
Indication of Solution Temperature and Conductivity During
Electrochemical Oxidation of Hydrazine Wastewater
Zhen Zhou, Shouzhong Wang, Xiaojun Fang, Wen Ma, Ningran Hou and Yuan Cheng
*a
State Key Laboratory of Technologies in Space Cryogenic Propellants, Beijing 100028, China
*
Keywords: Electrochemical Oxidation, Unsymmetrical Dimethylhydrazine, Conductivity, Temperature.
Abstract: The electrochemically activated persulfate process has been demonstrated to enhance the efficacy of the
degradation of unsymmetrical dimethylhydrazine (UDMH) in wastewater. The concentration of UDMH was
typically determined through amino sodium ferrocyanide spectrophotometry. Nevertheless, the lengthy
chromogenic reaction time and intricate operational procedure impede the efficiency of the analysis. The
utilization of solution conductivity and temperature as indicators to ascertain the extent of UDMH degradation
can enhance the efficiency of the analytical process. The impact of varying voltage, initial pH, ionic strength,
Na₂S₂O₈ dosage, circular flow, and UDMH concentration on the observed patterns of UDMH concentration,
pH, solution temperature, and conductivity were investigated. A correlation was identified between the
transition points for the rapid and gradual changes in conductivity and temperature, which occurred five
minutes earlier than those observed in the UDMH concentration and pH. During the sampling process, the
degree of UDMH removal can be predicted online by monitoring the solution temperature and conductivity,
thereby reducing the duration of the test and enhancing the efficiency of the analysis.
1 INTRODUCTION
The generation of propellant wastewater from rocket
launches is a significant environmental concern. Each
launch produces over 300 tons of wastewater (Li et
al., 2017), with high-density launches generating
even greater quantities. This wastewater has the
potential to overwhelm wastewater treatment systems
and negatively impact the surrounding ecological
environment at satellite launch centers. While the
advent of novel green propellants, such as liquid
hydrogen and liquid oxygen, has mitigated the
environmental impact, conventional propellants,
particularly unsymmetrical dimethylhydrazine
(UDMH), retain a pivotal role in thruster attitude
adjustment (Zheng et al., 2006). UDMH is classified
as a Class II highly toxic substance, and it has the
potential to cause a deficiency of vitamin B6, which
can lead to disease. Furthermore, it has been
identified as a teratogenic, carcinogenic, and
mutagenic hazard to humans (Zeng et al., 2019a;
Deng et al., 2015).
a
https://orcid.org/
0000-0002-3191-3270
The current treatment methods for UDMH can be
broadly classified into the following categories:
adsorption, ion exchange, biological treatment,
advanced oxidation processes, and so forth. In a study
conducted by (Gu et al., 2019), UDMH was adsorbed
using oxalic acid-modified attapulgite, resulting in an
adsorption efficiency of 95%. Nevertheless, the
adsorption method is primarily applicable to the
treatment of propellant wastewater with low
concentration and significant fluctuations in water
quality (Gu et al., 2019). Li et al. (2006) synthesized
a strong acid cation exchange fiber, which
demonstrated a treatment capacity that was 3.86 times
greater than that of 732 strong acid cation exchange
resin. Nevertheless, the ion exchange capacity is
susceptible to interference from background hardness
ions, and the regeneration rate is relatively slow
(Deng et al., 2015). Wang (2005) achieved 98%
removal of UDMH and 89% removal of COD
Cr
through the domestication of activated sludge.
However, this method has a complex microbial
cultivation process and a lengthy degradation reaction
time, which restricts its promotion and application.
Zeng's team prepared nitric acid-modified g-C
3
N
4
Zhou, Z., Wang, S., Fang, X., Ma, W., Hou, N., Cheng and Y.
Indication of Solution Temperature and Conductivity During Electrochemical Oxidation of Hydrazine Wastewater.
DOI: 10.5220/0013573700004671
In Proceedings of the 7th International Conference on Environmental Science and Civil Engineering (ICESCE 2024), pages 71-77
ISBN: 978-989-758-764-1; ISSN: 3051-701X
Copyright © 2025 by Paper published under CC license (CC BY-NC-ND 4.0)
71

(Zeng et al., 2019b), TiO
2
/g-C
3
N
4
(Zeng et al., 2018),
and modified TiO
2
/g-C
3
N
4
(Zeng et al., 2019a), which
demonstrated a notable degradation effect on UDMH.
