
Method to Reduce the Cost of Proton Exchange Membrane Water
Electrolysis
Yongqi Hu
Water Supply and Drainage, East China Jiaotong University, Luoshi South Road, Hongshan District, Wuhan City, Hubei
Province, 430070, China
Keywords: PEM Water Electrolysis, Low Iridium Catalyst, Water Electrolysis, Stainless Steel Bipolar Plate, Hydrogen
Production Efficiency.
Abstract: Hydrogen energy is a kind of clean, pollution-free, long-term storage of secondary energy. It plays a pivotal
role in an energy system dominated by renewable energy sources to relealize emissions peaking and carbon
neutrality goals. The production of green hydrogen is a prerequisite for decarbonization. Proton exchange
membrane (PEM) hydroelectrolysis technology is an important way to produce green hydrogen using green
electricity. However, the high cost restricts the popularization of PEM electrolysis technology. To reduce the
cost of hydrogen fuel, it is necessary to strengthen the research on hydrogen production technology. The study
found that the cost of electrolysis is mainly composed of two parts: equipment cost and energy cost, replacing
expensive materials with low-cost materials to make electrolytic equipment and catalysts, and changing the
current density and temperature can reduce the cost of pem electrolytic water. However, how to make the
electrolytic equipment made of cheap materials meet or exceed the requirements of electrolysis still needs
further research, but how to make the electrolytic equipment made of cheap materials meet or exceed the
requirements of electrolysis still needs further research. This paper summarizes and analyzes the cost structure
of PEM electrolysis and the ways to reduce the cost and looks forward to the improvement direction of PEM
electrolysis system from two aspects of equipment cost and energy cost.
1 INTRODUCTION
Nowadays, the water electrolysis of hydrogen
production process mainly includes alkaline water
electrolysis (ALK), proton exchange membrane
water electrolysis (PEM), anion exchange membrane
water electrolysis (AEM) and solid oxide water
electrolysis (SOEC). Among them, the proton
exchange membrane water electrolysis hydrogen
production technology has developed rapidly in
recent years and is considered as one of the most
promising water electrolysis technologies to produce
hydrogen at this stage. PEM has compact structure,
high current density, small floor area, fast response
speed, electrolytic hydrogen production efficiency
can reach more than 85%, wide power regulation
range, good adaptation with fluctuating wind power
and photovoltaic, high integration degree, can
achieve long-term stable operation, and simple
opening and closing operation (Ge et al., 2024).
However, because the PEM electrolytic cell is
working in an acidic environment, platinum and
iridium need to be used as catalysts, the bipolar plate
and diffusion layer use titanium-based material (Sun
et al., 2024), the device cost is about 3~5 times of the
alkaline electrolytic cell, and the proton exchange
membrane mainly depends on import, and the service
life is short. This research looks for ways to lower the
cost of PEM water electrolysis and advance the
technology to produce more green hydrogen using
PEM water lysis.
2 PROTON EXCHANGE
MEMBRANE PRINCIPLE
PEM electrolysis of water for hydrogen production is
an efficient electrolysis technology based on ion
exchange technology. Membrane electrode assembly
(MEA), which comprises of anode and cathode
Hu, Y.
Method to Reduce the Cost of Proton Exchange Membrane Water Electrolysis.
