
Research Advances on Electrocatalysts for Oxygen Reduction
Reaction in Fuel Cells
Wangjie Shangguan
School of Materials and Environmental Engineering, Hangzhou Dianzi University, Zhejiang, 310018, China
Keywords: Fuel Cell, ORR, Electrocatalyst.
Abstract: Nowadays, fuel cells have attracted much attention because they produce almost no carbon dioxide and air
pollutants, help reduce greenhouse gas emissions and air pollution, and mitigate climate change. Among them,
seeking electrocatalysts for the oxygen reduction reaction (ORR) is a top priority. This article aims to
introduce platinum group metals, transition metal compounds, and carbon-based non-noble metal
electrocatalysts with novel nanostructures. At the same time, it summarizes the research progress of various
electrocatalysts in recent years. The optimization strategies of catalytic processes are reviewed and their
development prospects are prospected. In addition, problems existing in the research on oxygen reduction
electrocatalysts are summarized. In the future, these contents can help researchers develop new catalysts and
electrolyte materials, optimize manufacturing processes, enhance system integration, study fuel cells for
portable and large-scale applications, explore hydrogen production and storage technologies, and integrate
fuel cells with smart grids and distributed Integrated energy systems.
1 INTRODUCTION
In today's society, energy consumption is increasing,
and concurrently, the issue of environmental
degradation is progressively intensifying.
Consequently, the advancement of alternative and
renewable energy sources has ascended to an
imperative priority status. A fuel cell typically
comprises three components: a cathode, an anode,
and an electrolyte. It uses hydrogen, methanol,
ethanol and other fuels to react with oxygen to
produce electrical energy and water. In a fuel cell, it
can be divided into ORR and Hydrogen Oxidation
Reaction (HOR) are direct reactions that occur at the
cathode and anode, respectively. ORR refers to a
sequence of reactions involving oxygen that transpire
within an electrochemical system, in which oxygen
accepts electrons and chemically reacts with water or
other substances. HOR refers to a series of reactions
that occur with water in an electrochemical system in
which water molecules lose electrons and form
hydroxides or other products with protons. In fuel
cells, the hydrogen oxidation reaction usually refers
to the reaction of hydrogen gas at the anode, in which
the hydrogen molecule loses electrons and combines
with protons to form water.
Compared with traditional internal combustion
engines, its advantages are more obvious. In practical
applications, the electrical energy conversion
efficiency of fuel cells is usually between 40% and
60%. However, traditional internal combustion
engines convert the thermal energy generated by fuel
combustion into mechanical energy, which is then
converted into mechanical energy through a
generator. Electric energy has low efficiency, only
20%~40%. More importantly, fuel cells have a lower
environmental impact. However, the slow kinetic
factor in the fuel cell results in a low oxygen reduction
reaction efficiency on the cathode, which in turn is
affected by the O=O bond energy (498 kJ/mol). Even
though platinum and its alloys have been validated as
the preeminent electrocatalysts for oxygen reduction,
their exorbitant cost and the complexities associated
with their preparation impede their large-scale
utilization. Hence, it is urgent to find a new cathode
electrocatalyst capable of supplanting the traditional
platinum-group metal-based counterparts, and non-
precious metal (NPM)-based oxygen reduction
electrocatalyst has emerged as an exceptional
alternative. The purpose of this paper is to introduce
platinum group metals, transition metal compounds
and carbon-based non-noble metal electrocatalysts
58
Shangguan, W.
Research Advances on Electrocatalysts for Oxygen Reduction Reaction in Fuel Cells.
DOI: 10.5220/0013845000004914
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 2nd International Conference on Renewable Energy and Ecosystem (ICREE 2024), pages 58-63
ISBN: 978-989-758-776-4
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

with new nanostructures. Strategies for optimizing
the catalytic process are also reviewed, and finally
their development prospects are prospected. In
addition, the existing problems in the research of
oxygen reduction electrocatalysts are summarized.
2 ORR ELECTROCATALYTIC
MECHANISM
The mechanism of ORR mainly includes the
following steps. Firstly, O
2
molecules become
attached to the catalytic surface, engaging with the
active site on the surface to form adsorbed oxygen
(O
ads
). Adsorbed oxygen (O
ads
) accepts electrons and
is converted into peroxy intermediates (OOH
-
).
