
Preparation and Application Research of Carbon Nanotubes
Mohan Zhang
1,*
and Siyu Zhang
2
1
Sino-European School of Technology, Shanghai University, Shanghai, 200444, China
2
College of Chemistry and Chemical Engineering, Central South University, Changsha, 410083, China
*
Keywords: Carbon Nanotubes, Chemical Vapor Deposition (CVD) Method, Laser Ablation Method, Arc Discharge
Method, Lithium Batteries.
Abstract: In recent years, carbon nanotubes have shown broad application prospects and become the research frontier
and hotspot in the international new materials field. This paper mainly discusses the classification,
mechanical, and electrical properties of carbon nanotubes and their preparation methods, and analyzes their
application examples in fields such as lithium batteries. The results show that the mechanical properties of
carbon nanotubes are far superior to traditional materials and they also have excellent electrical properties.
Regarding the preparation methods of carbon nanotubes, the arc discharge method is simple, fast, and high-
yield, the laser ablation method has relatively higher costs, while the equipment used in chemical vapor
deposition (CVD) method is simple and capable of large-scale production. In practical applications, carbon
nanotubes have achieved results in areas such as cathode materials in lithium batteries, solid-phase extraction
adsorbents, and catalytic materials. This study holds significant practical significance in exploring low-cost,
large-scale production of carbon nanotubes and provides a scientific basis for their future applications in other
fields.
1 INTRODUCTION
In 1991, Professor Iijima, an electron microscopy
expert from a Japanese electronics company,
discovered for the first time under a high-resolution
transmission electron microscope some needle like
structures composed of 2-50 concentric tubes, while
preparing C60 using an arc method μm. This is carbon
nanotubes.
Carbon nanotubes are a type of material with a
radial size in nanometers and an axial size in
micrometers, which has a structure different from
other materials. The hexagonal carbon atoms form the
carbon nanotubes, which curl along certain axes to
form coaxial circular tubes. The distance between
carbon atomic layers is about 0.34 nm, with most
diameters ranging from 2-20 nm. It is precisely
because its many properties, such as mechanical
properties, are better than other materials that it has
been widely used and has attracted the attention of
scientists in many fields.
This article analysed and studied the preparation
and application of carbon nanotubes. Firstly, the
*
Corresponding author
classification of carbon nanotubes and their
mechanical and electrical properties were explained.
Then, the preparation of carbon nanotubes was
introduced from three methods: arc discharge
method, laser burning method, and chemical vapor
deposition method. Finally, the application examples
of carbon nanotubes in lithium batteries, fuel cells,
and other fields were elaborated.
2 CLASSIFICATION OF CARBON
NANOTUBES
2.1 Single Walled Carbon Nanotubes
The incredible strength and flexibility of single-
walled carbon nanotubes (SWCNTs) make them a
fascinating material for researchers and engineers.
These nanotubes are entirely composed of carbon
atoms. Their shapes are mainly divided into
armchairs, serrations, and chirality, each with its own
unique electronic properties.
Zhang, M. and Zhang, S.
Preparation and Application Research of Carbon Nanotubes.
DOI: 10.5220/0013847500004914
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 2nd International Conference on Renewable Energy and Ecosystem (ICREE 2024), pages 103-108
ISBN: 978-989-758-776-4
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
103

When it comes to mechanical properties, carbon
atoms in SWCNTs are bonded through extremely
strong C-C covalent bonds, indicating flexibility. In
addition, these nanotubes exhibit incredible high axial
strength, approximately 100 times that of steel. Their
elastic strain can reach up to 5%, with a maximum of
12%.
SWCNTs have received much attention for their
superior properties and applications in composites.
SWCNTs can give composites extraordinary
mechanical properties, significantly improving their
strength, toughness, elasticity and fatigue resistance.
SWCNTs also possess properties including
anisotropy and diamagnetism. SWCNTs have
extremely strong capillary action and wetting
features. They are the materials with the largest
hydrogen storage capacity that are expected to
promote the development of hydrogen fuel cells
compared to other traditional hydrogen storage
materials.
2.2 Multi Walled Carbon Nanotubes
Carbon nanotubes are connected by stable C=C
covalent bonds and thus have excellent mechanical
properties. Theoretical calculations indicate that
carbon nanotubes have extremely high strength and
toughness. The theoretical value estimates that the
Young's modulus can reach 5 TPa, with a strength
about 100 times that of steel, while the weight density
is only 1/6 of steel. Treacy et al. used Transmission
Electron Microscope (TEM) for the first time to
measure the mean square amplitude of multi walled
carbon nanotubes in the temperature range from room
temperature to 800 degrees.