DOI: 10.5220/0013850300004914
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 2nd International Conference on Renewable Energy and Ecosystem (ICREE 2024), pages 127-132
ISBN: 978-989-758-776-4
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
127
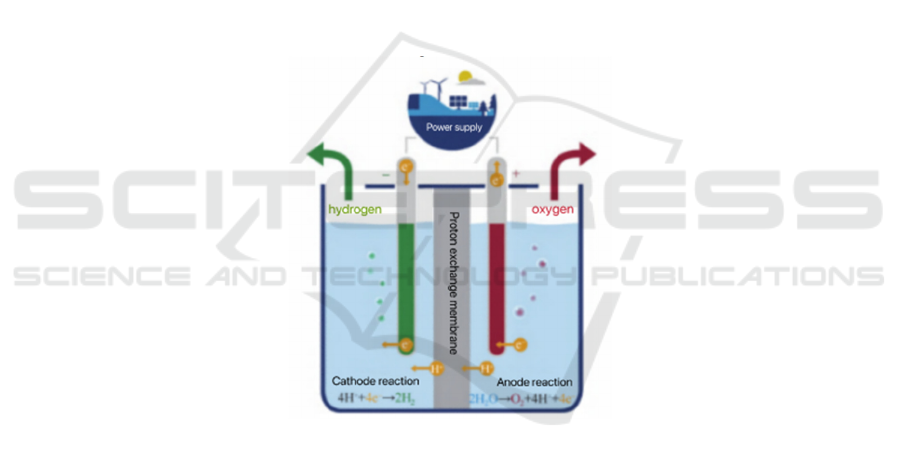
diffusion layer, anode and cathode catalytic layer, and
proton exchange membrane, is the essential
component of PEM electrolytic hydrogen production.
Schematic of PEM electrolysis cell can be seen in
figure 1. Proton exchange membrane is a kind of
polymer material, mostly using Nafion membrane
(Song et al., 2024), which plays an important role in
isolating the cathode and anode. The membrane used
in electrolysis has selective permeability, which can
conduct protons, but blocks the transmission of
electrons and gases. The catalytic layer is where
electrochemical reactions take place, and precious
metals are usually used as catalysts. The diffusion
layer is the intermediate layer between the collecting
plate and the catalytic layer, which transmits water,
gas and current. The electrochemical reaction occurs
at the three-phase interface (Wen et al., 2023), at the
junction of the proton exchange membrane, catalyst
and water.
Water is decomposed with electricity, generating
O2 and H+ on the anode side. The reaction occurring
in the PEM electrolytic cell is performed as in
equation (1) to equation (3).
Anode: 2H
2
O → 4H
+
+ O
2
+ 4e
-
(1)
Cathode: 4H
+
+ 4e
-
→ 2H
2
(2)
Whole reaction: 2H
2
O → 2H
2
+ O
2
(3)
Specifically, in the proton exchange membrane
electrolytic cell engineering, under the action of input
power and catalyst, water molecules in the anode is
decomposed into oxygen, H
+
and electron e
-
, H
+
and
water molecules into hydration ion H
3
O
+
, through the
membrane to the cathode under the electric field,
while the electron through the external circuit to leave
the electrolytic cell to the cathode, hydration ion
H
3
O
+
and electron e-in the cathode and solution
interface reduction reaction to produce hydrogen.
Figure 1: Schematic of PEM electrolysis cell (Song et al., 2024)
3 INFLUENCING FACTOR FOR
PEM PERFORMANCE
The cost of hydrogen production consists of four
parts: equipment cost, energy cost, other operating
cost and raw material cost (Zhao et al., 2021). Among
them, the energy cost, namely the electricity cost,
accounts for the largest proportion, generally
40%~60% (Guo et al., 2020; H, 2021 & Zhang et al.,
2021), or even up to 80% (The China Hydrogen
Energy Alliance, 2020), which is mainly affected by
the efficiency factor of electrolytic hydrogen
production. As a result, one crucial metric to capture
the economics of hydrogen production is its
efficiency of production. In order to lower the cost of
producing hydrogen from PEM water, it is crucial to
optimize the system's operating parameters and boost
production efficiency. The price of essential parts like
bipolar plates largely determines the equipment cost.
Because bipolar plates typically need to be coated
with Au or Pt, their cost makes up roughly 53% of the
total. The innovation of material technology to ensure
the performance of bipolar plates is of great
significance in terms of equipment cost reduction.
