guo Guocong, Sun Shigang 2014 Cyclic
voltammetric electrodeposition of cobalt platinum alloy
thin films and their anomalous infrared properties.
Electrochemistry 20 410-415
Zhao Zipeng, Xue Wang, Liu Zeyan, etc. 2022 Graphene-
nanopocket-encaged PtCo nanocatalysts for highly
durable fuel cell operation under demanding ultralow-
Pt-loading conditions. Nat. Nanotechnol 17 968-975
Effect of Cobalt-Platinum Nanoalloy Combination with Non-Metallic Catalyst Fe-N-C on Anti-Poisoning Performance of Hydrogen Fuel
Cell Catalyst
143

Min Jiang, Fei Wang, Fan Yang, etc. 2021 Rationalization
on High-Loading Iron and Cobalt Dual Metal Single
Atoms and Mechanistic Insight into the Oxygen
Reduction Reaction. Nano Energy 93 106793
Ran Lei, Wang Luyuan, Zhang Xingyu, etc 2023 Research
progress on non-precious metal Fe-N-C catalysts for
proton exchange membrane fuel cells. Chemical
Bulletin 86 1426-1433
Li Ziyao 2023 Nanostructure design and ORR-OER
electrocatalytic performance study of iron cobalt based
electrocatalysts. Anhui University of Science and
Technology.
Guo Jiangnan 2024 Preparation and electrocatalytic
properties of PtM (M=Co, Ni) nanocomposites. Central
South University
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
144
