
Analysis of the Preparation-Storage-Use of Hydrogen
Weisen Ma
Detroit Green Technology Institute, Hubei University of Technology, Wuhan Hubei, 430068, China
Keywords: Hydrogen, Electrolysis of Water, Hydrogen Energy Storage, Full Cells.
Abstract: In the past few decades, the production of electricity generated by chemical fuels has always dominated, but
the greenhouse gases produced by fossil fuels have become a major problem affecting the global climate.
Therefore, hydrogen energy, as the clean energy most used by mankind at present, has extremely high
exploration value. This article analyzes the use process of hydrogen energy in detail from three parts. First
part is the preparation of hydrogen, second part is the storage of hydrogen, and the last is how to use hydrogen.
Proton exchange membrane electrolyzer (PEMWE) technology is the main preparation technology of
hydrogen energy use process, which uses photovoltaic power generation as the main source of electricity. In
this article the principle of PEMWE’s hydrogen production technology and technical components and
functions of PEM electrolyzers, but also compare the more mature Proton exchange membrane (PEM)
technology with the Anion exchange membrane (AEM) electrolyzed water technology with great potential in
the future, and analyze the pros and cons of both. In terms of storage, this paper analyzes the advantages and
disadvantages of gas compressed storage, liquid storage, metal hydride storage and underground storage. In
terms of hydrogen energy treatment. Cogeneration technology, especially in hydrogen fuel cells, is the
primary method for converting hydrogen into electricity, and the remaining hydrogen will play a role in
different fields for different purposes.
1 INTRODUCTION
According to the "World Energy Statistical Yearbook
2023" report, primary energy consumption will
increase in most regions of the world in 2022 (except
Europe and the CIS). Despite rising prices for all three
fossil fuels, natural gas, oil and coal, only natural gas
has seen demand fall. Fossil fuels still account for 82%
of the world's total energy supply (Energy institute,
2023). This will lead to the production of greenhouse
gases, affecting climate change and global warming
(Baroutaji et al., 2023). The increase in the share of
renewable electricity generation has also become a
significant trend, with renewable electricity
(excluding hydropower) growing by 14% in 2022,
meeting 84% of the net increase in electricity demand.
In the 21st century, more and more people believe that
hydrogen is an important energy source that can
change the future energy system (Falcone, Hiete and
Sapio, 2021). Hydrogen fuel cells are the green energy
source most likely to make the "hydrogen economy" a
reality (Cheng et al., 2007). The development
potential of hydrogen energy is reflected in that it is a
green, clean and pollution-free energy source. This
article proposes a hydrogen energy usage process and
analysis of hydrogen production-storage-power
generation, with the purpose of using hydrogen energy
in an environmentally friendly and energy-saving
way. In the process of promoting the development of
hydrogen energy utilization, scientific and reasonable
measures should be taken to realize the dual benefits
of economy and environment.
2 PEM ELECTROLYSIS WATER
HYDROGEN PRODUCTION
TECHNOLOGY
2.1 Technical Principle of Hydrogen
Generation by PEM Electrolytic
Water
The main hydrogen generation technologies of PEM
are divided into two types as PEMFC and PEMWE.
Ma, W.
Analysis of the Preparation-Storage-Use of Hydrogen.
DOI: 10.5220/0013850700004914
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 2nd International Conference on Renewable Energy and Ecosystem (ICREE 2024), pages 133-139
ISBN: 978-989-758-776-4
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
133
PEMFC hydrogen generation technology produces
water as a byproduct of the chemical interaction
between hydrogen and oxygen, which transforms
chemical energy into electric energy (Baroutaji et al.,
2023). The chemical principle of the latter PEMWE
technology is as follows. The technical principle of
PEMWE is to inject deionized water into an
electrolytic cell, and the electrolytic cell consists of
two parts, and a is used to separate the two parts.
When an external power source is energized on the
cell, water (H
2
O) breaks down at the cathode and
anode. The reaction formula of cathode and anode is
shown below (Kya et al., 2020).