Less affordable alternative materials are currently
being studied. The rare metal Ir is indispensable for
making membrane electrodes. Although the cost of
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
128
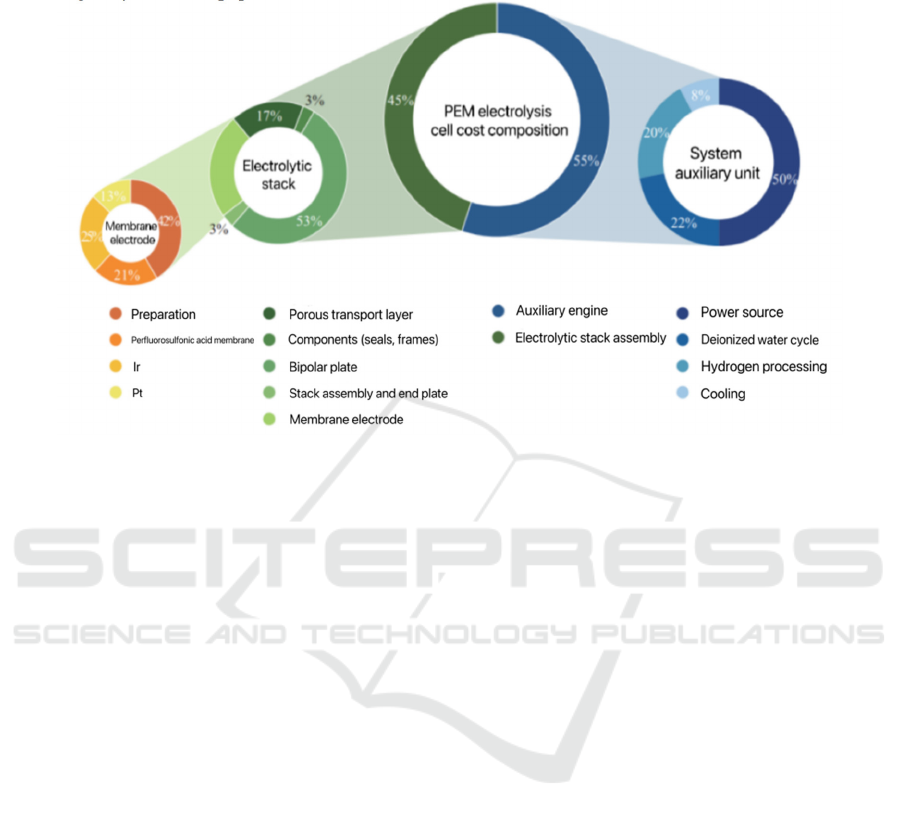
anode catalyst is not large in the total cost of the
electrolytic cell is large, the demand for iridium will
increase greatly with the popularization of iridium
technologies, such as PEM hydro electrolysis for
hydrogen production (Figure 2).
Figure 2: Cost break down for 1MW PEM electrolyser (International Renewable Energy Agency, 2020)
4 RESOLVENT FOR PEM
PERFORMANCE
ENHANCEMENT
4.1 Reduce the Equipment Cost
4.1.1 Change the Material of the Bipolar
Plate
The bipolar plate functions to provide mechanical
support to separate the membrane electrodes in the
stack, conduct heat and current in the stack, and
disperse water inside the PEM stack to deliver the
generated gas to the outlet. Generally, with gold-
plated or platinum-coated titanium plate as a bipolar
plate. The oxide film on the surface of the titanium
plate is low, and the oxide film on the surface must be
removed before coating. In addition, electroplating
needs to be punched on the surface, which will reduce
the corrosion resistance, and the coating thickness
needs to be increased to reduce the impact of the
pinhole. The thick coating and the complex
manufacturing process make the manufacturing cost
of the bipolar plate high, accounting for 18%~21% of
the total cost of the electrolytic cell (GAOAS et al.,
2026). In recent years, researchers have tried to use
non-precious metal material as the main material of
bipolar plate and modify its surface. Rojas et al.