Secondly, the peroxy intermediate (OOH
-
) further
accepts electrons and protons and is reduced to water
(H
2
O).
The influence of catalysts on ORR is mainly
reflected in increasing the reaction rate and reducing
the activation energy. Excellent ORR catalysts should
have high active site density and catalytic activity,
and can reduce the energy loss of reaction. Current
challenges include issues such as catalyst stability,
cost and durability. In response to these challenges,
researchers are seeking to design more efficient and
stable catalysts and explore novel materials and
structural designs.
2.1 Dynamic Influencing Factors
The adsorption/desorption of oxygen-containing
intermediates, e.g. O
2
*
,
*
O,
*
OH,
*
OOH and HOOH
*
on the catalyst surface is a key factor affecting ORR
kinetics (Nørskov et al., 2004). O
2
→O
2
*
(The
adsorption process of O
2
) as the initial step of the
reaction determines the subsequent reaction process,
and the initial potential of the reaction is determined
by the adsorption/desorption of
*
OH (at high
potential), and the effective number of active centers
is determined by the adsorption amount of
*
OH. More
importantly, the adsorption of the catalyst to the
intermediate can directly affect the catalytic activity,
too weak adsorption strength will hinder the
proton/electron movement, too strong adsorption
strength will cause desorption.
2.2 Promotion Mechanism of Catalyst
Performance
By adjusting the diameter and shape of the catalyst
particles, the exposure degree of the active site of the
catalyst and oxidation reaction can be increased, so as
to increase the specific surface area of the catalyst, so
as to improve the performance of the ORR. The
synthesis of core-shell structured catalysts and the
choice of catalyst support possessing comparatively
expansive specific surface areas can markedly
enhance catalytic efficacy. Simultaneously,
modulating the electronic structure of Pt induces a
downward shift in the D-band center, thereby altering
the binding energy between the catalyst and aerobic
species. Appropriately tuning this binding energy can
thereby bolster catalyst activity at its fundamental
source.
2.3 Inhibition and Strategies for
Stability Enhancement
The decline in the activity stability of Pt catalyst can
be attributed to two primary factors: Initially, the
dissolution, aggregation, and dispersion of Pt
particles result in the depletion of active constituents.
Secondly, corrosion of the carbon carrier attenuates
the interaction between catalytic particles and the
carrier. The stability of catalyst activity can be
achieved through the fabrication of Pt-transition
metal alloys and intermetallic compounds.
Nevertheless, Pt electrode will generate PtO after a
long period of operation, thereby overlaying the
original active sites. Alternatively, the
oxidation/dissolution of other metals resulted in the
thickening of the Pt layer and subsequent reduction in
activity. Hence, when selecting various carriers to
enhance catalyst activity, the corrosion resistance of
the carrier stands as a pivotal consideration.
Based on the above, the search for a carrier with
high corrosion resistance is essentially to extend the
service life and reduce costs. Previous studies have
shown that the oxidation of Pt on the catalyst’s
surface will cause Pt to corrode and then chemically
dissolve. Therefore, in order to avoid that, it is an
effective means to cover the surface with a layer of
oxygen-bound substances. However, there is also the
possibility of electrochemical dissolution acting
directly on the unmasked Pt nanoparticles and thus
degrading the catalyst. Research in this area shows
that the quality of Pt does not change within the
working potential range of 0-0.8V vs. RHE. However,
in order to prevent Pt from being interfered by
oxidation factors, under highly acidic conditions and
the working potential is greater than 0-0.8V vs. RHE,
the quality of Pt was found to decrease significantly
(Liu et al., 2010 & Rinaldo et al., 2010). It can be seen
that if you want to enhance stability, you must
optimize the size and crystal structure of Pt, because
Research Advances on Electrocatalysts for Oxygen Reduction Reaction in Fuel Cells
59

the thermodynamic properties of tiny particles are
inherently unstable, and at the same time, the surface
energy is relatively high, making them easier to
aggregate. Not only that, the carbon corrosion
phenomenon caused by high potential is also not
conducive to maintaining the Pt/C system. Although
increasing the size has the potential to reduce ECSA
and catalyst activity because it results in a lower
surface-to-volume ratio, economic considerations
require sacrificing some activity to extend the
operational longevity of the catalyst. The following
related research is conducted based on the principle
of developing multi-dimensional Pt nanostructures.
