
Study of the Temperature and Molarity Ratio Effects in Geraniol
Esterification and Testing Its Antibacterial Activity
Stefanie Sugiarto
1a
, Novita Ariani
2 b
, Elvina Dhiaful Iftitah
1c
and Galuh Widiyarti
3d
1
Department of Chemistry, Brawijaya University, Veteran, Malang, Indonesia
2
Research Center for Chemistry, National Research and Innovation Agency (BRIN) Puspiptek, South Tangerang, Indonesia
3
Research Center for Pharmaceutical Ingredients and Traditional Medicine, BRIN, Puspiptek, South Tangerang, Indonesia
Keywords: Geraniol, Geranyl Isobutyrate, Disc Diffusion Method.
Abstract: This study modifies the molarity ratio of geraniol to isobutyric acid (1:1, 1:1.1, and 1:1.3) and temperature
(RT, 40°C, 60°C, and 80°C) in the synthesis of geranyl isobutyrate ester using 5% (w/w) NaOH as a base
catalyst. The antimicrobial activity was tested against both gram-positive and gram-negative bacteria. Ester
products were separated and purified using column chromatography, and identified using Fourier Transform
Infrared Spectroscopy (FTIR) and Gas Chromatography-Mass Spectrometry (GCMS). The antimicrobial
activity was assessed using the disk diffusion method. The results showed that the esterification product with
a 1:1.1 molar ratio at 80°C had the best separation based on thin-layer chromatography (TLC) and
antimicrobial properties. GCMS analysis of the purified product revealed five compound peaks with geranyl
isobutyrate at R
T
= 13.376 minutes (2.77%). FTIR confirmed the presence of C=O ester carbonyl groups at
1717.82 cm
-1
and C-O groups at 1080.37 cm
-1
. Antimicrobial tests showed inhibition zones on gram-positive
bacteria of 18.33±2.62 mm for Bacillus subtilis and 15.67±0.47 mm for Staphylococcus aureus, and against
gram-negative bacteria of 10.67±0.47 mm for Pseudomonas aeruginosa and 16.67±2.36 mm for Escherichia
coli.
1 INTRODUCTION
The development of essential oils in Indonesia is
progressing rapidly due to their diverse benefits in the
pharmaceutical and medicinal fields. Essential oils,
commonly derived from plants, include lemongrass
oil, which is currently popular with global
consumption reaching around 2,000-2,500 tons per
year (Direktorat Jendral Perkebunan, 2020). The
diverse benefits of lemongrass oil are its antiseptic
properties and medicinal uses, make it a highly
valuable commodity. Lemongrass oil is rich in
beneficial compounds such as citronellal, citronellol,
and geraniol.
In Indonesia, there is a significant demand for
geraniol derived from lemongrass essential oil,
especially in the pharmaceutical and perfume
industries. Furthermore, geraniol exhibits a variety of
a
https://orcid.org/0009-0002-9067-4326
b
https://orcid.org/0000-0002-1639-9058
c
https://orcid.org/0000-0002-6615-1851
d
https://orcid.org/0000-0002-0235-596X
beneficial medical properties, including antioxidant,
anti-inflammatory, antimicrobial, antitumor,
hepatoprotective, cardioprotective, and
neuroprotective effects (Pavan et al., 2018). Some
researchs have shown that geraniol has strong
antimicrobial activity due to its lipophilic properties,
which allow it to bind to the lipid membranes of
microorganisms, demonstrating effectiveness against
various bacteria, including Candida and
Staphylococcus(Lira et al., 2020). Nevertheless,
geraniol exports from Indonesia decreased from
11,789.3 million USD in 2019 to 8,251.1 million
USD in 2020 (Badan Pusat Statsitik, 2021).
Therefore, efforts are needed to increase the market
value of geraniol by discovering its derivatives to
enhance bioactivity and application potential through
derivatization into geranyl esters.
38
Sugiarto, S., Ariani, N., Iftitah, E. D. and Widiyarti, G.
Study of the Temperature and Molarity Ratio Effects in Geraniol Esterification and Testing Its Antibacterial Activity.