3 PERFORMANCE OF CARBON
NANOTUBES
Carbon nanotubes are a type of nanomaterial with
excellent performance and broad application
prospects, and their unique structural characteristics
determine their potential for application in multiple
fields. Carbon nanotubes, as an important
nanomaterial, have many unique properties,
including structural characteristics, mechanical
properties, electrical properties, and thermal
properties. Studying its performance will help
promote the application of nanomaterials in various
fields.
3.1 Mechanical Properties
The mechanical properties of carbon nanotubes refer
to their mechanical response and performance under
external forces. When studying the mechanical
properties of carbon nanotubes, indicators such as
elastic modulus, yield strength, fracture strength, and
tensile properties are usually considered.
Carbon nanotubes have excellent elastic modulus,
usually above 1 TPa, and even up to hundreds of TPa.
This makes carbon nanotubes have important
application potential in fields such as nanomaterial
reinforcement and nanocomposites. The introduction
of carbon nanotubes (CNTs) into NC will reduce the
percolation threshold and resistivity of composite
materials, and improve the tensile strength of
composite materials (Zhang et al., 2024).
Due to the nanoscale characteristics of carbon
nanotubes, their yield strength is usually much higher
than traditional materials, reaching tens of GPa or
even higher. This makes carbon nanotubes have
important application prospects in fields such as
nanodevices and nanosensors. Meanwhile,
due to the influence of structural defects, external
environment, and other factors on the yield behaviour
of carbon nanotubes, researchers are also committed
to exploring the yield behaviour laws and influencing
factors of carbon nanotubes, in order to better apply
them in engineering practice.
3.2 Electrical Performance
Carbon nanotubes have good electrical properties due
to their helical tubular structure. Research has shown
that the current carrying capacity of single-walled
carbon nanotubes is 1000 times that of copper,
approximately 109A/cm2.The conductivity of single-
walled carbon nanotubes also changes with the
change of their diameter and helical mode. CNTs
dispersed in polymer matrices are more likely to form
conductive networks, and composite materials
prepared with CNTs as conductive fillers have a
percolation threshold of less than 5%, or even less
than 1% (Qiu et al., 2020).
3.3 Thermal Conductivity
Like conductivity, carbon nanotubes are also
excellent thermal conductors, with an axial thermal
conductivity of approximately 6600 W/m·K or
higher, far greater than diamond and graphite. Their
application in composite materials more extensive,
and the study of their properties and applications has
important theoretical and practical significance
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
104
(Zheng et al., 2024). It can become a thermal
conductive agent for the heat sink of Central
Processing Unit (CPU) chips in computer central
processing units. The carbon nanotube CPU heat sink
produced by OCZ Company, as shown in Fig. 1, is a
physical image of the central processing unit and an
example of the CPU. Its thermal conductivity
efficiency is 5 times that of ordinary copper materials.
In addition, single-walled carbon nanotubes can also
be used in high thermal conductivity composite
materials, with good application prospects.
Figure 1: Physical image of central processing unit (Chen
et al., 2024).
4 CARBON NANOTUBE
PREPARATION METHODS
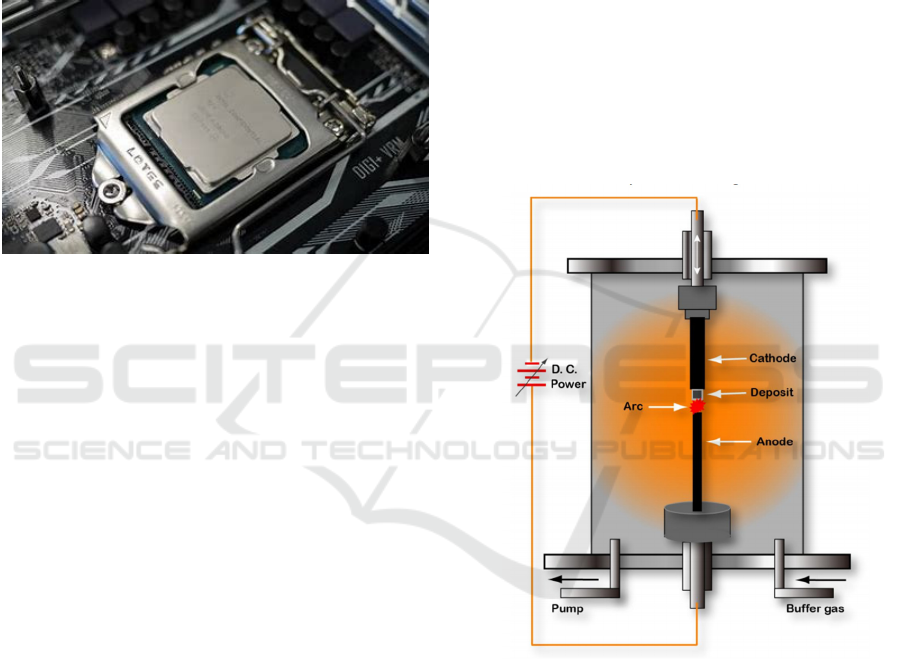
4.1 Arc Discharge Method
The main principle of the arc discharge method is to
place an anode and a cathode in a vacuum-sealed
container, fill it with an inert gas such as argon, and
apply a high voltage between the two poles to
generate an arc, creating high temperature and high
pressure conditions. In the high temperature
environment, carbon arc discharge generates high
energy, causing some atoms on the surface of the
conductive material to evaporate and ionize to form
high-energy ions. At the same time, the polarity of the
carbon source determines the type of product.