Hou et al. (2019) employed a near-critical water
oxidation process to facilitate the harmless treatment
of high-concentration UDMH waste liquid.
Plasma is the fourth state of matter, occurring
when gases are ionized to a sufficient extent. It can be
produced either completely or partially, and only
under specific conditions. The process of high-
pressure discharge results in the formation of a
considerable number of free electrons, which are
subsequently accelerated to attain a high level of
energy. Ultraviolet light irradiation, shockwaves and
other physicochemical effects are produced by high-
energy electrons and non-elastic collisions between
atoms or molecules. The prevailing hypotheses
regarding the mechanisms by which plasma removes
water pollutants are as follows: high-energy electron
action, ozone oxidation, and ultraviolet light
radiation. The utilization of plasma in wastewater
treatment offers a number of advantages, including a
compact structure, a small footprint, convenient
operation, wide adaptability to raw water, the absence
of the need to add chemicals during the reaction
process, and the absence of risk of secondary
pollution.
Qiu et al. (2020) employed nanosecond pulse
power to achieve the degradation of 83.2% of acid red
73 dyes. Rong et al. (2020) applied low-temperature
plasma to treat polyacrylamide, achieving a
degradation rate of 85.74%. Liu et al. (2020)
exploited the strong reductive ·H and oxidative ·OH
generated during low-temperature plasma discharge
to achieve the simultaneous reduction of Cr(VI) and
oxidation of phenol. Yi et al (2019) utilized dielectric
barrier discharge low-temperature plasma to achieve
the degradation of 82.1% of UDMH. Nevertheless,
the fundamental theory and empirical findings
pertaining to the utilization of plasma for the
remediation of hydrazine propellants are
comparatively scarce within both domestic and
international academic circles. Furthermore, the
direct application of plasma in industrial wastewater
treatment is characterized by high energy
consumption and a low energy throughput, which
constrains the practical deployment of this
technology (Wu et al., 2019).
The Fenton-like advanced oxidation technology
based on sulfate radicals has recently attracted
attention due to several favorable characteristics. Its
oxidation-reduction potential (2.5-3.1 V) is higher
than that of ·OH (1.8-2.7 V), the half-life of ·SO
4
-
is
long and difficult to affect by pH, and it has strong
oxidation selectivity. At room temperature, the
oxidizing power of persulfate is limited and requires
activation by an activator to produce ·SO
4
-
. The
activation of persulfates can be achieved through the
application of ultraviolet visible light, heat, alkali,
microwave radiation, carbon materials, and transition
metals. Of these, ultraviolet light radiation and heat
produced under plasma action have been
demonstrated to be particularly effective in this
regard.
Based on this, the combination of plasma and
persulfate to remove UDMH can be used to activate
persulfate, producing ·OH and ·SO
4
-
, in two ways.
Firstly, ultraviolet light and heat produced by plasma
can be used to activate persulfate. Secondly, ozone
and high-energy electrons formed by plasma
discharge enable direct attack on pollutants. This
approach allows the complementary advantages of
both methods to be achieved.
In the preceding stage, the parameters, including
voltage, initial pH, background ion concentration,
persulfate dosage, circulation flow rate, and pollutant
concentration, were optimized to identify the optimal
operating conditions for the process (Zhou et al.,
2023). These conditions were found to result in a
93.8% removal efficiency for 100 mg/L UDMH.
However, the measurement of UDMH concentration
in the effluent was obtained by the GB/T 14376-1993
amino ferrocyanide sodium spectrophotometry
method, which has a lengthy coloration reaction and
complex operational steps. Accordingly, we explored
the potential of utilizing convenient test indicators as
proxies for the degree of reaction, with a view to
employing them as alternative indicators for UDMH
concentration. In this regard, we examined the
fluctuations in solution temperature and conductivity
throughout the UDMH treatment process.
2 METHOD
2.1 Reagents
Na
2
S
2
O
8
, NaCl, NaOH, HgSO
4
, Ag
2
SO
4
,
(NH
4
)
2
Fe(SO
4
)
2
·6H
2
O, Na
2
HPO
4
·12H
2
O, Na
2
[Fe
(CN)
5
NO]·2H
2
O, 1,10-phenanthroline, acetic acid,
acetylacetone, ammonium acetate were all purchased
from the National Pharmaceutical Group, analytical
pure. K
2
Cr
2
O
7
was provided by the National
Pharmaceutical Group as a guarantee reagent.
