
Explore the Application of Waste Gypsum in Intelligent Construction
Yang Wen
1a
, Zixin Ai
2,* b
and Yaoyu Wang
1c
1
Key Laboratory for Prediction & Control on Complicated Structure System of the Education Department of Liaoning
Province, Dalian University, Dalian 116622, China
2
School of Transportation Engineering, Dalian Jiaotong University, Dalian 116622, China
*
Keywords: Phosphogypsum, 3D Printing, Mechanical Properties, Resource Utilization.
Abstract: This study explores the potential application of waste gypsum, particularly phosphogypsum (PG) in the field
of intelligent construction. Through calcination pretreatment and 3D printing technology,the physical and
chemical properties of phosphogypsum and their impact on 3D printing performance were investigated.
Initially,the chemical composition and microstructure of the raw phosphogypsum were analyzed using X-ray
fluorescence spectrometer (XRF), X-ray diffractometer (XRD), and scanning electron microscope (SEM).
Subsequently, the effect of different calcination temperatures on the dehydration reaction and phase
composition of phosphogypsum was studied through calcination treatment. Then, using 3D printing
technology, the influence of different water-to-cement ratios (w/c) on the printability and mechanical
properties of phosphogypsum paste was explored.The results indicated that the dehydration reaction of
phosphogypsum was most significant at a calcination temperature of 175℃, and the 3D printing effect of
phosphogypsum paste was optimal at w/c = 0.67,with the printed components being continuous, uniform,and
smooth without defects. Additionally, calcined phosphogypsum exhibited the highest mechanical
performance at w/c = 0.70. This study provides new ideas for the resource utilization of waste gypsum and is
of great significance for promoting the development of intelligent construction technology.
1 INTRODUCTION
Phosphogypsum is a solid waste generated by
phosphochemical enterprises in the production of
phosphoric acid fertilizers (Tayibi et al., 2009).
Statistics show that for every 1 ton of phosphoric acid
produced globally, 4.5 to 5.5 tons of phosphogypsum
is generated (Shakor et al., 2020). The composition of
phosphogypsum is similar to that of natural gypsum,
with CaSO
4
·2H
2
O accounting for more than 90%
(Sun et al., 2023). Its chemical composition is
influenced by the composition of phosphate ore and
production processes, often containing fluorides,
water-soluble phosphates,heavy metals, and other
harmful substances,and it has a strong acidity (Wu et
al., 2022). It may also contain radioactive elements
such as radium and thorium (Xiao et al., 2021). It is
a
https://orcid.org/0009-0003-9136-9208
b
https://orcid.org/0009-0009-6501-8950
c
https://orcid.org/0000-0002-9556-6765
hygroscopic and can react with water to form acidic
solutions (Yuvaraj et al., 2021). The long-term
stacking of phosphogypsum not only occupies a large
amount of land resources but also poses a serious
threat to regional ecological safety (Zhang et al.,
2021). These issues exert great pressure on the long-
term development of China's phosphochemical
industry (Zhang et al., 2019). Therefore, it is
necessary to promote the comprehensive utilization
of phosphogypsum and improve the level of resource
utilization technology (Zhang et al., 2020).
Provinces(cities) are actively promoting the resource
utilization of phosphogypsum (Zhao et al., 2020;
Zhou et al., 2023; Rashad, 2017), based on
which,various new types of building materials are
continuously innovated and transformed, striving to
achieve sustainable development.
Wen, Y., Ai, Z., Wang and Y.
Explore the Application of Waste Gypsum in Intelligent Construction.
DOI: 10.5220/0013627900004671
In Proceedings of the 7th International Conference on Environmental Science and Civil Engineering (ICESCE 2024), pages 213-219
ISBN: 978-989-758-764-1; ISSN: 3051-701X
Copyright © 2025 by Paper published under CC license (CC BY-NC-ND 4.0)
213
At the same time,with the rapid development of
urbanization and intelligent industries worldwide, 3D
printing technology has become a global research and
application hotspot. 3D printing concrete technology
has characteristics such as low construction costs,
free structural design, high molding precision, low
construction noise, and less dust pollution, playing an
irreplaceable role in the transformation and upgrading
of China's construction industry.