DOI: 10.5220/0013553900004612
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of BRIN’s 2nd International Conference for Health Research (ICHR 2024), pages 38-46
ISBN: 978-989-758-755-9
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

Numerous studies have shown that derivatives
of geraniol, such as geranyl formate, geranyl acetate,
geranyl butyrate, and geranyl isobutyrate, possess
antibacterial properties. For instance, a study
indicated that organophilic bentonite incorporated
with geranyl acetate exhibited antibacterial activity
against Staphylococcus aureus and Salmonella
typhimurium (Capelezzo et al., 2023). Additionally,
geraniol derivatives such as geranyl butanoate have
shown potential as anticancer agents (Widiyarti et al.,
2019). On the other hand, geranyl isobutyrate has
been proven to have superior antimicrobial properties
compared to other geraniol derivatives, as
demonstrated in studies testing antimicrobial activity
against Escherichia coli, Staphylococcus aureus,
Pseudomonas aeruginosa, and Staphylococcus
epidermidis. The antibacterial effect of geranyl
isobutyrate against E. coli was the strongest among
the 12 compounds tested (Zhaoshuang et al., 2016).
However, further research is needed due to the limited
studies examining the antimicrobial activity of
geranyl isobutyrate, and most existing studies have
not included B. subtilis as one of the bacteria.
Moreover, additional research is required regarding
the synthesis method of geranyl isobutyrate, as its
quantity derived from plants is usually limited, such
as in Conyza incana, which yields only about 2.5%
(Zabin, 2018). The synthesis of geranyl isobutyrate
generally involves an esterification reaction between
alcohol and carboxylic acid compounds to form the
desired ester.
Conceptually, the esterification reaction requires
high temperatures or heating above 55°C (Tolvanen
et al., 2014). Additionally, catalysts play a role in
accelerating the reaction. Acid catalysts are generally
used, but recent research has shown that the use of
base catalysts can be an effective alternative. Base
catalysts are known to increase reaction efficiency, be
environmentally friendly, and reduce water formation
(Khan et al., 2021). Although the use of base catalysts
for esterification is still in the research phase,
previous studies have successfully produced geraniol
derivatives using a base catalyst of NaOH. These
derivatives include geranyl butyrate, geranyl
caproate, and geranyl caprylate, with yields of
66.94%, 76.72%, and 54.92%, respectively
(Widiyarti et al., 2019). This finding indicates the
potential use of heterogeneous base catalysts in the
esterification process, which has not been widely
reported.
Based on the above issues, this research aims to
synthesize geranyl isobutyrate using sodium
hydroxide (NaOH) as a base catalyst to enhance the
antibacterial activity of geraniol derivatives. The
geraniol esters will be identified using TLC, FTIR,
and GCMS. The geranyl isobutyrate product is then
antibacterial activity tested against B. subtilis, S.
aureus, E. coli, P. aeruginosa. These four bacteria are
bacterial models that are usually used for antibacterial
activity tests for natural products screening and their
derivative compounds that have the potential to be
antibacterial.
2 RESEARCH METHODS
2.1 Synthesis of Geranyl Isobutyrate
(GI)
The esterification of GI based on 1:1 molarity ratio is
carried out by reacting 20 mmol (3.086 g) of geraniol,
which is placed into a 250 mL round-bottom flask and
mixed with NaOH catalyst at 5% of the weight of
geraniol. After 4 hours, 20 mmol (1.762 g) of
isobutyric acid is added. The solution mixture is
reacted at room temperature (RT) for 24 hours. The
same procedure is repeated for each molar variation
of isobutyric acid for 1:1.1 (22 mmol, 1.939 g) and
1:1.3 (26 mmol, 2.290 g).
Next, the synthesis product is extracted using a
100 mL separatory funnel with a solvent solution of
ethyl acetate and distilled water (1:1). The mixture is
shaken and allowed to stand until two phases form,
and the organic phase is collected. This process is
performed in triplicate. The collected organic phase
is evaporated using a rotary evaporator until crude GI
ester product is obtained. The yield of the crude ester
is calculated using the following formula:
% 𝑌𝑖𝑒𝑙𝑑 =
()
𝑥100% (1)
The product ester is then purified using column
chromatography with eluent n-hexane, ethyl acetate,
and methanol eluted gradually. The separated
compound is identified using TLC, FTIR, and
GCMS.
This procedure is repeated for each molar
variation at different temperatures of 40
0
C, 60°C and
80°C.
2.2 Purification and Characterization
of GI Ester
The purification of GI crude ester is carried out using
gradient column chromatography with n-hexane and
ethyl acetate as solvents. The purified product is
subsequently analyzed by GCMS Agilent
Study of the Temperature and Molarity Ratio Effects in Geraniol Esterification and Testing Its Antibacterial Activity
39

Tecnologies 7890C and FTIR Shimadzu prestige 21
using KBr pellets.