Anhydrous ethanol, citric acid, 95-98% H
2
SO
4
were
provided by the Beijing Chemical Factory. UDMH
was provided by the Beijing Aerospace Test
Technology Research Institute (98.7%), and all steps
ICESCE 2024 - The International Conference on Environmental Science and Civil Engineering
72
involving the dilution, degradation, and analysis of
UDMH were carried out in a fume hood. All reagents
were dissolved and diluted with ultrapure water
prepared by Milli-Q (18.2 MΩ, USA) and stored in a
refrigerator set at 4°C after preparation.
2.2 Experimental Apparatus
The entire experimental system consists of the
following components: a tubular reactor, a blower, a
power supply, an oscilloscope (MSO7104B, Agilent
Technologies), a peristaltic pump (LongerPump), a
water tank, and so forth. The UDMH solution in the
water tank is forced into the inlet by the peristaltic
pump (LLS PLUS-B163, Kachuaner Fluid
Technology Co., Ltd.), enters the quartz inner tube
from the bottom, overflows from the top of the inner
tube, and enters the gap between the inner and outer
tubes. Upon traversing the 110 mm-long discharge
zone, the wire mesh assumes the role of the high-
voltage pole, while the UDMH wastewater serves as
the ground pole. The treated solution is then conveyed
to the lower water tank, where it is subjected to a
further treatment cycle via the peristaltic pump.
During the sampling process, it is essential to ensure
that the power supply is deactivated and that the
reaction is terminated with the addition of 0.1ml of
anhydrous ethanol. The pH, conductivity, and
solution temperature of the samples should be
measured. Once the experiment has concluded, the
liquid in the central tube should be emptied, rinsed
with ultrapure water on several occasions and the
remaining water should be drained away. The
concentration of UDMH in the solution is determined
by the GB/T 14376-1993 amino ferrocyanide sodium
spectrophotometry method, with the measurement
instrument being the SHIMADZU UV-2550
spectrophotometer. Meanwhile, the temperature and
conductivity are recorded with the HACH HQ14d
apparatus.
3 RESULTS AND DISCUSSION
3.1 Influence of Initial pH
Changing the pH of the solution will change the form
of existence of UDMH, and it is necessary to study
the changes in solution temperature and conductivity
under different pH conditions. Degradation of 100
mg/L UDMH waste water under the environment of
maintaining a NaCl background ionic strength of 50
mmol/L, a discharge voltage of 12.5 kV, a
Na
2
S
2
O
8
/UDMH (mol/mol) ratio of 1.0 and a
circulation flow rate of 140 mL/min.
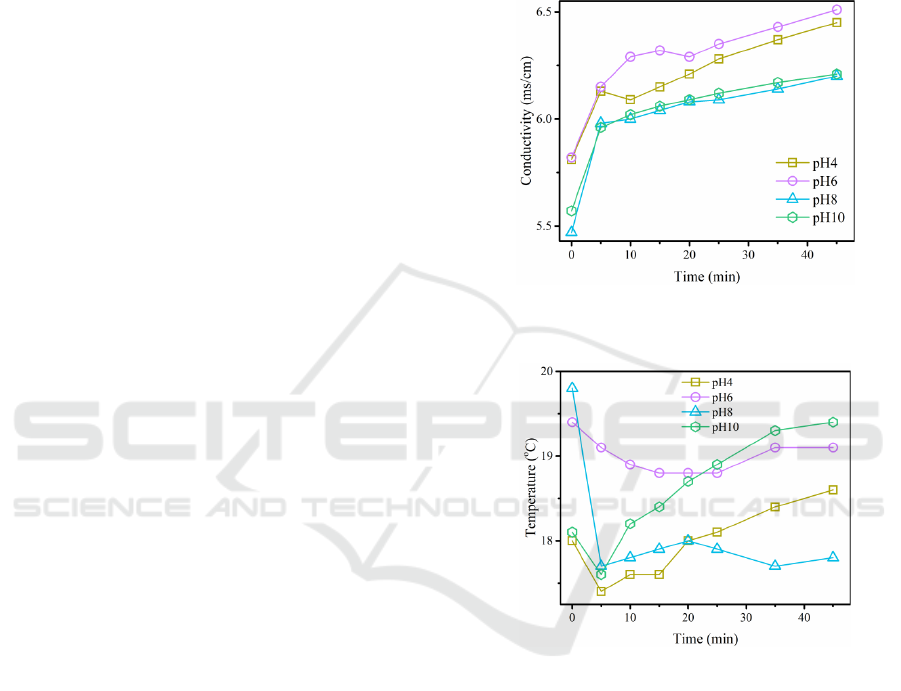
The change in conductivity of the solution is
shown in Figure 1. As can be seen from the figure, the
solution conductivity corresponding to the initial pH
of 4-10 increases as the reaction time is increased,
rising rapidly within the first 5 minutes and then
slowing down.