Compared with conventional isotropic nanoparticles,
this complex structure remains more stable. Among
them, adding metals with higher oxidation potential
to improve the stability of Pt is one of the most
promising methods. For example, attaching Au
particles to the surface of Pt, after 30,000 potential
cycles, the catalyst still maintains good stability and
almost no decay (Zhang et al., 2007). In addition, you
can also consider using a protective coating, which
can effectively prevent the non-precious metals inside
from being corroded by the environment. However,
protective coatings, especially carbon coatings under
high-temperature conditions, will inhibit the
exchange process between reactants and active sites.
Although the size and structure of the catalyst are
optimized, its stability is still limited. Therefore, there
is an urgent need to find other methods to solve the
above problems, and alloying is one of them. The
alloy has stronger dissolution resistance and is
relatively cheap. Alloying can improve durability, but
it will still be accompanied by corrosion of the metal,
which will cause particles to overflow, leading to
redeposition and aggregation problems. This
phenomenon was found on the PtAu surface of the
PtAuCo catalyst, with only 25% loss after 100,000
potential cycles (Tan et al., 2015). Such excellent
stability is attributed to the self-healing mechanism of
Au to fine-tune the surface electronic structure.
Isolated Au with low surface energy can improve the
stability of the PtAu surface, thereby mitigating the
corrosion and dissolution process of Co (Sasaki et al.,
2012).
3 ELECTROCATALYSTS
Electrocatalysts are capable of diminishing the
activation energy associated with electrochemical
reactions, thereby augmenting the rate of these
reactions. In addition, it can improve the efficiency of
electrochemical reactions, resulting in higher product
output at the same voltage or current. Moreover, some
electrocatalysts can promote certain reaction
pathways, thereby improving product selectivity and
reducing the occurrence of side reactions.
3.1 Platinum Group Metals
The platinum group metals have the characteristics of
high melting point, high density, good corrosion
resistance, catalytic performance and oxidation
resistance, so they are excellent choices for use as
electrocatalysts. Platinum group metal
electrocatalysts mainly include platinum-based alloys
and core-shell structures. The former option has the
potential to diminish the utilization of the precious
metal platinum and concurrently mitigate the
likelihood of catalyst poisoning. The central layer of
the latter consists of base metals, while the outer layer
comprises platinum. Employed as an electrocatalyst,
it exhibits traits such as minimal Pt loading,
exceptional endurance, and commendable cost-
effectiveness.
3.1.1 Pt Alloy
While Pt exhibits highly efficacious catalytic activity
for ORR, its limited abundance and expensive nature
have emerged as primary obstacles impeding the
advancement of fuel cell technology. Therefore, the
use of platinum-based binary alloy or ternary alloy
electrocatalyst constitutes an efficacious method to
improve both the activity and stability of ORR. Pt-
based alloys serve to curtail the consumption of Pt
elements while concurrently mitigating the likelihood
of catalyst poisoning occurrences, and improving the
catalytic efficiency of ORR. Employing the soft
template method, Yang et al. successfully engineered
Pt-Co nanowire networks that serve as
electrocatalysts for ORR (Yang et al., 2016). Tang et
al. synthesized ultrafine Pt nanoparticles, doped with
minute amounts of Co and modified them on carbon
black carrier via modified ethylene glycol reduction
and chemical etching techniques (Tang et al., 2019).
The uniform distribution of Pt-Co nanoparticles was
successfully achieved without the addition of extra
surfactants, leading to enhanced catalytic efficacy and
stability of the Pt-Co nanoparticles.
3.1.2 Pt-Based Core-Shell Structure
While the fabrication of alloy-type catalysts helps to
minimize the financial expenditure of catalysts, the
potential loss of metal atoms through dissolution
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
60
during continuous operation may diminish the
catalytic activity of the catalyst. Core-shell Pt-based
catalysts exhibit attributes such as low Pt loading,
cost-effectiveness, and high durability, thus
showcasing broad prospects for application. Core-
shell Pt-based catalysts typically consist of two
components: the core layer is mainly composed of
non-precious metals, while the shell primarily
comprises precious metals. The non-precious metal
core layer is wrapped by a Pt layer of precious metal.