2.3 Antibacterial Test Using Disc
Diffusion Method
The bacterial culture of B. subtilis ATCC 6633, S.
aureus ATCC 25923, E. coli ATCC 8739, and P.
aeruginosa ATCC 9027 was prepared by swiped of 1
ose of bacteria culture from stock culture on the
sterile Nutrient Agar (NA) agar slant. The culture is
stored in an incubator at 37°C for 24 hours. Bacterial
culture on rejuvenated agar slants was then added
with 10 mL of 0.9% NaCl solution, shaken until all
colonies on the surface are loose and suspended in
NaCl solution 0.9%. The bacteria as much as 100 μl
were put into the petri dish, and then an amount of 10
mL of NA is added, and allow it to solidify.
Afterwards, the discs (approximately 6 mm in
diameter) are placed on the NA. Finally, 5 μl of the
sample at a desired concentration is added on top of
each disc. For each sample, a triplicate test was
carried out. The petri dishes are then incubated under
suitable conditions at 37
0
C for 18-24 hours, then the
resulting diameters of inhibition zone was measured
(Yusmaniar et al., 2017; Hossain, 2024).
3 RESULTS AND DISCUSSION
3.1 Synthesis of GI
Esters are produced through the reaction of
carboxylic acids with alcohols, forming water as a by-
product and replacing the hydroxyl group (-OH) with
an alkoxy group (-OR) (Melvine et al., 2021). The
esterification of geraniol with isobutyric acid, aided
by the base catalyst NaOH, results in the formation of
geranyl isobutyrate (GI). The presence of NaOH is
used to increase the reaction rate without undergoing
chemical change and to lower the activation energy
(Ea) (Setyaningsih et al., 2017). The synthesis and
mechanism reaction of GI as shown in Figure 1 and
Figure 2.
Figure 1: Synthesis Reaction of GI.
Figure 2: Esterification Reaction Mechanism of GI.
The hydroxyl group (-OH) reacts with the catalyst,
resulting in proton abstraction and the formation of an
alkoxide ion. This nucleophilic alkoxide ion attacks
the carbon atom of the carbonyl group, leading to
resonance stabilization and the formation of GI ester.
The mechanism occurs because the alcohol acts as a
good nucleophile, making the reaction with
carboxylic acid more effective in forming the geranyl
ester. This aligns with previous research, which states
that heterolytic cleavage occurs in the presence of the
catalyst, followed by the alcohol attacking the
catalyst, enhancing its nucleophilicity and forming an
intermediate. Subsequently, this intermediate
decomposes, eliminating the catalyst and forming the
ester (Kohsaka et al., 2018). However, this reaction
may produce side products if the alkoxide ion reacts
with other compounds in the mixture.
The reaction product exhibits a pH of 5-6,
indicating that it is non-corrosive. Liquid-liquid
extraction is then performed to separate the organic
phase from the aqueous phase (Figure 3). Ethyl
acetate (EtOAc) is used as the solvent to ensure the
efficient distribution of the geranyl ester in the
organic phase. EtOAc is soluble in various
compounds and has relatively low viscosity,
facilitating separation (Schneider et al., 2021).
Figure 3: Liquid-liquid extraction separation of geranyl
ester.
Subsequently, the crude ester as a yellowish-brown
substance was produced by evaporating the extracted
material as shown in Figure 4.