(2021) used CrN/TiN, Ti/TiN, Ti and TiN as coatings,
and different models of stainless steel SS321,
SS316L, and SS904L as substrates. They were made
by PVD method. After testing the performance of the
electrolytic cell, they found that the performance of
CrN/TiN combination was comparable to that of
titanium-based bipolar plates coated with platinum.
4.1.2 Low-Cost HER Catalysts
Cathodic catalyst catalyzes the hydrogen evolution
reaction (HER) in the process of water electrolysis,
generally using platinum-based precious metal. The
platinum-catalyzed hydrogen evolution is three
orders of magnitude faster than the order of anode
(Hong, Gu and Zhen, 2024). Although the amount of
cathodic platinum is much lower than that of anodal
iridium, the amount of Pt still will increase
significantly with the large-scale application of PEM
hydroelectrolysis. Therefore, the development of low
Pt catalysts such as single atom catalyst, Pt alloy
catalyst and non-PT catalyst can effectively reduce
the cost of HER catalyst. Recently, it was found that
materials such as phosphide (CoP), sulfide (MoS2)
and nitride may replace Pt in the manufacture of
cathode catalysts, which have higher activity, better
stability and lower cost, although their performance
is still inferior to that of Pt (Ma et al., 2022).
Method to Reduce the Cost of Proton Exchange Membrane Water Electrolysis
129

4.1.3 The OER Catalyst for the Low Ir
Compared with the cathode hydrogen evolution
reaction, the reaction dynamics is slower and the
working potential is higher (> 1.23V) (Hong, Gu and
Zhen, 2024). The catalyst material needs to withstand
the high potential, strong oxidation and strong acidic
environment of the anode, which only some precious
metals can meet.
The anode catalyst of PEM electrolytic cell
commonly used in industry is mainly IrBlack and its
oxide IrO
2
. Considering the small Ir reserves and high
price, the anode catalyst becomes one of the main
obstacles to reduce the cost of PEM electrolytic cells.
Research and development of high activity, high
stability of PEM low iridium catalyst, is the key to
realize the commercial application of PEM
electrolytic cell. In recent years, doping or loading Ir
and oxides become the mainstream research
direction, usually known as low Ir catalyst, through
suitable preparation process, screening and
preparation performance of excellent anode catalyst,
effectively reduce the amount of precious metal Ir.
Doping other metallic elements with Iridium to
Form binary or ternary composite is a method to
directly reduce the Ir content in the catalyst. Its form
can be abbreviated as IrxMyNzOa, where M and N
are other precious metals or non-precious metals. The
addition of non-precious metals can effectively
expand the surface area of the catalyst and further
reduce the amount of precious metals without
reducing the activity. Commonly used non-precious
metals include Sn, Ta, Mo, Gd, Ce and other (Wang
et al., 2020 & Wang et al., 2021).
In addition to direct bonding, the doped
components can also adjust the electronic structure of
Ir by introducing oxygen vacancies, such as doped Fe,
Co, Ni, Zn, etc., which can produce large amounts of
oxygen vacancies. The crystal phase changes caused
by the introduction of other elements into the iridium-
based catalyst can also effectively enhance the OER
activity of the catalyst, such as the preparation of
perovskite-type and pyrochlorite-type iridium-based
catalyst (Hong, Gu and Zhen, 2024).
Loading the precious metal on the carrier is
another effective way to improve the dispersion and
reduce the dosage. It can also improve the utilization
rate of Ir through the carrier and the carrier to improve
the intrinsic activity of the precious metal. Due to the
harsh OER reaction conditions, the electrode catalyst
carrier needs to have both oxidation resistance,
corrosion resistance and high electrical conductivity
properties. However, common electrochemical
carriers cannot meet the above requirements at the
same time, such as cheap metals Ni, Fe, Co have good
electrical conductivity but poor corrosion resistance;
SnO
2
, TiO
2
, SiO
2
and others have acid resistance but
are all semiconductors or insulators with poor
electrical conductivity. Despite their corrosion
resistance and high conductivity, carbon carriers can
easily oxidize at high potentials. Therefore, materials
such as doped metal oxides, metal carbide and metal
nitrides have become the focus of supported iridium
catalyst carriers in recent years (Hong, Gu and Zen,
2024).