2𝐻
𝑂
→𝑂
4𝐻
4𝑒
𝐸
1.23𝑉
(1)
2𝐻
2𝑒
→𝐻
𝐸
0.00𝑉 (2)
Of the following two equations, formula (1) is
called oxygen evolution reaction (ORE) and formula
(2) is called hydrogen evolution reaction (HER). In an
acidic environment, the total reaction composed of
two half reactions is shown in formula (3). When a
DC power supply is connected to the electrode and
the applied voltage is higher than the thermodynamic
potential, water begins to be decomposed (Falcão and
Pinto, 2020).
Whole reaction:
𝐻
𝑂→𝐻
𝑂
(3)
In summary, the technical principle of PEMWE
is that the cathode gains electrons to produce
hydrogen, and the anode loses electrons to produce
oxygen. The intermediate PEM (proton exchange
membrane) plays two roles, one is to allow hydrogen
ions to pass and block electrons, and the other is to
separate hydrogen and oxygen to prevent mixing.
And then end up with hydrogen and oxygen to collect
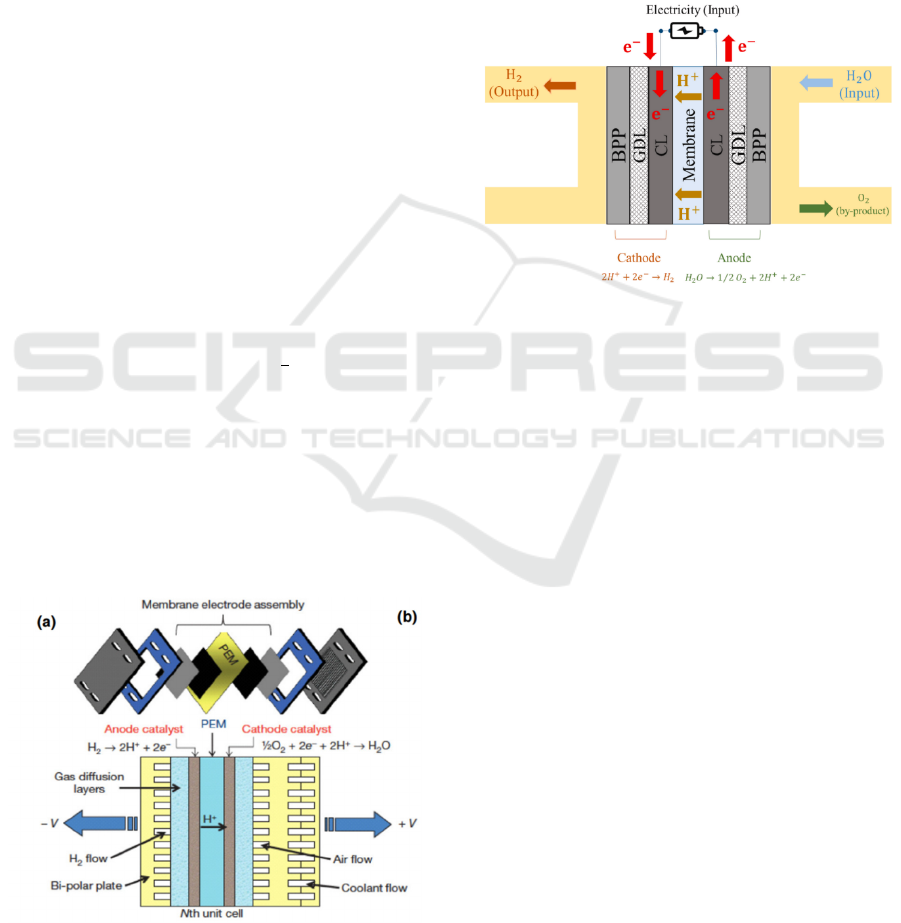
as shown in figure 1.
Figure 1: The structure of PEM fuel cell (Wang et al.,
2020)
2.2 PEM Electrolysis Water Hydrogen
Production Technology
Components
In addition to the DC power supply on the top, there
are four components in PEMWE electrolyzer device
including Bipolar Plates (BPP), Gas Diffusion Layer
(GDL), Catalyst Layer (CL) and Membrane, as
shown in figure 2.