By establishing a thin Pt shell encapsulating the core,
the exposed surface area of platinum particles can be
effectively augmented, thereby significantly
improving the utilization rate of platinum. Owing to
the electronic interaction between the core and shell,
the existence of different metal clusters accelerates
the oxidation of Pt, thus alleviating the dissolution
and loss of Pt particles at high electric potential.
G.M.Leteba et al. synthesized binary Pt-Ni
nanoparticles via a co-pyrolysis approach and
optimized the synthesis parameters, consequently
yielding a substantial enhancement in catalytic
performance (Leteba et al., 2020). F. Zhou et al.
successfully synthesized Pt/C catalyst possessing a
core-shell architecture via an one-step self-assembled
calcination process (Zhou et al., 2020). In this
procedure, commercial platinum carbon underwent
treatment with isopropyl alcohol and Nafion. The
commercial platinum carbon underwent combustion
and decomposition in a nitrogen atmosphere,
resulting in the formation of a porous coating devoid
of sulfonate. The results show that there is a thin
porous layer between Pt particles and Nafion, which
effectively improves the performance of the catalyst
without affecting the electrical conductivity and
oxygen conduction rate.
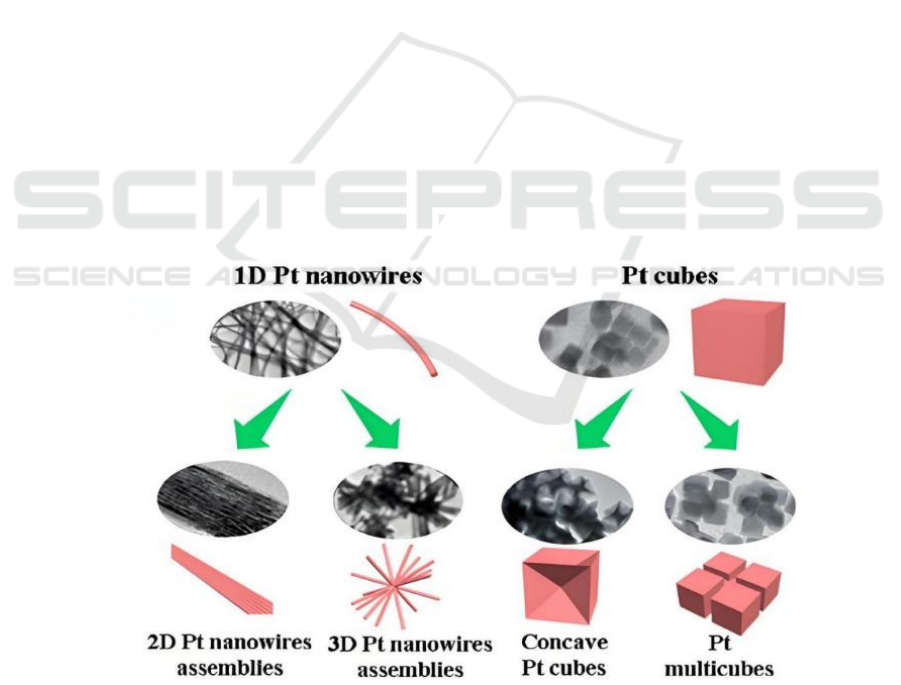
3.1.3 Pt-Based Nano-Structure
In recent years, researchers have devoted themselves
to designing and preparing different Pt
nanostructures, including 1D nanowires, 2D
nanoplates, and 3D nanoframes. The above-
mentioned nanostructures serve to effectively
augment the utilization rate of Pt, thereby improving
catalytic performance, accelerating electron transfer,
and exposing more active sites, shown as Figure 1. It
is worth noting that such nanostructures are
anisotropic and have stronger crystallinity, allowing
the catalyst to remain active for a longer period of
time. Ultra-long and ultra-thin platinum nanowires
not only facilitate the unimpeded conveyance of
electrons, but can also serve as the basic material for
forming 2D nanoplates and 3D nanoframes because
these nanowires are malleable. Moreover, this will
have a more stable structure and larger space, which
will help the stable transfer of electrons.
Figure 1: Structure control of Pt catalysts (Huang et al., 2021)
3.2 Transition Metal Electrocatalysts
Nowadays, Pt-based electrocatalysts have been
commonly used in ORR, with pronounced
initialization and robust half-wave potential.