4.1.4 Change the Proton Exchange
Membrane Material
As a key component of PEM cell, the proton
exchange membrane is one of the determinants of the
cost and performance of PEM cell, and it acts as a
barrier to the conduction of protons and the resulting
gas produced. The key indicators of the proton
exchange membrane include electrical conductivity,
gas permeability, dimensional stability, and chemical
stability. Generally composed of polymer backbone
and negatively charged ion exchange groups,
additional additives and enhancers can be added to
improve membrane stability and reduce gas cross-
diffusion.
The most widely used in the PEM electrolytic cell
is the perfluoro sulfonic acid (PFSA) membrane, also
known as the Nafion membrane. The membrane
internal resistance is higher, the required electrolytic
voltage is higher, and the electrolytic efficiency of the
electrolytic cell is lower, but reducing the thickness
of the proton exchange membrane will lead to gas
penetration, reduce the purity of hydrogen
production, reduce the chemical and mechanical
stability of the membrane, and curtail the life of the
electrolytic cell. In addition, Nafion film also has
disadvantages such as high cost and fluorine pollution
(Beyraghi et al., 2020). Therefore, it is significant to
develop low-cost proton exchange membrane
materials with high conductivity.
Hydrocarbon based membrane not only low cost,
high conductivity, but also has high chemical stability
and dimensional stability, but also can reduce gas
penetration, is the first choice to replace Nafion
membrane.
The development of hydrocarbon-based
membrane and ionomer has great potential for cost
reduction, which is of great significance for the
construction of low-cost and high-performance PEM
electrolytic cell (Ma et al., 2022). Among them,
sulfonated polyaromatic ether is easy to synthesize
and modify and has excellent film formation, which
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
130
has wide applications in the preparation of proton
exchange membrane.
4.2 Improve the Efficiency of
Hydrogen Production
4.2.1 Relationship Between Current Density
and Hydrogen Production Rate
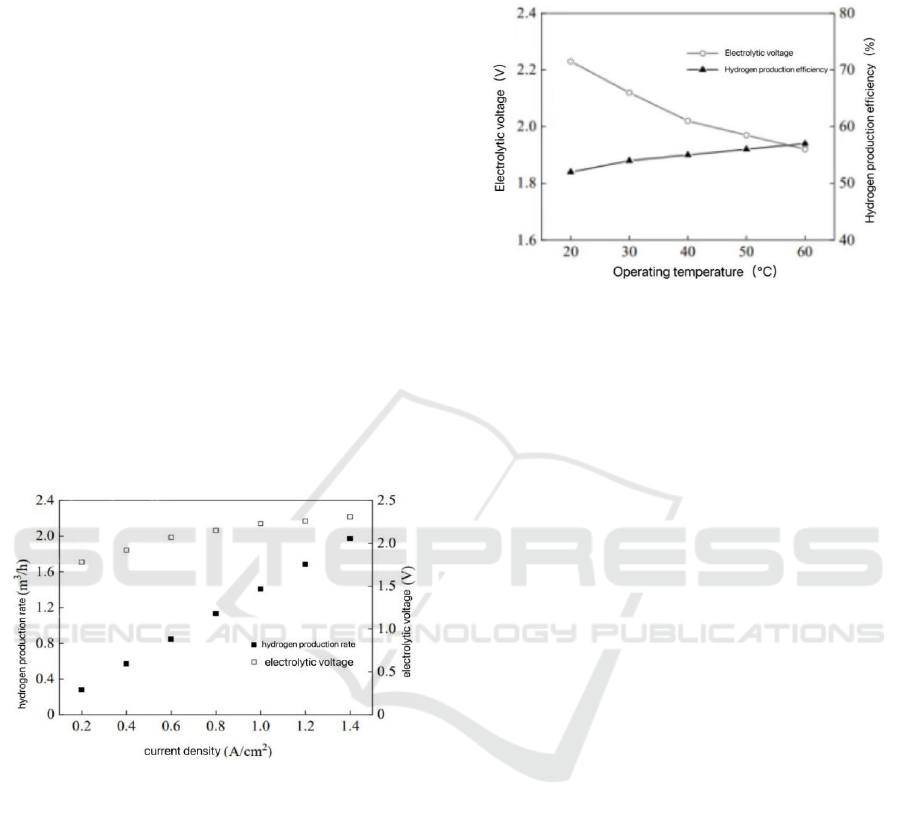
According to ZHU et al. (2020), when current density
rises, the electrolytic cell chamber's average
electrolytic voltage rises steadily and the rate at which
hydrogen is produced rises progressively as well.