Figure 1: Conductivity variation with time as initial pH
fluctuation.
Figure 2: Temperature variation with time as initial pH
fluctuation.
The change in solution temperature is shown in
Figure 2. Except for the solution corresponding to the
initial pH of 6, the solution temperature decreases
rapidly within the first 5 minutes and then increases.
When the initial pH is 6, the solution temperature
decreases slowly and starts to increase after 25
minutes.
Compared with previously reported patterns of
change in UDMH concentration and pH (Zhou et al.,
2023), the rapid response phase of solution
temperature and conductivity also appeared to
precede the initial pH change by 5 min, i.e. the cut-
off point between rapid and slow responses for
solution temperature and conductivity was 5 min, and
Indication of Solution Temperature and Conductivity During Electrochemical Oxidation of Hydrazine Wastewater
73
10 min was used as the cut-off point between rapid
and slow responses for UDMH concentration and pH.
3.2 Influence of Background Ion
Concentration
The changes in solution temperature and conductivity
were investigated as the background ionic strength of
NaCl was varied at a molar ratio of Na
2
S
2
O
8
to
hydrazine hydrate of 1.0, an initial pH = 8, a discharge
voltage of 12.5 kV, a recirculation flow rate of 140
mL/min and an initial concentration of meta-
dihydrazine of 100 mg/L.
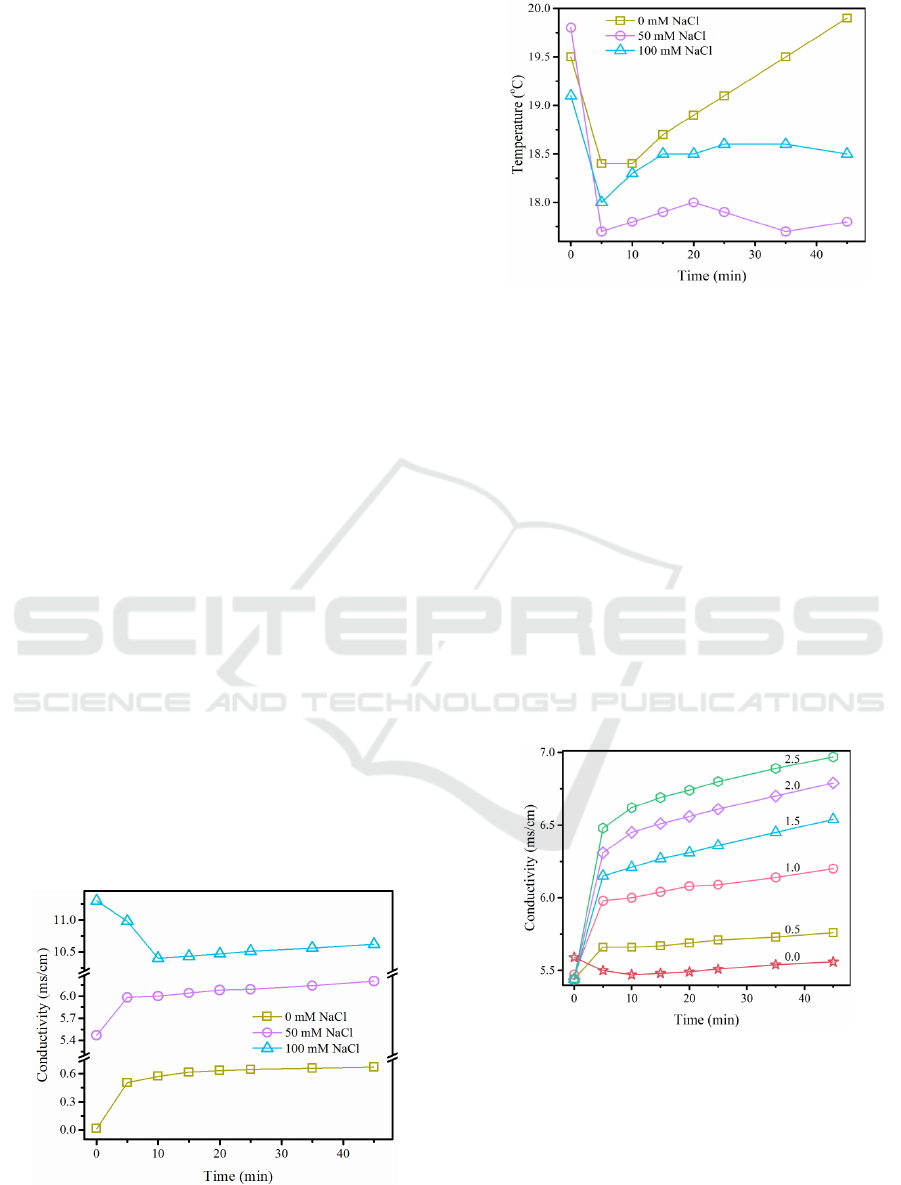
The change in conductivity of the solution is
shown in Figure 3. As can be seen from the figure, the
solution conductivity corresponding to 0 mM and 50
mM NaCl increases as the reaction time is increased,
rising rapidly within the first 5 minutes and then
slowing down. In contrast, the conductivity of the
solution corresponding to 100 mM NaCl decreases