ICHR 2024 - BRIN’s International Conference for Health Research (ICHR)
40
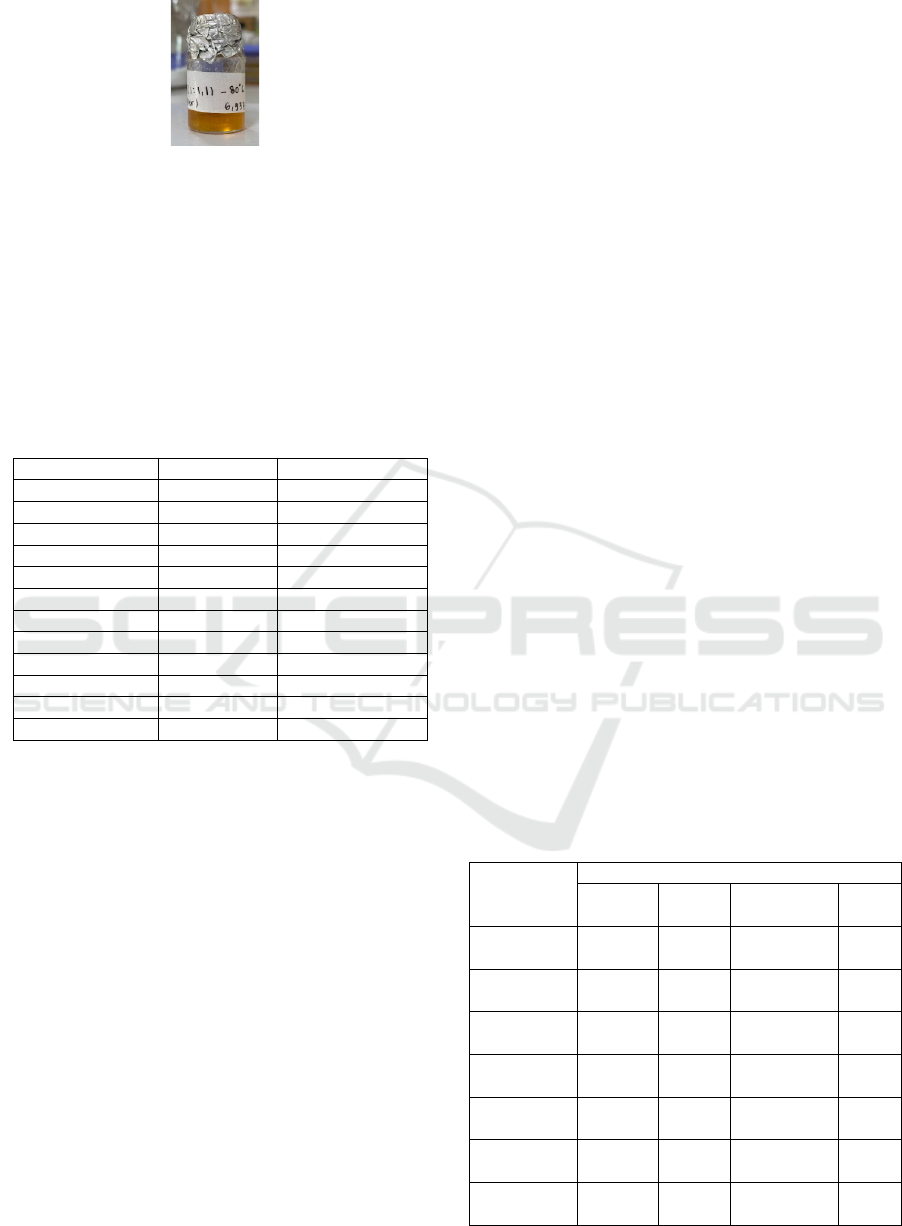
Figure 4: Crude GI product.
The percentage yields of GI crude esters were
calculated based on the percentage molarity of GI
divided by the molarity of starting material/reactan
from geraniol.
The relatively high yield is attributed to the
presence of impurities and geraniol that have not been
completey synthesized into GI ester. The crude ester
yield under various reaction conditions is presented in
Table 1.
Table 1: Crude ester yield.
Sample Weight (g) Yield (%)
GI (1:1)
–
RT 3.480 77.28
GI
(
1:1,1
)
–
RT 4.011 89.11
GI
(
1:1,3
)
–
RT 4.208 93.53
GI
(
1:1
)
- 40°C 4.143 92.15
GI (1:1,1) - 40°C 3.472 77.10
GI (1:1,3) - 40°C 3.791 84.46
GI (1:1) - 60°C 3.433 76.43
GI
(
1:1,1
)
- 60°C 3.522 78.29
GI
(
1:1,3
)
- 60°C 4.035 89.46
GI
(
1:1
)
- 80°C 4.104 90.98
GI (1:1,1) - 80°C 4.248 94.78
GI (1:1,3) - 80°C 4.173 92.95
The highest yield of 94.78% was produced in the
esterification of geraniol at a temperature of 80℃ and
a reactant molarity ratio of 1:1.1.
Afterthat, esterification results were analyzed by
TLC using silica gel plates (Kiesel gel 60F254 0.25
mm), with an eluent ratio of n-hexane to ethyl acetate
(9:1) at λ = 254 nm. The results showed that at RT,
the Retention factor (R
f
) value for GI matches the R
f
of geraniol of 0.73, indicating esterification has not
occurred yet. However, there is a gradual decrease in
the R
f
value from 0.65 to 0.6 and finally to 0.55 at
80°C, suggesting that 80°C is the optimal temperature
for forming the geranyl ester product.