However, it confronts challenges such as elevated
Research Advances on Electrocatalysts for Oxygen Reduction Reaction in Fuel Cells
61

costs and limited stability. Consequently, there is an
imperative necessity to identify cost-effective non-
noble metal catalysts (NPMC) to replace them.
Transition metal catalysts are cheap, have high
catalytic activity, and have better controllability,
thereby achieving precise control of the reaction.
More importantly, it has better stability and can
maintain its catalytic activity for a long time under
reaction conditions, extending the service life of the
catalyst.
3.2.1 Carbides
To find a catalyst at a lower cost, metal carbide
materials such as tungsten carbide (WC) were
initially evaluated. Tungsten carbide possesses
surface electronic properties akin to those of metal Pt
and is naturally occurring in various formations.
At present, the vast majority of research focuses
on its application as an anode electrocatalyst, and has
demonstrated exceptional catalytic performance.
However, the stability of WC is affected when it
encounters acids and high concentrations of oxygen.
Therefore, it seems difficult to realize the pristine WC
material as an ORR electrocatalyst and utilize it in
fuel cell cathodes without changing its composition
and structure.
Recently, Yu and his team conducted unbiased
structure searches and first-principles calculations for
the 2D TiC
2
, VC
2
, NbC
2
, TaC
2
and MoC
2
to
comprehensively investigate the electrocatalytic
properties (Yu, Zhou and Sun, 2020). Owing to their
excellent conductivity, these materials can facilitate
rapid charge transfer kinetics in catalytic reactions.
3.2.2 Oxides
Oxides have better stability in acidic, alkaline and
oxidising environments than transition metal
carbides. As well as demonstrating certain ORR
activity in alkaline solutions, however, enhancing
their ORR performance remains a formidable
challenge. Due to the altered atomic coordination
environment and electronic structure of the metal
oxides, a certain strain is generated on the catalyst
surface, which leads to a significant increase in its
adsorption capacity with ORR intermediates. Of
these, manganese oxide has garnered considerable
interest on account of its cost-effectiveness,
environmental protection, polyvalent states and
diverse crystal structures. Previous studies have
shown that MnO
x
activity follows a trend as Mn
5
O
8
<Mn
3
O
4
<Mn
2
O
3
. This shows that the MnO
x
catalyst
performance changes with its different crystalline
morphology (Chu et al., 2014). Thus, Zhang et al.
adopted the method of calcination of manganese
glycolate to synthesize Mn
3
O
4
with manganese
defects. In Mn
3
O
4
, the change in electronic structure
makes it a better conductor. Moreover, Wang and his
team prepared a new 3D oriented monolithic
integrated electrode. The electrode consists of Fe
3
O
4
cores and N-doped C shell composite nanostructure
(Wang et al., 2020). Owing to its judicious pore
architecture and N-Fe synergy, as well as the
optimized combination of metal species modification
of the N species catalytic site, the material exhibits
considerable ORR activity in acidic solutions.
3.3 Carbon-Based Electrocatalysts
A carbon-based electrocatalyst is a catalyst composed
of carbon-based materials that is used to promote
electrochemical reactions. The carbon nanotubes,
graphene, carbon black, and porous carbon have good
electrical conductivity, chemical stability and
structural tunability. Compared with transition metal
electrocatalysts, carbon-based electrocatalysts have
lower raw material costs; they do not contain heavy
metal elements and are environmentally friendly.
3.3.1 Metal-Organic Framework Carbon
Materials (MOFs)
MOFs represent a novel class of microporous
materials, constructed from the assembly of metallic
ions and organic linker molecules. The intrinsic
porosity of MOFs endows the resulting carbonaceous
materials with a wealth of pores and elevated specific
surface areas, facilitating enhanced mass transport
capabilities and, consequently, manifesting superior
electrocatalytic properties (Kalaj et al., 2020). Qiao
and colleagues employed a method of dissolve-
induced heteronucleation to create layered ordered
porous carbon on a polystyrene sphere (PS) template,
which was subsequently carbonized to yield a
material doped with atomically dispersed FeN
4
(FeN
4
/HOPC). Following this, they developed Fe-
doped ordered macroporous/microporous ZIF-8
(referred to as OMSFe-ZIF-8) (Xu et al., 2020).