rapidly during the first 10 minutes and then increases
slowly. The rapid decrease in conductivity is due to
the high concentration of Cl
-
which is rapidly
oxidized and gasified in the electrochemical reaction.
The variation in solution temperature is shown in
Figure 4. The solution temperature decreased rapidly
for the first 5 min, then the solution temperature
increased. The solution temperature increased
linearly with the fastest growth at 0 mM NaCl
background ionic strength. The increase in
temperature may be due to the low conductivity and
high resistivity of the solution resulting in high heat
generation.
Similarly, the cut-off points for fast and slow
solution temperature, conductivity were 5 min earlier
than the cut-off points for fast and slow UDMH
concentration, acidity and alkalinity as the
background ionic strength was varied.
Figure 3: Conductivity changing with time as NaCl
concentration fluctuates.
Figure 4: Temperature changing with time as NaCl
concentration fluctuates.
3.3 Influence of Na
2
S
2
O
8
Dosage
Optimization of Na
2
S
2
O
8
as a key factor in the
degradation of UDMH wastewater is necessary.
UDMH effluent of 100 mg/L was degraded by
maintaining the initial pH = 8, a recirculation flow
rate of 140 mL/min, a background NaCl ionic
strength of 50 mmol/L and a loaded voltage
environment of 12.5 kV.
The variation in solution conductivity is shown in
Figure 5. As can be seen from the figure, without
Na
2
S
2
O
8
the solution conductivity decreased slightly
in the first 10 minutes and then slowly increased.
After the addition of Na
2
S
2
O
8
, the solution
conductivity increased rapidly in the first 5 minutes
and then the growth rate slowed down.
Figure 5: Conductivity changing with time as Na
2
S
2
O
8
fluctuates.