In this study, we systematically investigated the
impact of different calcination temperatures on the
dehydration reaction,phase transformation, and
mechanical properties of phosphogypsum, and
further explored the influence of w/c on the
morphology,structure, and strength of
phosphogypsum hydration products.In addition,this
paper also assessed the feasibility of 3D printing of
phosphogypsum, providing new ideas for the
application of phosphogypsum in the construction
field. Through this study,we aim to provide a
scientific basis for the high-value utilization of
phosphogypsum, promote the transformation of
phosphogypsum from waste to resources, and
contribute to environmental protection and
sustainable development.
2 METHOD
2.1 Raw Materials
Phosphogypsum, a byproduct generated during
phosphoric acid production, served as the primary
material in this investigation. Predominantly
comprised of CaSO
4
·2H
2
O, PG also contains
impurities like phosphorus oxide and silica. The
chemical composition of the initial PG sample was
assessed using a Rigaku ZSX Primus IV XRF
spectrometer, with the findings detailed in Table 1.
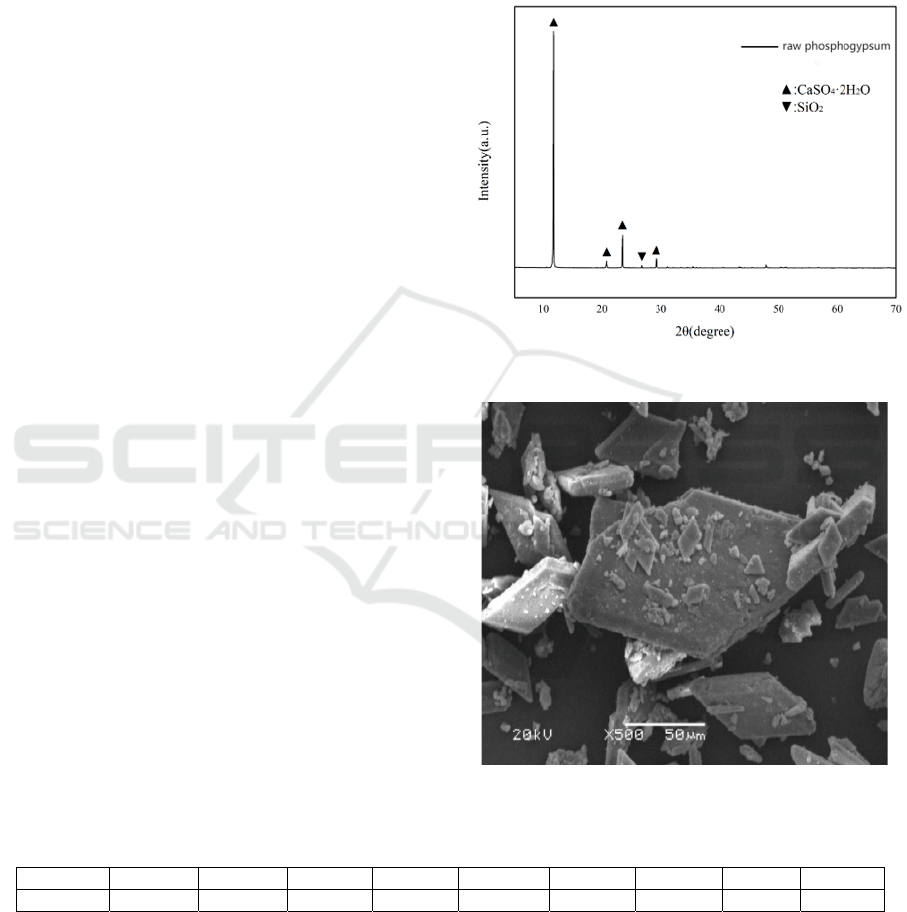
Qualitative analysis of the phase composition of the
original PG was conducted using XRD. Notably,
distinct peaks were observed in the X-ray diffraction
pattern within the 10°-30° 2θ range, as depicted in
Figure 1. The morphology of the initial
phosphogypsum was examined through SEM. The
crystal structure of phosphogypsum primarily
exhibited rhombus or parallelogram plate shapes,
with fine granular and flocculent impurities adhering
to the surface, as illustrated in Figure 2.