Figure 2: Main components and working principles of
PEMWE (Baroutaji et al., 2023)
2.2.1 Bipolar Plate (BPP)
Also known as the collector plate, the main function
of bipolar plate is to provide two channels of air flow
and current conduction. The former serves to prevent
hydrogen and oxygen from combining in the fuel
electrolyzer, while the latter creates a channel for
electricity to flow between the cathode and anode
(Xiong et al., 2021). BPP is mainly divided into two
types, one is a metal bipolar plate, one is a graphite
bipolar plate. The main disadvantages of graphite
bipolar plates are that their strength is low and the
material is relatively fragile. The thickness of the
material needs to be increased to ensure the strength
of the material. However, increasing the thickness of
the material will lead to the increase of the quality of
the electrolyzer, which is not conducive to the
conditions of lightweight and convenient
transportation. In addition, the use condition of
graphite bipolar plates is high-temperature
graphitization treatment, which requires a long
production cycle. Hence, the aforementioned
shortcomings result in a subpar commercial impact of
graphite bipolar plate. The raw materials of metal
bipolar plates are usually corrosion-resistant metals,
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
134

such as titanium, aluminum, nickel alloys. Despite
challenges stemming from the acidic environment
and corrosion by reducing substances like hydrogen
and oxygen within the electrolytic cell, metal bipolar
plates offer significant advantages including high
strength, excellent conductivity, cost-effectiveness,
and easy scalability in production. Hence, metal
bipolar plates retain considerable application
potential and commercial viability (Wang et al.,
2020).
2.2.2 Gas Diffusion Layer (GDL)
Gas diffusion layer (GDL) is one component of PEM.
Ensuring that the gas can diffuse evenly into the
catalyst layer is the main goal of the gas diffusion
layer. Its porous construction makes it possible for the
gas to pass through and arrive at the intended
location. GDL also has a certain degree of
conductivity and thermal conductivity, which not
only helps to maintain the normal flow of current in
the electrolytic cell, but also helps to evenly distribute
and diffuse the heat inside the electrolytic cell,
thereby providing a suitable working temperature for
the electrolytic cell. The mechanical strength of GDL
provides support and protection for more fragile
membranes and catalyst layers. Its structure can also
drain the water by-product of the electrolyzer, and it
ensures that moisture does not accumulate inside the
electrode, thereby avoiding blocking the gas channel
and affecting performance (Ozden et al., 2019).
2.2.3 Catalyst Layer (CL)
The specific position of the catalytic layer is attached
to both ends of the proton exchange membrane, which
is divided into a cathode catalytic layer and an anode
catalytic layer. Currently, the cathode mainly uses
platinum carbon (Pt/c) catalyst to accelerate the
hydrogen evolution reaction (HER). The platinum-
carbon catalyst is composed of platinum
nanoparticles and carbon carrier, in which the
platinum nanoparticles are efficient hydrogen
precipitation catalysts, while the surface of the carbon
carrier has more pores, which increases the surface
area and stability of the catalyst. However, platinum-
based catalysts have a higher cost than what,
constituting approximately 35% of the total cost of
PEMWE (Baroutaji et al., 2023). Iridium oxide
(IrO
2
), which has good oxygen evolution reaction
(OER) activity and stability and can successfully
encourage oxygen precipitation, is the primary
material used in the anode. Reducing the activation
energy needed for water electrolysis is the catalytic
layer's purpose. As a result, the pace at which
hydrogen and oxygen evolve is accelerated.
Enhancing the electrolysis efficiency is possible by
increasing the electrode's current density through the
design and optimization of the catalytic layer. Lastly,
the catalytic layer's strong chemical stability
guarantees PEMWE's steady operation over the long
run (Sui, Zhu and Djilali, 2019).
2.3 Comparison of Technology of PEM
and AEM
In the field of producing hydrogen through water
electrolysis, PEM and AEM technologies currently
hold a dominant position. These two technologies
differ in their features and benefits. The solid
electrolyte utilized in PEM electrolysis cells ensures a
high energy density and efficiency, which is PEM's
advantage. As a result, PEM technology plays a big
role in the field of producing hydrogen by water
electrolysis. The benefit of AEM technology stems
from the utilization of non-precious metal catalysts,
which significantly reduces material costs and makes
the implementation of AEM technology for hydrogen
production via water electrolysis more economically
feasible (Pushkareva et al., 2020). However, the
current high cost has not affected the competitiveness
of PEM technology, because of the technical maturity
and wide application. The widespread application of
PEM fuel cells provides rich experience and technical
support for hydrogen production, further promoting
the development of PEM technology. Although AEM
technology has advantages in material cost, further
efforts are still needed in terms of technology maturity
and large-scale application (Santoro et al., 2022).