In comparison to monometallic MOFs, bimetallic
MOFs exhibit enhanced catalytic performance due to
synergistic effects between the incorporated metallic
species, thereby demonstrating superior activity. Han
et al. successfully prepared a new type of binary Co-
Ni sites by pyrolysis of MOFs containing dopamine
(DPA) coating. These sites are atomically dispersedly
embedded into N-doped hollow carbon nanotubes,
showing a highly reactive single-atom dispersed state.
At the same time, the synergistic catalytic effect of the
bimetallic Co-Ni sites reduces the energy barrier of
the reaction and accelerates the reaction. the reaction
ICREE 2024 - International Conference on Renewable Energy and Ecosystem
62

kinetics. Consequently, it exhibits exceptional ORR
performance similar to Pt/C (Han et al., 2019).
4 CONCLUSION AND OUTLOOK
This article comprehensively reviews the research
progress of Pt noble metal, transition metal and
carbon-based material ORR electrocatalysts and
reviews strategies to optimize the catalytic process.
Although the ORR mechanism of some materials has
been extensively studied, for most emerging
materials, the mechanism of ORR remains
incompletely elucidated. Therefore, it’s urgent to
have an in-depth comprehension of the mechanism.
Although the overall scientific research strength is
increasing, the physical characterization methods are
becoming more and more perfect. Some ideal
catalysts can be prepared and constructed accurately
by in-situ characterization techniques, but there are
still some limitations in the study of these catalysts in
the field of theoretical calculation. Therefore, in the
future, it is necessary to further improve the research
capabilities by combining theoretical calculations and
experiments on Pt-based catalysts, and explore the
ability to operate within a wider pH range. Efficiently
working ORR electrocatalyst. All things considered,
designing a robust, efficient and eco-friendly
electrocatalyst is the current challenge that needs to
be solved.
REFERENCES
Nørskov J K, Rossmeisl J, Logadottir A, Lindqvist L,
Kitchin J R, Bligaard T and Jonsson H 2004 J. Phys.
Chem. B 108 17886-92
Liu X, Chen J, Liu G, Zhang L, Zhang H and Yi B 2010 J.
Power Sources 195 4098-103
Rinaldo S G, Stumper J r and Eikerling M 2010 J. Phys.
Chem. C 114 5773-85
Zhang J, Sasaki K, Sutter E and Adzic R 2007 Science 315
220-2
Tan X, Prabhudev S, Kohandehghan A, Karpuzov D,
Botton G A and Mitlin D 2015 ACS Catal. 5 1513-
24
Sasaki K, Naohara H, Choi Y, Cai Y, Chen W-F, Liu P and
Adzic R R 2012 Nat. Commun. 3 1115
Yang D, Yan Z, Li B, Higgins D C, Wang J, Lv H, Chen Z
and Zhang C 2016 Int. J. Hydrogen Energy 41
18592-601
Tang X, Fang D, Qu L, Xu D, Qin X, Qin B, Song W, Shao
Z and Yi B 2019 Chin. J. Catal. 40 504-14
Leteba G M, Mitchell D R, Levecque P B, Van Steen E and
Lang C I 2020 RSC Adv. 10 29268-77
Zhou F, Yan Y, Guan S, Guo W, Sun M and Pan M 2020 Int.
J. Energy Res. 44 10155-67
Huang L, Zaman S, Tian X, Wang Z, Fang W and Xia B Y
2021 Acc. Chem. Res. 54 311-22
Yu Y, Zhou J and Sun Z 2020 Adv. Funct. Mater. 30
2000570
Chu W, Higgins D, Chen Z and Cai R 2014 Non‐Noble
Metal Fuel Cell Catalysts (New York: Wiley) pp 357-
88
Wang Y, Wu M, Wang K, Chen J, Yu T and Song S 2020
Adv. Sci. 7 2000407
Kalaj M, Bentz K C, Ayala Jr S, Palomba J M, Barcus K S,
Katayama Y and Cohen S M 2020 Chem. Rev.
120 8267-302
Xu X, Yang T, Zhang Q, Xia W, Ding Z, Eid K, Abdullah A
M, Hossain M S A, Zhang S and Tang J 2020 Chem.
Eng. J. 390 124493
Han X, Ling X, Yu D, Xie D, Li L, Peng S, Zhong C, Zhao
N, Deng Y and Hu W 2019 Adv. Mater. 31 1905622
Research Advances on Electrocatalysts for Oxygen Reduction Reaction in Fuel Cells
63