Figure 1: XRD of Phosphogypsum.
Figure 2: SEM of Phosphogypsum.
Table 1: Chemical composition of gypsum.
Sam
p
le SO
3
CaO SiO
2
P
2
O
5
Al
2
O
3
K
2
OM
g
O F Cl
PG/% 43.40 33.28 6.4 0.71 1.04 0.29 0.16 0.33 0.035
2.2 Method
This study investigates a low-energy pretreatment
approach to efficiently eliminate impurities and alter
the composition of phosphogypsum to increase its
utility. Calcination is chosen as the pretreatment
technique, employing an SX2-4-10A muffle furnace.
The calcination process involves controlling the
temperature to dehydrate the dihydrate gypsum in
phosphogypsum, converting it into hemihydrate
ICESCE 2024 - The International Conference on Environmental Science and Civil Engineering
214

gypsum and anhydrous gypsum. Specifically,
calcination temperatures of 150°C, 175°C, 200°C,
225°C, and 250°C are utilized.
In this experiment, a small gantry integrated
concrete rapid-setting 3D printing equipment with
mixing and extruding functions as shown in the figure
was used. The pipeline of this equipment is not for
conveying mixed slurry,but separates the dry material
and liquid material conveying routes to achieve
dedicated pipe usage, and eliminates the pumping
device found in conventional 3D printing equipment.
Unlike conventional mixing methods, dry materials
and water can come into contact and mix rapidly in
the air within the printing system,allowing materials
such as phosphogypsum powder to undergo hydration
reactions quickly. The work of material
mixing,stirring, and extruding molding is all
completed within the printing system.This ensures
that the material can enter the wet extrusion from dry
powder,thereby breaking through the problem that
cement-based materials cannot be pumped and
extruded due to short setting times. In this
experiment, the water temperature was controlled
between 15°C and 20°C, and different w/c were
set,such as 1:1, 1.5:1, 2:1, 4:1, etc. The 3D printer was
used to strictly follow the standard ratios.
A TG test was conducted using a TGA/DSC 1
thermal analyzer from the STARe system by
METTER TOLEDO Group to measure the mass
change of samples with temperature under
programmed temperature control. The phase
composition of PG was determined using XRD.
Hydration kinetics of PG were investigated using a
TAM air C80 isothermal calorimeter at a constant
temperature of 25℃ and a water/cement ratio of 4.
Compressive and flexural strengths of PG were
assessed using a DYE-300S computerized constant
stress testing machine from Wuxi Huaxi Building
Materials Testing Instrument Co., Ltd., with sample
dimensions of 20mm × 20mm × 20mm and 40mm ×
40mm × 160mm, respectively. The microstructure of
the samples was analyzed using a Zeiss SUPRA 55
field emission SEM. Printability of PG was evaluated
with an architectural 3D printer, where the water-
cement ratio of printed components was adjusted by
controlling water output while maintaining a
consistent discharge speed.
3 RESULT
3.1 Thermal Analysis Characterization
Figure 3 illustrates the thermogravimetric analysis of
PG. The dehydration process of PG initiates at
approximately 100°C, with a gradual release of water
until around 300°C, marking the completion of
dehydration, resulting in a weight loss of
approximately 19.5%. The derivative
thermogravimetric (DTG) curve displays two distinct
dehydration peaks. The first peak, occurring between
100°C and 166°C with a peak at 157°C, corresponds
to an initial mass loss of 14.7%. The second peak,
observed between 157°C and 300°C with a peak at
175°C, corresponds to an initial mass loss of 4.8%.
The transformation of CaSO
4
·2H
2
O to
CaSO
4
·1/2H
2
O involves the removal of 1.5H
2
O in the
first stage, followed by the conversion of CaSO
4
to
CaSO
4
·1/2H
2
O by eliminating the remaining 1/2H
2
O
in the second stage.