ICESCE 2024 - The International Conference on Environmental Science and Civil Engineering
74
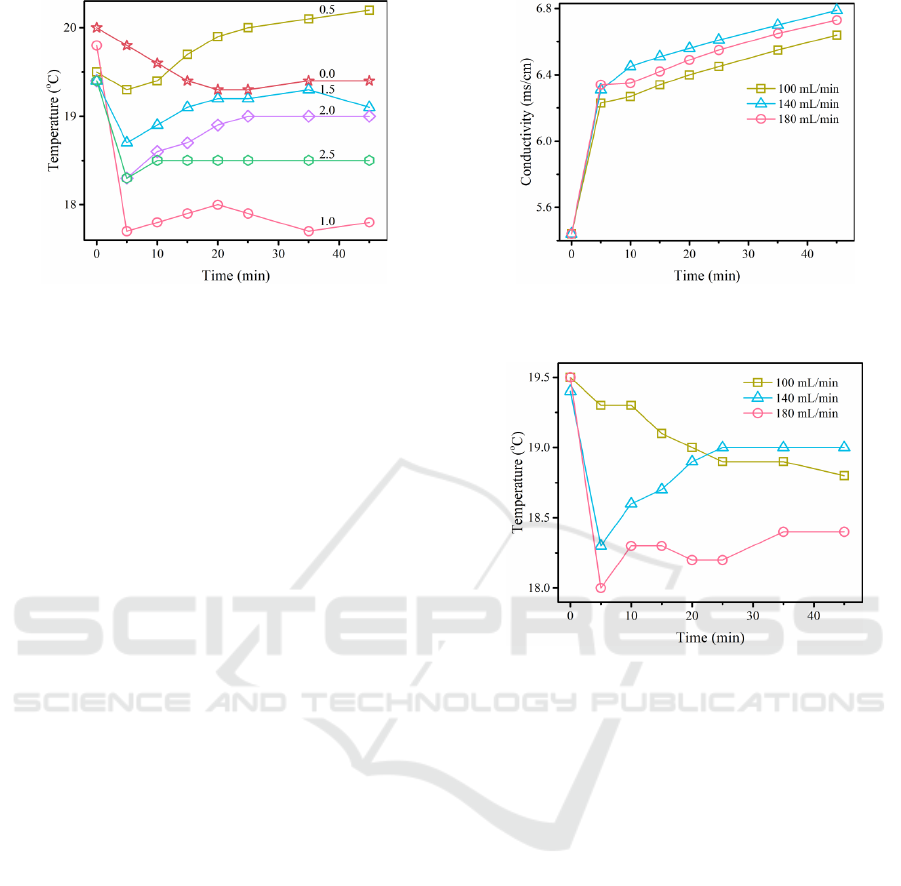
Figure 6: Temperature changing with time as Na
2
S
2
O
8
fluctuates.
The variation of the solution temperature is shown
in Figure 6. After the addition of Na
2
S
2
O
8
, the
solution temperature decreased for the first 5 minutes,
after which the solution temperature increased. In
contrast, when Na
2
S
2
O
8
was not added, the solution
temperature continued to decrease slowly and then
reached a steady state.
The cut-off point for fast and slow solution
temperature and conductivity was 5 min when
Na
2
S
2
O
8
dosage was varied.
3.4 Influence of Circulation Flow Rate
The influence of the circulation flow rate on the
solution temperature and conductivity was
investigated under the conditions of maintaining an
initial pH of 8, a NaCl background ionic strength of
50 mmol/L, a Na
2
S
2
O
8
/UDMH (mol/mol) ratio of 2.0
and a voltage of 12.5 kV.
The variation of the solution conductivity is
shown in Figure 7. As can be seen from the figure, the
solution conductivity corresponding to the three
different recirculation flow rates all increased with
reaction time. The conductivity increased rapidly in
the first 5 min and then the growth rate slowed down
and the final conductivity followed the pattern of the
UDMH removal rate, reaching a maximum value at
140 mL/min.
The variation in solution temperature is shown in
Figure 8. 140 mL/min and 180 mL/min correspond to
solutions with a rapid decrease in temperature during
the first 5 min, after which the solution temperature
increases. In contrast, the temperature of the solution
corresponding to 100 mL/min decreased
continuously.
The cut-off point for the fast and slow changes in
solution temperature and conductivity is 5 minutes
when the cyclic flow rate is varied.
Figure 7: Conductivity changing with time as circular flow
fluctuates.
Figure 8: Temperature changing with time as circular flow
fluctuates.
3.5 Influence of UDMH Concentration
The effects of fluctuations in the initial concentration
of UDMH on the temperature and conductivity of the
solution were investigated under the environment of
maintaining the initial pH = 8, circulating flow rate of
140 mL/min, NaCl background ionic strength of 50
mmol/L, Na
2
S
2
O
8
/UDMH (mol/mol)=2.0 and
loading voltage of 12.5 kV, and the results are shown
in Figures 9-10.
The variation of the solution conductivity is
shown in Figure 9. As can be seen from the figure, the
solution conductivities corresponding to the three
different initial UDMH concentrations all increased
with reaction time, with a rapid increase in
conductivity in the first 5 minutes and a slower rate
of increase thereafter.