Figure 3 illustrates that the rate of hydrogen
production is about 2.2 m3/h when the current density
is 1.4A / cm
2
. This is because the auxiliary equipment
gradually reaches the rated load operation state, and
the power utilization efficiency is gradually
improved.
In general, the PEM hydroelectrolysis hydrogen
production system has obvious technical economy
when the current density is high than when the current
density is low, which is consistent with the conclusion
that GRESPI et al (2023) PEM system running 60kW.
Figure 3: Relationship between current density and
hydrogen generation rate (Zhu et al., 2024)
4.2.2 Relationship Between Operating
Temperature and Hydrogen
Production Efficiency
The holding current density is 1.4A / cm
2
. The
electrolytic cell's average chamber voltage sharply
drops as the operating temperature rises, and the
system's efficiency in producing hydrogen is likewise
enhanced. Figure 4 illustrates that the efficiency of
hydrogen production is around 57% at an operating
temperature of 60 ℃ . This is because raising the
temperature lowers the active overpotential, quickens
the rate of the electrochemical reaction, and enhances
the electrolytic cell's performance. As a result, the
system uses less energy to produce hydrogen per unit.
OZDEMIR et al (2023) uses laboratory PEM
electrolytic cell at different temperatures, and the test
results are consistent with this test.
Figure 4: Cell voltage and hydrogen production efficiency
(Zhu et al., 2024)
5 CONCLUSION
This paper aims to reduce the cost of pem electrolysis
water, make hydrogen as a common fuel in the future,
and promote the application and development of
green hydrogen hydrogen production technology and
equipment. In terms of equipment costs, bipolar
plates, proton exchange membranes, and precious
metal catalysts make the equipment cost of pem water
electrolysis systems extremely high. Using stainless
steel as the main material and surface modification to
make bipolar plate, using cheap materials to make
proton exchange membrane, using low-cost, low
iridium catalyst play important roles in reducing the
equipment cost. In terms of energy cost, current
density and operating temperature are effective
control parameters for optimizing hydrogen
production efficiency. Hydrogen production
efficiency is influenced by both current density and
operating temperature. The peak working parameter
of hydrogen production efficiency appears at the
current density of 1.4A /cm2 to 1.7A/cm2, and the
best working temperature is about 60℃. Under these
conditions, the hydrogen production efficiency can
reach 50%-70%. Maintaining the optimal operating
temperature and current density, and making the low-
cost equipment achieve the same electrolytic effect as
the original expensive equipment is a problem that
needs to be overcome.