3.2 XRD
Figure 4(a) shows the XRD patterns of the PG sample
at different calcination temperatures ranging from
150°C to 250°C. When 2θ is 11.7°, 14.8° and 38.3°,
respectively, the diffraction peak falls on the peak
with the largest difference between CaSO
4
·2H
2
O and
CaSO
4
·1/2H
2
O and CaSO
4
. As can be seen from
Figure 4(b), (c) and (d), when 2θ is 11.7°C, the
diffraction peak decreases gradually with the increase
of calcination temperature, and the diffraction peak
disappears when the calcination temperature reaches
175°C. It can be concluded that CaSO
4
·2H
2
O is
gradually depleted after the calcination temperature
of the sample reaches 175°C, and the sample is
mainly composed of CaSO
4
·1/2H
2
O. In addition,
when 2θ is 14.8° and 29.7°, the diffraction peak of PG
increases first and then decreases with the increase of
calcination temperature, and reaches the maximum
value when the calcination temperature is 200°C,
which may be due to the fact that the CaSO
4
·1/2H
2
O
content of the sample first increases and then
decreases, and the CaSO
4
·1/2H
2
O content reaches the
maximum value at 200°C, and then the content
gradually decreases, and with the increase of
temperature, the content of CaSO
4
gradually
increases, when the calcination temperature is 250°C,
The diffraction peak appears at 2θ=38.3°, and the
sample is a mixture of CaSO
4
·1/2H
2
O and CaSO
4
.
Explore the Application of Waste Gypsum in Intelligent Construction
215
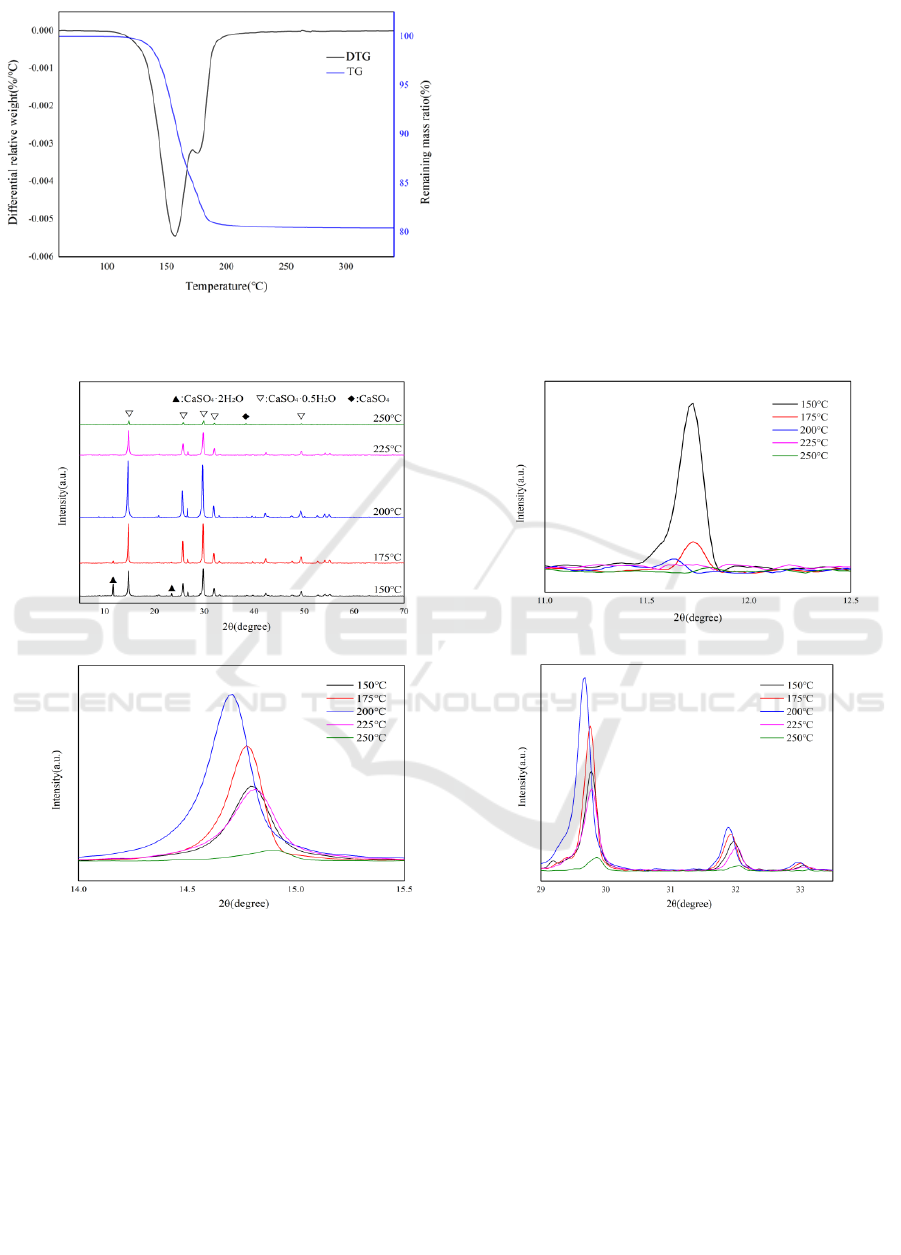
Figure 3: TG-DTG curves of the original PG.