However, with the increasing focus on
environmental awareness and the rapid advancement
of renewable energy technologies, there is a growing
demand for electrolytic water hydrogen production.
This trend provides ample opportunities for the
development of AEM technology in this field (Li and
Baek, 2021). With its flexibility and low cost, AEM
technology is expected to emerge as a key technology
in future water electrolysis-based hydrogen
production (C
hina Energy News Network, 2024). In
summary, PEM and AEM have their own advantages
in hydrogen production technology through water
electrolysis. PEM technology is favored for its high
efficiency and maturity, while AEM technology
shows great potential for its economy and flexibility.
These two technologies will probably become
Analysis of the Preparation-Storage-Use of Hydrogen
135

increasingly significant in the field of hydrogen
production by electrolytic water in the future as
technology develops and application scenarios grow.
2.4 Source of Electricity for Hydrogen
Production
2.4.1 Generating Electricity from
Non-Renewable Sources
According to the report from the World Energy
Statistics Review, global energy demand will grow by
1% in 2022, with a record growth in renewable
energy. However, the dominant position of fossil fuels
has not changed, making up 82% of the world’s
energy supply. Natural gas contributes 27% to the
overall electricity generation from fossil fuel power
globally (Leonard, Michaelides and Michaelides,
2020). However, coal still ranks first in the total
power generation, accounting for approximately
35.4%.
2.4.2 Generating Electricity from
Renewable Sources
According to data from British Petroleum’s latest BP
World Energy Outlook, by the end of April 2023,
China had amassed a combined installed capacity of
820 GW from wind and solar power, constituting a
substantial portion of the nation's total power
generation capacity, which is 31% (Leonard,
Michaelides and Michaelides, 2020). Of this 31% of
total renewable energy capacity, wind power
accounted for 14 percent, while solar power
accounted for 17 %. Of the new installed capacity in
2023, wind added 14.2 GW and solar added 48 GW,
which accounted for 16.8% and 57.2% of the new
installed capacity, respectively. This data further
indicates that China's installed capacity of renewable
energy has increased by 11.5% year-on-year. By
2050, the share of wind and solar power in China's
total power generation will increase from less than
10% today to more than 50%, and may even increase
to more than 65%.
2.4.3 Cost Comparison
The cost of different method for hydrogen production
is summarized in table 1. The cost of electrolyzing
water is more than three times that of fossil fuels,
making it expensive. Additionally, the electricity cost
of electrolyzed water accounts for a significant
proportion of the total cost (>50%).
Table 1: Production costs from various energy
sources (China Energy Storage Network, 2024)
Traditional
method
Cost of raw
materials
(dollars/kg)
Cost of hydrogen
production
(dollars/m3)
Coal 8.68-18.33 1.36-1.90
Natural gas 0.23-0.28(m3) 1.36-1.89
Electrolysis of
water
Proportion of
electricity
consumption cost
Cost of hydrogen
production
(dollars/m
3
)
Proton
exchange
membrane
50.52 5.54
Alkaline
water
electrolysis
74.91 5.54
3 HYDROGEN ENERGY
STORAGE
3.1 Gaseous Compression Storage
Currently, among various hydrogen storage
technologies, room temperature compressed gas
hydrogen storage technology (CGH2) is the most
mature one. More than 80% of the 215 hydrogen
stations operating worldwide in 2010 used CGH2
technology. At present, the pressure of CGH2's on-
board storage tank is as high as 70MPa, which
changes the physical state of hydrogen and reduces
the distance between gas molecules, thereby
compressing and storing hydrogen into the hydrogen
storage tank. CGH2 technology requires low
equipment costs, high safety, and strong flexibility.
The storage of compressed gas at room temperature
is not limited by geographical location and climate
conditions, and can be stored and transported in
various environments. However, the bulk density of
hydrogen does not increase with the increase of
pressure, which makes it difficult to increase the
density of hydrogen (Yanxing et al., 2019), and if the
pressure is too large, it will also bring safety
problems.
3.2 Liquid Storage
The operational principle of liquid storage technology
(LH
2
) is to use a compressor to compress hydrogen
gas to extremely high pressure, thereby increasing gas
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
136
density, and then reducing the temperature of high-
pressure gas to -253 ℃ to achieve liquefaction.