Ultimately, carbon atoms transfer in a free state at
high temperature and high energy under the action of
electrons and ions, condense, and deposit on the
cathode surface, forming carbon nanotubes. The
earliest carbon nanotubes were accidentally obtained
during the conventional arc discharge process used
for preparing fullerenes. In 1991, Iijima obtained
carbon nanotubes through arc discharge technology
in an argon environment (Iijima, 1991). In 1992,
Ebbesen et al. (1992) significantly increased the yield
of carbon nanotubes by increasing the helium gas
pressure in the arc chamber, reaching a level of
kilograms, making arc discharge one of the primary
methods for mass-producing carbon nanotubes. In
1993, Iijima and Ichihashi introduced a small amount
of transition metal iron into the graphite anode and
synthesized single-walled carbon nanotubes through
arc discharge in a methane and argon atmosphere
(Iijima, 1993).
The advantages of the arc discharge method are its
simplicity, speed, high yield, and high degree of
graphitization. Moreover, the carbon nanotubes
produced by this method exhibit excellent properties.
However, the arc discharge in this method is often
very intense, making it difficult to control the process.
Additionally, carbon nanotubes in the deposition on
the cathode during arc discharge are prone to sintering,
resulting in the presence of numerous carbon
nanograins. Fig. 2 shows the device for preparing
carbon nanotubes by arc discharge method.
Figure 2: Arc discharge apparatus for preparing CNTs
(Zhao, 2014).
4.2 Laser Ablation Method
This method was first discovered by Smalley et al.
(Guo et al., 1995), involves using high-energy lasers
as a heat source in an inert gas environment to
vaporize solid graphite for the synthesis of carbon
nanotubes. Initially, the graphite target is placed in a
tube furnace and heated to 1200 ℃ . The laser
vaporizes the graphite target, which is then carried by
a carrier gas to a copper collector equipped with a
wate cooling device, where carbon vapor condenses
Preparation and Application Research of Carbon Nanotubes
105

and grows on the cooled surface, forming carbon
nanotubes. Laser ablation method allows for the
control of carbon nanotube diameter by adjusting the
reactor temperature. However, the use of high-energy
lasers increases its cost compared to the arc discharge
method and chemical vapor deposition method. Fig.
3 illustrates a schematic representation of the
equipment used in the laser ablation method for
synthesizing carbon nanotubes.
Figure 3: Schematic representation of a laser vaporization
apparatus for preparing CNTs (Zhao, 2014).
4.3 CVD Method
Chemical vapor deposition is a process that utilizes
gaseous substances to undergo chemical reactions on
a solid surface, leading to the deposition of gaseous
products. In the context of carbon nanotube synthesis,
CVD involves the catalytic cracking and deposition
of carbon-containing gases on catalyst particles at
temperatures ranging from 700 ℃ to 1000 ℃
within a high-temperature tube furnace filled with a
carbon source and carrier gas.
Common carbon sources used in CVD include
ethylene, acetylene, ethanol, among others, while
commonly used carrier gases are mixtures of
hydrogen and argon. The Smalley research group, for
example, employed CO as the carbon source and
ferrocene as the catalyst, conducting continuous
synthesis of high-purity single-walled carbon
nanotubes with a diameter of 0.7 nm at temperatures
ranging from 1073 to 1473K and pressures from one
to ten atmospheres (Nilolave et al., 1999).