(a) (b)
(c) (d)
Figure 4: XRD patterns of PG at different calcination temperatures.
3.3 Compressive Strength and Flexural
Strength
Figure 5 illustrates the 7-day compressive and
flexural strength of calcined PG under varying water-
to-ash ratios. The intensity of PG initially rises and
then declines as the water-to-ash ratio increases. For
ratios below 0.70, both compressive and flexural
strength of PG increase with the ratio. At a ratio of
0.70, peak values are reached at 15.62 MPa and 3.86
MPa for compressive and flexural strength,
respectively. Beyond a ratio of 0.70, both strengths
decrease. Specifically, at a ratio of 0.85, compressive
and flexural strengths are 28.87% and 27.20% lower
than those at a ratio of 0.70, measuring 11.11 MPa
and 2.81 MPa, respectively. The highest hydration
rate for calcined PG is observed at a water-to-ash ratio
of 0.70, indicating a direct correlation between
hydration level and strength. Excessive hydration
beyond this ratio leads to increased formation of
pores and microcracks within the material,
ICESCE 2024 - The International Conference on Environmental Science and Civil Engineering
216
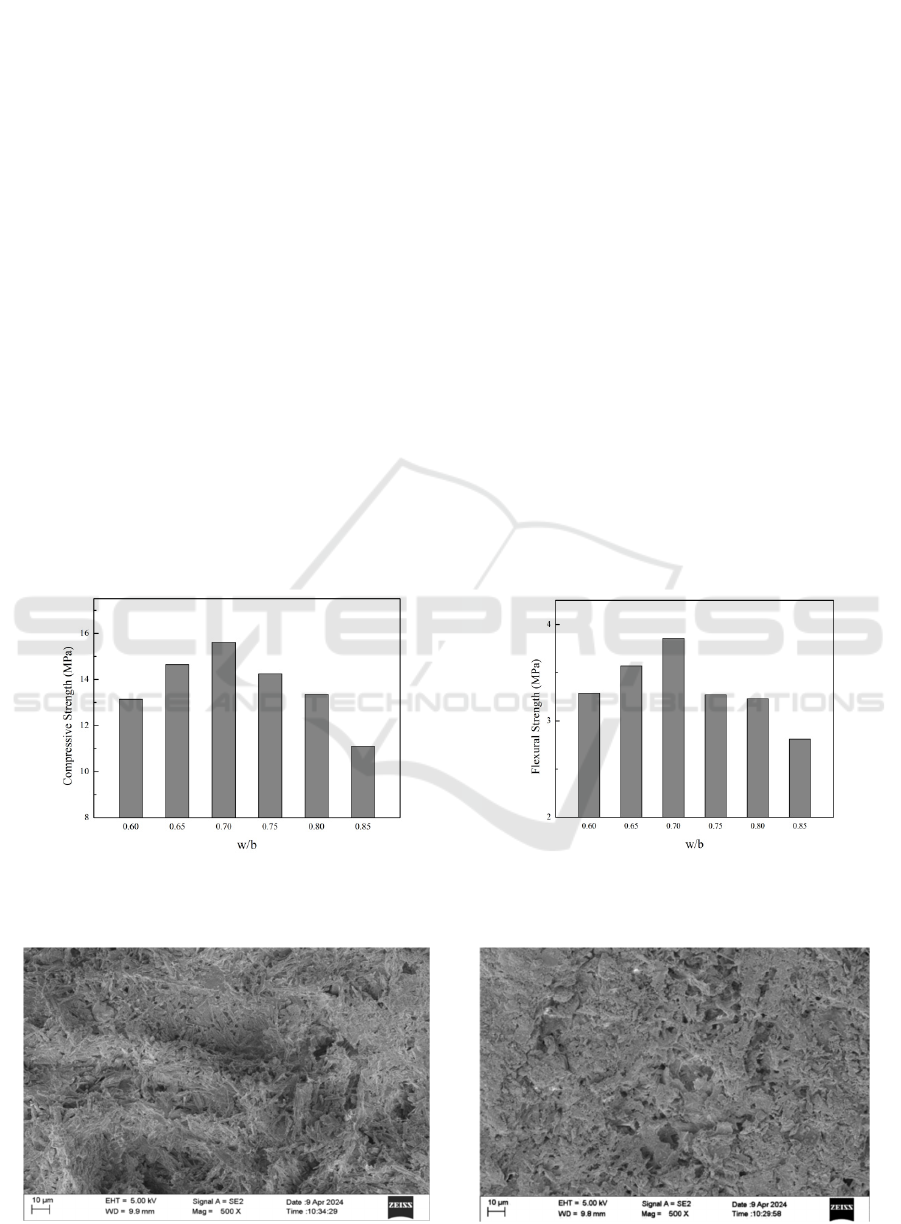
consequently diminishing its overall strength and
stability.
3.4 SEM
Figure 6 shows SEM photos of phosphogypsum after
natural curing for 7 days under different water-
cement ratio conditions. The strength of PG is
provided by the molecular forces connected to each
other through crystal contact. From the SEM cross-
sectional image with a magnification of two thousand
times, it can be seen that the microstructure of the
dihydrate PG crystal is relatively clear, the internal
morphology and lines are disordered, and the large
crystals The gaps between them are filled with small
crystals or small granular substances. When the
water-cement ratio is less than or equal to 0.70, PG