Indication of Solution Temperature and Conductivity During Electrochemical Oxidation of Hydrazine Wastewater
75
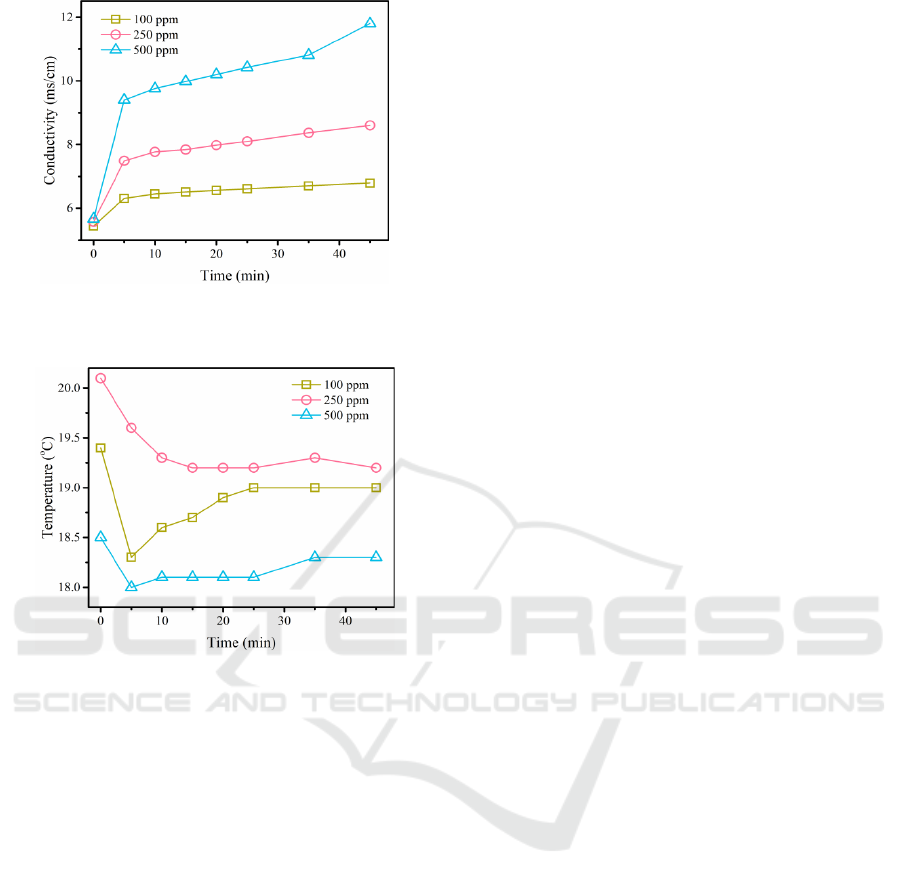
Figure 9: Conductivity variation with time as UDMH
concentration fluctuates.
Figure 10: Temperature variation with time as
UDMH concentration fluctuates.
The changes in solution temperature are shown in
Figure 10. 100 ppm and 500 ppm correspond to
solutions with a rapid decrease in temperature during
the first 5 minutes, after which the solution
temperature increases. In contrast, the temperature of
the solution corresponding to 250 ppm continued to
decrease.
The cut-off point for the fast and slow changes in
solution temperature and conductivity is 5 min when
the initial concentration of UDMH is changed.
4 SUMMARY
In order to solve the problems of long colour
development reaction time and complicated operation
steps of spectrophotometric measurement of UDMH
concentration, the conductivity and temperature of
the solution, which are convenient for testing, were
used as the indicators to judge the degree of
electrochemical degradation of UDMH. During the
degradation of UDMH wastewater by Na
2
S
2
O
8
synergistic plasma, when the variables such as
voltage, initial pH of solution, background ion
concentration, Na
2
S
2
O
8
dosage, recycle flow rate, and
initial concentration of UDMH were changed, the
change rules of the UDMH concentration, acidity and
alkalinity with the temperature of the solution, and the
electrical conductivity were checked in turn, and the
results showed that: under different reaction
conditions, the change of the UDMH concentration
and the acidity and alkalinity were fast and slow. The
results showed that under different reaction
conditions, the cut-off point of UDMH concentration,
acidity and alkalinity appeared at 10 min, and the
corresponding transition point of solution
temperature and conductivity was 5 min. Based on
this, the transition point of UDMH rapid degradation
to slow degradation can be predicted online by
collecting the solution temperature and conductivity
during the sampling process, so as to shorten the
duration of the test and improve the efficiency of the
test.