The equipment used in the CVD method is known
for its simplicity, easy controllability of conditions,
scalability for large-scale production, and the ability
to directly grow carbon nanotubes on suitable
substrates. Fig. 4 is a CVD apparatus for growing
CNTs.
Figure 4: Schematic representation of a CVD apparatus for
growing CNTs (Zhao, 2014).
5 APPLICATIONS OF CARBON
NANOTUBES IN PRACTICE
Carbon nanotubes possess unique electrical
conductivity, mechanical properties, and
physicochemical properties, which have attracted
widespread attention since their inception, and in
recent years, they have been widely applied in
numerous scientific research fields.
5.1 Applications in Lithium Batteries
Lithium-ion battery performance indicators such as
capacity and voltage largely depend on the
performance of the cathode material. However,
common cathode materials like lithium iron
phosphate and lithium cobalt oxide suffer from low
electrical conductivity, poor rate capability, and
cycling stability issues (Shi and Ding, 2012). These
problems mainly stem from structural changes
occurring within the material during the repetitive
lithium-ion intercalation and deintercalation
processes. Simultaneously, there is a decrease in
electron transport capability, ultimately leading to
cathode material pulverization and increased internal
resistance (Lu, 2022).
Carbon nanotubes exhibit excellent electron
conductivity and mechanical properties. They not
only provide good electron transport pathways for
cathode materials but also further enhance the
electrode's mechanical performance. Consequently,
carbon nanotubes are widely used in cathode
materials. Due to their superior electrical conductivity
and ion diffusion capabilities compared to traditional
materials like carbon black, carbon nanotubes are
commonly used as conductive additives in cathode
materials. Additionally, high-strength carbon
nanotubes can also enhance the mechanical
performance of electrodes.
Yang et al. prepared a LiFePO4@CNT composite
material with core and shell structure. By reducing the
radius of LiFePO4, the process can greatly reduce the
transmission path of lithium ions. At the same time,
the introduction of CNT can improve the conductivity
of the electrode material. The electrode material is
recycled at a large doubling rate of 50 C, and the
specific discharge capacity can still reach 65 mAh/g.
5.2 Applications of Solid Phase
Extraction Adsorbents
Carbon nanotubes have a large specific surface area
and strong adsorption capacity, making them
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
106

promising materials for solid-phase extraction.
Literature reports indicate that carbon nanotubes
exhibit high enrichment capabilities for organic
compounds (Liang et al., 2004), metal ions (Muñoz et
al., 2005), organic metal compounds (Cai et al., 2003),
and other environmental pollutants. Cai et al.
employed multi-walled carbon nanotubes to enrich
organic pollutants from environmental water samples,
such as bisphenol A, 4-nonylphenol and 4-
octylphenol. The results showed that multi-walled
carbon nanotubes had high enrichment efficiency,
surpassing or equaling that of solid-phase extraction
adsorbents like C18-bonded silica gel, XAD-2 resin,
and C60 fullerene (Liang et al., 2004; Cai et al., 2003
& Cai et al., 2005).
The development of novel adsorption devices
based on carbon nanotubes is becoming a research
hotspot in this field. Yuan Dongxing et al. (2004)
tested the performance of multi-walled carbon
nanotubes for enriching volatile organic pollutants
and compared them with common adsorbents
Carbopack B and VOCARB 3000, demonstrating the
superior enrichment performance of carbon
nanotubes. Saridara et al. (2005) used ethylene and
carbon monoxide as carbon sources to generate
carbon nanotube films on the inner surface of
stainless steel using chemical vapor deposition and
fabricated them into microtrap devices for volatile
organic compound detection. Experimental results
showed that carbon nanotubes synthesized from
ethylene had higher density and larger adsorption
capacity, exhibiting strong adsorption capabilities for
volatile organic small molecules.