mainly takes the form of strips or needles after
hydration. The particles are cemented together and
interlaced to form a network structure. As the water-
cement ratio gradually increases, the structure
becomes loose and porous. There are obvious cracks
in the cross section. This should be the reason why
low water-cement ratio has higher strength compared
with high water-cement ratio.
3.5 The Printability
Figure 7 illustrates the printing effects of calcined
phosphogypsum (PG) under varying water-cement
ratios of 0.60, 0.65, 0.67, and 0.70. In (a), at a water-
cement ratio of 0.60, the PG slurry exhibits severely
restricted fluidity, leading to discontinuous extrusion,
material breaks, and uneven surfaces with significant
cracking. Increasing the water-cement ratio to 0.65 (b)
reduces cracking but results in a rough surface with
noticeable flaws. At a ratio of 0.67 (c), the printing
quality improves markedly, with continuous and
uniform extrusion, and a smooth, flawless surface.
However, at 0.70 (d), the slurry becomes excessively
fluid, failing to maintain structural integrity and
preventing the formation of viable printed
components. These findings indicate that calcined PG
slurry is printable, with a water-cement ratio of 0.67
being optimal for achieving continuous, uniform, and
smooth printed structures.
(
a
)
(
b
)
Figure 5: 7d compressive and flexural strength of PG naturally cured: (a) compressive strength (b) flexural strength.
(a) w/c = 0.65 (b) w/c = 0.70
Explore the Application of Waste Gypsum in Intelligent Construction
217

(c) w/c = 0.75 (d) w/c = 0.80
Figure 6: SEM images of phosphogypsum under different w/c at 7 days.
(a) (b) (c) (d)
Figure 7: Printing effects of calcined PG under different
water-cement ratios: (a) w/c = 0.60 (b) w/c = 0.65 (c) w/c =
0.67 (d) w/c = 0.70.