REFERENCES
Li, Y., Li, B., Huang, H., Gong, C., Wan, X. 2017. Research
Progress on Propellant Wastewater Treatment. In The
8th National Conference on Chemical Propellants.
China Chemical Society, vol. 4.
Zheng, M., Chen, X., Cheng, R., Li, N., Sun, J., Wang, X.,
Zhang, T. 2006. Catalytic decomposition of hydrazine
on iron nitride catalysts. Catal. Commun., 7(3): 187-
191.
Zeng, B.P., Jia, Y., Xu, G., Li, M., Feng, R. 2019a.
Preparation of TiO
2
/g-C
3
N
4
under the action of CTAB
and its photocatalytic degradation of unsymmetrical
dimethylhydrazine wastewater. Material Engineering,
47(09): 139-144.
Deng, X., Liu, X., Liu, Y., Cao, L. 2015. Progress in the
treatment technology of unsymmetrical
dimethylhydrazine wastewater. Chemical Propellants
and Polymer Materials, 13(3): 21-25,34.
Bu, X., Liu, X. 2013. Experimental study on the adsorption
of unsymmetrical dimethylhydrazine by oxalic acid-
modified attapulgite. Chemical Propellants and
Polymer Materials, 11(01): 55-58.
Gu, D. 2019. Study on the degradation mechanism of
typical psychoactive substances by advanced oxidation
technology. China University of Mining and
Technology, Xuzhou.
Li, X., Zeng, Q., Feng, C., Zhou, S. 2006. Adsorption
performance of ion exchange fiber on unsymmetrical
dimethylhydrazine. Chemical Engineering, 6(1): 23-
27.
Wang, L. 2005. Aerobic biodegradation and kinetics of
unsymmetrical dimethylhydrazine wastewater.
Chongqing University, Chongqing.
ICESCE 2024 - The International Conference on Environmental Science and Civil Engineering
76

Zeng, B. P., Xu, G., Jia, Y., Feng, R. 2019b. Nitric acid-
modified graphite phase carbon nitride photocatalytic
degradation of unsymmetrical dimethylhydrazine
wastewater. Environmental Pollution and Control,
41(02): 160-163,169.
Zeng, B. P., Xu, G., Jia, Y., Li, M., Ma, J. 2018. Preparation
of TiO
2
/g-C
3
N
4
and its photocatalytic degradation of
unsymmetrical dimethylhydrazine wastewater. Applied
Chemical Industry, 47(04): 771-774,779.
Hou, R., Liu, Z., Ma, W., Fang, X. 2019. Experimental
study on the near-critical water oxidation treatment of
high-concentration unsymmetrical dimethylhydrazine
waste liquid. Water Supply and Drainage, 45(4): 82-87.
Qiu, C., Gu, X., Liu, Z., Zhu, A., Yan, K. 2020.
Experimental study on the treatment of organic dye
wastewater by nanosecond pulse discharge. High
Power Laser and Particle Beams, 32(2): 025010.
Rong, J., Li, L., He, Y., Zhang, Y., Li, F., Shi, T. 2020.
Study on the low-temperature plasma process for
efficient purification of PAM-containing wastewater.
Chemical New Materials, 48(3): 269-273.
Liu, H., Song, Z., Song, C., Wang, S. 2020. Study on the
simultaneous removal of Cr(VI) and phenol from water
by low-temperature plasma technology. Industrial
Water Treatment, 40(4): 35-43.
Yi, Z., Qing, Z., Wang, D., Jiang, M., Wang, Y., Huang, Y.
2019. Study on the degradation of unsymmetrical
dimethylhydrazine wastewater by low-temperature
plasma. Integrated Technology, 8(6): 65-74.
Wu, H., Chen, W., Fang, Z., Liu, F., Fan, J., Yin, B. 2019.
Optimization of dielectric barrier discharge for
activating persulfate to treat tetracycline wastewater.
High Voltage Technology, 45(5): 1387-1395.
Zhou, Z., Zheng, Z., Li, H., He, R., Hou, R., Yu, Y., Yang,
Y. 2023. Activated persulfate by dielectric barrier
discharge plasma for the degradation of unsymmetrical
dimethyl hydrazine as wastewater pollutants. Pol. J.
Environ. Stud., 32(4): 3447-3454.
Indication of Solution Temperature and Conductivity During Electrochemical Oxidation of Hydrazine Wastewater
77