Compared to gaseous compression technique, liquid
storage technology has a higher hydrogen density.
High energy density and long-term stability are
features of liquid storage technology. Because of its
higher liquid storage density and comparatively
stable physical characteristics, more hydrogen may be
held in each container. Nevertheless, the liquefaction
process is energy-intensive. It is inevitable that heat
will enter the container, causing a hydrogen loss of
2% -3%. Therefore, liquid storage technology is more
suitable for high-tech industries like the aerospace
industry (which consider performance more than
cost) (Yanxing et al., 2019).
3.3 Metal Hydride Storage
Metal hydride storage is the use of metal or alloy with
hydrogen reaction to produce metal hydride, when
hydrogen and metal or alloy contact, hydrogen atoms
will enter the metal or alloy lattice, thus forming a
stable compound, the metal hydride. Heating the
compound until hydrogen is needed breaks the
chemical bond between the hydrogen atoms and the
metal, releasing hydrogen (Klopčič et al., 2023).
Metal hydrides have small space requirements and
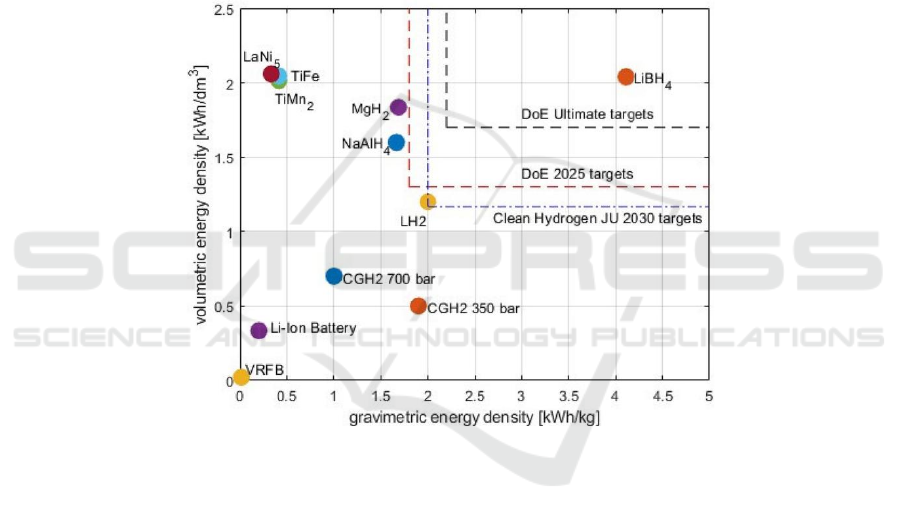
strong ductility. Figure 3 lists the volume and weight
energy density of different metal hydride hydrogen
storage systems.
Figure 3. Volumetric and gravimetric energy densities of storage systems (Klopčič et al., 2023).
3.4 Ground Storage
In June 2019, underground storage was identified by
the International Energy Agency (IEA) as the optimal
storage mode for long-term and large-scale storage
(Elberry et al., 2021). It is a method to store hydrogen
by using the physical characteristics of underground
space. The injection of hydrogen gas into the ground
by drilling holes or pipelines into depleted oil and gas
reservoirs, aquifers, or cave storage (excavation or
dissolution of mined rock, such as salt coal, igneous
and metamorphic rocks). These underground media
have many pores and good permeability, and
hydrogen enters underground materials through
adsorption, dissolution, etc. A buffer gas such as N
2
or CH
4
is injected before hydrogen is injected. The
buffer gas is periodically expanded and compressed
to ensure the ground pressure and transportation rate
required for transportation (Zivar et al., 2021). The
underground hydrogen storage has a large capacity,
long storage time, and strong safety, because the
underground structure acts as a natural barrier,
separating hydrogen from the outside world and
greatly reducing risks. And this storage method does
not produce harmful substances, making it more
environmentally friendly.
Analysis of the Preparation-Storage-Use of Hydrogen
137

4 HYDROGEN PROCESSING
METHOD
4.1 Hydrogen Fuel Cell
Currently, hydrogen fuel cell technology primarily
finds application in the realm of new energy vehicles.