Method to Reduce the Cost of Proton Exchange Membrane Water Electrolysis
131

REFERENCES
Ge Shuqiang, Bai Jie, Ding Yongchun, et al. 2024
Development status and prospect of renewable energy
hydrogen production technology and its main
equipment Journal of Taiyuan University of
Technology 1-39
Sun Hao, Wu Weining, Chen Lijie, et al. 2024 Overview on
the development of new energy water hydrogen
production technology Journal of Power 1-18
Song Jie, Gao Jie, Liang Danxi, et al. 2024 Study of proton
exchange membrane Power Construction, 45 58-78
Wen Chang, Zhang Bohan, Wang Yaqin, et al. 2023
Research progress of high efficiency proton exchange
membrane water electrolysis technology Journal of
Huazhong University of Science and Technology
(Natural Science Edition) 51 111-122
Zhao Xueying, Li Gendi, Sun Xiaotong, et al. 2021 Key
technology and application progress of electrolytic
hydrogen production under the target of "double
carbon" The Global Energy Internet, 4 436-446
Guo Xiuying, Li Xianming, Xu Zhuang, et al. 2020 Cost
analysis of hydrogen production by electrolysis of
renewable energy. Energy Storage Science and
Technology, 9 688-695
Hu Daling. SIEMENS energy Power-to-X solution.2021
Renewable Energy Hydrogen Production Forum,
Shanghai
Zhang Xuan, Fan Xinye, Wu Zhenyu, et al. 2021 Hydrogen
supply chain cost analysis and suggestions Chemical
industry progress 41 2364
The China Hydrogen Energy Alliance. 2020 China
Hydrogen energy and fuel cell industry manual. Beijing
International Renewable Energy Agency. 2020 Green
hydrogen cost reduction: scaling up electrolysers to
meet the 1.5℃climate goal. AbuDhabi
Gagoas, Ansarsa, Saruhanb, et al. 2026 Protective coatings
on stainless steel bipolar plates for proton exchange
membrane ( PEM ) electrolysers. Journal of power
sources, 307 815-825
Rojasn, Sánchez-Molinam, Sevillag, et al. 2021 Coated
stainless steels evaluation for bipolar plates in PEM
water electrolysis conditions. International journal of
hydrogen energy, 46 25929-25943
Hong Siqi, Gu Fangwei, Zhen GJinyu. 2024 Development
status and prospect of low-iridium hydrogen catalyst
produced by PEM water electrolysis Chemical progress
1-15
Ma Xiaofeng, Zhang Shuhan, He Yong, et al. 2022
Research status and application prospect of PEM water
electrolysis for hydrogen production technology The
Journal of Solar Energy 43
420-427
Wang Yahui, Hao Shaoyun, Liu Xiangnan, et al. 2020 Ce-
dopedIrO(2)electrocatalysts with enhanced
performance for water oxidation in acidic media ACS
Applied Materials & Interfaces 12 37006-37012
Wang Yibo, Hou Shuai, Ma Rongpeng, etal. 2021
Modulating crystallinity and surface electronic
structure of IrO2 via gadolinium doping to promote
acidic oxygen evolution. ACS Sustainable Chemistry &
Engineering 9 10710-10716
Beyraghi F, Mirfarsi S H, Rowshanzamir S, et al. 2020
Optimal thermal treatment conditions for durability
improvement of highly sulfonated poly (ether ether
ketone) membrane for polymer electrolyte fuel cell
applications International journal of hydrogen energy
45 13441-13458
Zhu Ningwei, Zhao Jinghui, Xie Haiyang, et al. 2024 Effect
of current density and operating temperature on the
energy consumption of hydrogen production by PEM
water electrolysis New energy progress 1-5
Crespi E, Guandalini G, Mastropasqua L, et al. 2023
Experimental and theoretical evaluation of a 60 kW
PEM electrolysis system for flexible dynamic operation
Energy conversion and management 277 116622
Ozdemir S N, Taymaz I, Okumus E, et al. 2023
Experimental investigation on performance evaluation
of PEM electrolysis cell by using a Taguchi method
Fuel 344 128021
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
132