5.3 Applications in Catalyst Materials
Due to recent advancements in the functionalization
of carbon nanotube walls, coupled with their
excellent electron conductivity, unique adsorption
and desorption properties towards reactants and
reaction products, special spatial stereoselectivity in
pore structure, strong metal-carbon interactions in
carbon-metal catalysts, as well as the specific
catalytic and photocatalytic properties induced by
quantum effects in carbon nanotubes, along with their
strong oxidizing and reducing capabilities, there has
been considerable interest in the application of carbon
nanotubes in catalytic chemistry. Currently, there are
not many examples of direct utilization of carbon
nanotubes as catalysts. Lou et al. reported the direct
application of carbon nanotubes with a surface area
of 180 m2/g in the catalytic reduction of NOx,
achieving 8% conversion of NO at 573K, which
increased to 100% at 873K. This represents a
successful application of nanomaterial quantum
effects in catalytic chemistry. Carbon nanotubes are
mainly used as carriers in catalytic processes due to
their unique electronic properties, pore structure and
adsorption capacity. Planeix was among the first to
utilize carbon nanotubes as catalyst supports. They
found that catalysts with multi-wall carbon nanotubes
loaded with Ru exhibited up to 90% selectivity and
an 80% conversion rate in the hydrogenation
synthesis of cinnamaldehyde to cinnamyl alcohol.
6 CONCLUSION
Carbon nanotubes possess unique electrical
conductivity, mechanical properties, and physical and
chemical properties, making them widely applied in
various scientific research fields in recent years.
Carbon nanotubes mainly include single-walled
carbon nanotubes and multi-walled carbon nanotubes.
The mechanical properties of carbon nanotubes far
exceed those of traditional materials, reaching tens of
GPa, while also exhibiting excellent electrical
conductivity, making them excellent thermal
conductors.
Among the methods for preparing carbon
nanotubes, the arc discharge method offers the
advantages of simplicity, speed, and high yield, while
the laser ablation method incurs relatively higher
costs. On the other hand, the equipment used in CVD
method is simple and capable of large-scale
production. Currently, carbon nanotubes are widely
used as cathode materials in lithium batteries,
showcasing broader application ranges and higher
mechanical performance. Additionally, their
application as solid-phase extraction adsorbents
presents promising prospects. However, there are
relatively few examples of direct application of
carbon nanotubes in catalytic materials.
In the future, researchers should focus on
improving the existing shortcomings of carbon
nanotubes and developing more diverse applications
to achieve their large-scale utilization. This study
holds significant practical significance in exploring
low-cost, large-scale production of carbon nanotubes
and provides a scientific basis for their future
applications in other fields.
AUTHORS CONTRIBUTION
All the authors contributed equally and their names
were listed in alphabetical order.
Preparation and Application Research of Carbon Nanotubes
107

REFERENCES
H.J. Zhang, Q.F. Wang, H.R. Wei, et al. Engineer. Plas.
Appl., 52, 7 (2024)
J. Qiu, Z.W. Jiang, H.P. Xing, et al. Chinese J. Appl.
Chem., 37, 8 (2020)
M. Zheng, J. Liu, K. Shi, et al., Molec. Cat., 554113737
(2024)
G. Chen, Y.Z. Hu, N. Chen, et al., Mater. Chem and Phys.,
314128842 (2024)
S. Iijima, Nature, 354, 56-58 (1991)
T.W. Ebbesen, P.M. Ajayan, Nature, 358, 220-222 (1992)
S. Iijima, T. Ichihashi, Nature, 363, 603-605 (1993)
T. Zhao, Shanghai Jiao Tong University (2014)
T. Guo, P. Nikolaev, A. Thess, et al., Chem Phys Lett, 243,
49-54 (1995)
P. Nilolave, M.J. Bromikowski, P.K. Bradley, Chem. Phys.
Lett., 313, 91-94 (1999)
J.Y. Shi, Y.S. Ding, Anhui Chemical Industry, 38, 37-39
(2012)
X.T. Lu, Close Ag. appl. mater., 51, 121-123 (2022)
P. Liang, Y. Liu, L. Guo, et al., J. Anal. At. Spectrom, 19,
1489–1492 (2004)
J. Muñoz, M. Gallego, M. Valcárcel, Anal. Chem., 77,
5389–5395 (2005)
Y.Q. Cai, G.B. Jiang, J.F. Liu, et al. Anal. Chem., 75, 2517–
2521 (2003)
Y.Q. Cai, G.B. Jiang, J.F. Liu, et al.l, Anal. Chim. Acta.,
494, 149–156 (2003)
Y.Q. Cai, Y.E. Cai, S.F. Mou, et al., J. Chrom. A., 1081,
245–247 (2005)
Q. Li, D. Yuan, Q. Lin, et al., J. Chrom. A., 1026, 283–288
(2004)
C. Saridara, R. Brukh, Z. Ipbal, et al., Anal. Chem., 77,
1183–1187 (2005)
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
108