With zero emissions while using hydrogen and
oxygen as fuel, PEMFC technology's cleanliness and
environmental friendliness are its greatest advantages
(Cheng et al., 2007). Combination of heat and power
(CHP) is currently a mature technology suitable for
industrial applications and large-scale commercial
use for a high efficiency in power generation. Within
the entire energy production and supply system,
cogeneration utilizes the heat generated by fuel cells
to generate electricity, which is then converted into
low-grade thermal energy in power engineering,
resulting in higher efficiency in the utilization of
hydrogen energy. Therefore, the efficient and
environmentally friendly energy utilization method
combining the advantages of fuel cell and
cogeneration has broad application prospects
(Arsalis, 2019).
4.2 Analysis of Utilization Scenarios
Of the hydrogen production process, about 90% is
currently used for ammonia synthesis, methanol
production and refineries. This ratio will gradually
decline over the next few years, until hydrogen
production increases and this energy source is
gradually combined with other energy sectors, such
as for grid power generation (Tarhan and Çil, 2021).
In the metallurgical, fertilizer, and chemical
industries, as well as the processing and upgrading of
crude oil, hydrogen has a very high commercial
value. Additionally, it is important for fuel upgrading,
aviation and marine fuels, and other sectors (Okolie
et al., 2021).
5 CONCLUSION
PEMWE water electrolysis technology is now a very
mature technology, has been widely used and studied.
Further research on PEMWE is currently focused on
the modeling of electrolyzers and the cost efficiency
of electrocatalysts. In comparison with AEM
electrolysis water technology, PEMWE currently has
rich experience and market support. However, as the
demand for hydrogen energy continues to rise, AEM
electrolysis water technology that doesn't necessitate
valuable metals as materials has shown advantages
such as low cost and flexibility, and has greater
potential in future. As for hydrogen storage, the
selection of different storage technologies depends on
different regions, different economic conditions,
different technical differences and so on. This paper
analyzes the advantages and disadvantages of four
different storage technologies, namely gas
compressed storage, liquid storage, metal hydride
storage and underground storage, which should be
comprehensively considered according to the
requirements of specific application scenarios, safety,
cost, efficiency and other factors, and make decisions
based on the maturity and sustainability of the
technology. Regarding the utilization of hydrogen
energy, current hydrogen fuel cells are predominantly
employed in the new energy automotive sector. The
specific suggestion is to utilize the heat generated by
PEMFC when converting hydrogen energy into
electric energy and employing the CHP mode, the
efficiency of hydrogen fuel cell utilization is greatly
improved. The process of using renewable energy to
generate electricity, producing hydrogen through
PEMWE technology, and finally generating
electricity through hydrogen fuel cells through
cogeneration mode has the characteristics of
sustainability, environmental protection, and
efficiency, and has broad application prospects.
REFERENCES
Energy institute. Statistical Review of World Energy
(2023). Retrieved on April 15 2024, retrieved from
https://www.energyinst.org/__data/assets/pdf_file/000
7/1408075/5456af7ed79ea2b1943197dce2f0b019e78f
3ee2.pdf
Baroutaji, A., Arjunan, A., Robinson, J., Abdelkareem, M.
A., & Olabi, A. G. 2023 Additive manufacturing
for Proton Exchange Membrane (PEM) hydrogen
technologies: merits, challenges, and
prospects. International journal of hydrogen energy 52
561-584
Falcone, P. M., Hiete, M., & Sapio, A. 2021 Hydrogen
economy and sustainable development goals: Review
and policy insights Current opinion in green and
sustainable chemistry 31 100506
Cheng, X., Shi, Z., Glass, N., Zhang, L., Zhang, J., Song,
D., ... & Shen, J. 2007 A review of PEM hydrogen fuel
cell contamination: Impacts, mechanisms, and
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
138

mitigation Journal of Power Sources 165 739-756
Kaya, M. F., Demir, N., Rees, N. V., & El-Kharouf, A. 2020
Improving PEM water electrolyser’s performance by
magnetic field application Applied Energy 264 114721
Falcão, D. S., & Pinto, A. M. F. R. 2020 A review on PEM
electrolyzer modelling: Guidelines for beginners
Journal of cleaner production 261 121184
Wang, Y., Diaz, D. F. R., Chen, K. S., Wang, Z., & Adroher,
X. C. 2020 Materials, technological status, and
fundamentals of PEM fuel cells–a review Materials
today 32 178-203
Xiong, K., Wu, W., Wang, S., & Zhang, L. 2021 Modeling,
design, materials and fabrication of bipolar plates for
proton exchange membrane fuel cell: A review Applied
energy 301 117443
Ozden, A., Shahgaldi, S., Li, X., & Hamdullahpur, F. 2019
A review of gas diffusion layers for proton exchange
membrane fuel cells-With a focus on characteristics,
characterization techniques, materials and
designs Progress in Energy and Combustion Science 74
50-102
Baroutaji, A., Arjunan, A., Robinson, J., Abdelkareem, M.