4 CONCLUSION
(1) Effect of calcination temperature on XRD pattern
of PG sample. With the increase of calcination
temperature, part of CaSO
4
·2H
2
O in PG is dehydrated
to CaSO
4
·1/2H
2
O at 157℃, and CaSO
4
·1/2H
2
O may
be transformed into CaSO
4
when the temperature
reaches 175℃. Finally, the optimum heat treatment
temperature of phosphogypsum is determined to be
175℃.
(2) The mechanical properties of calcined
phosphogypsum increase and then decrease with the
increase of water ash ratio. And the mechanical
properties of phosphogypsum are the highest when
the water ash ratio is 0.70 (the compressive strength
and folding strength are 3.86MPa and 15.62MPa
respectively).
(3) The shape of hydration products gradually
changed from strip cross network to lamella gradually
to paste, the structure changed from dense to loose
and porous, and the fracture appeared obvious cracks.
(4) The calcined phosphogypsum slurry is
printable, and the 3D printing effect of the slurry is
best when the w/c is 0.67. The printed components are
continuous and uniform, and the surface is smooth
and flawless.
REFERENCES
Shakor, P., Nejadi, S., Paul, G., Sanjayan, J. 2020.
Dimensional accuracy, flowability, wettability, and
porosity in inkjet 3DP for gypsum and cement mortar
materials. Automation in Construction, 110: 102964.
Sun, T., Li, W., Xu, F., Yu, Z., Wang, Z., Ouyang, G., Xu,
D. 2023. A new eco-friendly concrete made of high
content phosphogypsum based aggregates and binder:
Mechanical properties and environmental benefits.
Journal of Cleaner Production, 400: 136555.
Tayibi, H., Choura, M., López, F.A., Alguacil, F.J., López-
Delgado, A. 2009. Environmental impact and
management of phosphogypsum. Journal of
Environmental Management, 90: 2377–2386.
Wu, F., Ren, Y., Qu, G., Liu, S., Chen, B., Liu, X., Zhao,
C., Li, J. 2022. Utilization path of bulk industrial solid
waste: A review on the multi-directional resource
utilization path of phosphogypsum. Journal of
Environmental Management, 313: 114957.
Xiao, J., Ji, G., Zhang, Y., Ma, G., Mechtcherine, V., Pan,
J., Wang, L., Ding, T., Duan, Z., Du, S. 2021. Large-
scale 3D printing concrete technology: Current status
and future opportunities. Cement and Concrete
Composites, 122: 104115.
Yuvaraj, K., Mohamed Ismail, A., Nagarajan, P.,
Vigneshwaran, S. 2021. Design and fabrication of
gypsum prototypes based on binder jetting technology.
Materials Today: Proceedings, 45: 3085–3090.
Zhang, C., Nerella, V.N., Krishna, A., Wang, S., Zhang, Y.,
Mechtcherine, V., Banthia, N. 2021. Mix design
concepts for 3D printable concrete: A review. Cement
and Concrete Composites, 122: 104155.
Zhang, J., Tan, H., He, X., Yang, W., Deng, X., Su, Y.,
Yang, J. 2019. Compressive strength and hydration
process of ground granulated blast furnace slag-waste
gypsum system managed by wet grinding. Construction
and Building Materials, 228: 116777.
ICESCE 2024 - The International Conference on Environmental Science and Civil Engineering
218

Zhang, Y., Yang, J., Cao, X. 2020. Effects of several
retarders on setting time and strength of building
gypsum. Construction and Building Materials, 240:
117927.
Zhao, Y., Gao, J., Liu, C., Chen, X., Xu, Z. 2020. The
particle-size effect of waste clay brick powder on its
pozzolanic activity and properties of blended cement.
Journal of Cleaner Production, 242: 118521.
Zhou, S., Lu, Y., Pan, Y., Li, J., Qu, F., Luo, Z., Li, W.
2023. Flowability prediction of recycled α-hemihydrate
gypsum for 3D powder printing under combined effects
of different glidants using response surface
methodology. Developments in the Built Environment,
16: 100265.
Rashad, A.M. 2017. Phosphogypsum as a construction
material. Journal of Cleaner Production, 166: 732–743.
Explore the Application of Waste Gypsum in Intelligent Construction
219