A., & Olabi, A. G. 2023 Additive manufacturing for
Proton Exchange Membrane (PEM) hydrogen
technologies: merits, challenges, and prospects
International journal of hydrogen energy 52 561-584
Sui, P. C., Zhu, X., & Djilali, N. 2019 Modeling of PEM
fuel cell catalyst layers: status and outlook
Electrochemical Energy Reviews 2 428-466
Pushkareva, I. V., Pushkarev, A. S., Grigoriev, S. A.,
Modisha, P., & Bessarabov, D. G. 2020 Comparative
study of anion exchange membranes for low-cost water
electrolysis International Journal of Hydrogen Energy
45 26070-26079
Santoro, C., Lavacchi, A., Mustarelli, P., Di Noto, V., Elbaz,
L., Dekel, D. R., & Jaouen, F. 2022 What is next in
anion ‐ exchange membrane water electrolyzers?
Bottlenecks, benefits, and future ChemSusChem 15
e202200027
Li, C., & Baek, J. B. 2021 The promise of hydrogen
production from alkaline anion exchange membrane
electrolyzers Nano Energy 87 106162
China Energy News Network (cpnn),
https://cpnn.com.cn/news/baogao2023/202306/t20230
607_1607604.html, last accessed 2024/04/13
Leonard, M. D., Michaelides, E. E., & Michaelides, D. N.
(2020). Energy storage needs for the substitution of
fossil fuel power plants with renewables. Renewable
Energy, 145, 951-962.
China Energy Storage Network (ESCN),
https://www.escn.com.cn/2022-
11/01/c_1211743453.htm, last accessed 2024/04/13.
Yanxing, Z., Maoqiong, G., Yuan, Z., Xueqiang, D., & Jun,
S. 2019 Thermodynamics analysis of hydrogen storage
based on compressed gaseous hydrogen, liquid
hydrogen and cryo-compressed hydrogen International
Journal of Hydrogen Energy 44 16833-16840
Klopčič, N., Grimmer, I., Winkler, F., Sartory, M., &
Trattner, A. 2023 A review on metal hydride materials
for hydrogen storage Journal of Energy Storage 72
108456
Elberry, A. M., Thakur, J., Santasalo-Aarnio, A., & Larmi,
M. 2021 Large-scale compressed hydrogen storage as
part of renewable electricity storage
systems. International journal of hydrogen energy 46
15671-15690
Zivar, D., Kumar, S., & Foroozesh, J. 2021 Underground
hydrogen storage: A comprehensive review
International journal of hydrogen energy 46 23436-
23462
Cheng, X., Shi, Z., Glass, N., Zhang, L., Zhang, J., Song,
D., ... & Shen, J. 2007 A review of PEM hydrogen fuel
cell contamination: Impacts, mechanisms, and
mitigation Journal of Power Sources 165 739-756
Arsalis, A. 2019 A comprehensive review of fuel cell-based
micro-combined-heat-and-power systems Renewable
and Sustainable Energy Reviews 105 391-414
Tarhan, C., & Çil, M. A. 2021 A study on hydrogen, the
clean energy of the future: Hydrogen storage methods
Journal of Energy Storage 40 102676
Okolie, J. A., Patra, B. R., Mukherjee, A., Nanda, S., Dalai,
A. K., & Kozinski, J. A. 2021 Futuristic applications of
hydrogen in energy, biorefining, aerospace,
pharmaceuticals and metallurgy International journal of
hydrogen energy 46 8885-8905
Analysis of the Preparation-Storage-Use of Hydrogen
139