Temperature is a crucial parameter that regulates
the rate and extent of esterification. An increase in
reaction temperature significantly impacts
esterification, where higher temperatures lead to
faster conversion. However, higher reaction
temperatures risk increasing product darkness. It has
been observed that even a small temperature
difference of 10-20°C can significantly affect the
reaction rate and product yield (Mazubert et al.,
2014).
In the esterification process, the addition of
excess reactants can drive the esterification reaction
toward higher product yield, consistent with Le
Chatelier's principle (Peris, 2021). Based on TLC also
showed that (GI) with a concentration ratio of 1:1.1
has the best R
f
value, while other concentrations show
no significant change. Although the 1:1.3
concentration is higher than 1:1.1, the R
f
value
decreases because the 1:1.1 concentration achieves
the optimal stoichiometric level. There is a threshold
where further increases do not significantly enhance
conversion and may even reduce it due to reaction
mixture saturation and catalyst deactivation (Lade et
al., 2014).
The rate of esterification is influenced by the
structure of the carboxylic acid. Linear chain acids
esterify more readily than branched acids due to steric
hindrance. The presence of branched chains in the
acid slows the reaction rate. Adding more chains to
the acid structure further reduces the reaction rate.
However, branched chain acids offer higher
conversion rates than linear chain acids. Additionally,
certain substituents can either accelerate or decelerate
the reaction rate (Jin et al., 2012).
R
f
value for synthesized compound of GI is 0.55,
lower than R
f
value of 0.73 from the starting material
geraniol. This is allegedly because larger size and
structure of GI than geraniol, less polar and causing
more interaction with the stationary phase of silica
gel.
Furthermore, antibacterial tests were conducted to
determine the efficacy of the crude ester with the best
results, as shown in Table 2.
Table 2: Antibacterial test results of crude GI extract.
Sample
Inhibition Zone
B.
s
ubtilus
S.
aureus
P.
aeruginosa
E. coli
GI(1:1,1) -
80°C
16.67
±0.94
17.00
±1.63
16.33
±0.47
16.67±
1.25
GI(1:1) -
80°C
16.67
±0.47
15.33±0
.47
16.33
±1.70
16.00±
0.82
GI(1:1,3) -
80°C
16.33
±0.47
17.00±0
.82
15.33
±0.47
16.33±
1.25
GI(1:1,1) -
RT
17.67
±2.05
16.33±0
.94
16.00
±0.82
15.33±
1.25
GI(1:1,1) -
40°C
16.33
±0.94
16.67±0
.47
16.33
±0.47
16.00±
1.63
GI(1:1,1) -
60°C
17.00
±0.82
16.33±1
.25
16.67
±1.70
16.00±
1.41
Geraniol
12.33
±1.25
11.67±1
.25
11.33
±0.47
10.00±
0.00
Study of the Temperature and Molarity Ratio Effects in Geraniol Esterification and Testing Its Antibacterial Activity
41
Antibacterial test results indicate that the crude
ester with an isobutyric acid molar ratio of 1:1.1 at
80°C demonstrated the best antibacterial activity
against both gram-positive and gram-negative
bacteria. This condition proved to be more effective
compared to other molarity and temperature
variations. Therefore, the molar ratio of 1:1.1 at 80°C
is selected as the optimal synthesis condition. This
condition will be used for subsequent purification and
characterization stages. This is in accordance with the
yield produced, where the highest yield is produced
under these conditions.
3.2 Purification and Characterization
of GI by Using GCMS and FTIR
A total of 3500 mg of crude GI was purified using
gradient column chromatography, yielding 19
fractions. This method utilized silica gel 60 (230-400
mesh) and a gradient solvent mixture of n-hexane and
ethyl acetate. Fractions F6-F9 in n-hexane: ethyl
acetate (9:1), and F10-13 in n-hexane ; ethyl acetate
(8:2) were identified as geranyl isobutyrate, with
yields of 24 mg (0.682%), and 28 mg (0.795%)
respectively. The TLC of the purified ester as shown
in Figure 5.
Figure 5: The TLC of purified GI.
Tailing was observed in the TLC of the purified
product grom GI, likely due to the presence of
impurities or other by-products in the sample. To
confirm the presence of the compounds, further
analysis was conducted using GCMS and FTIR.
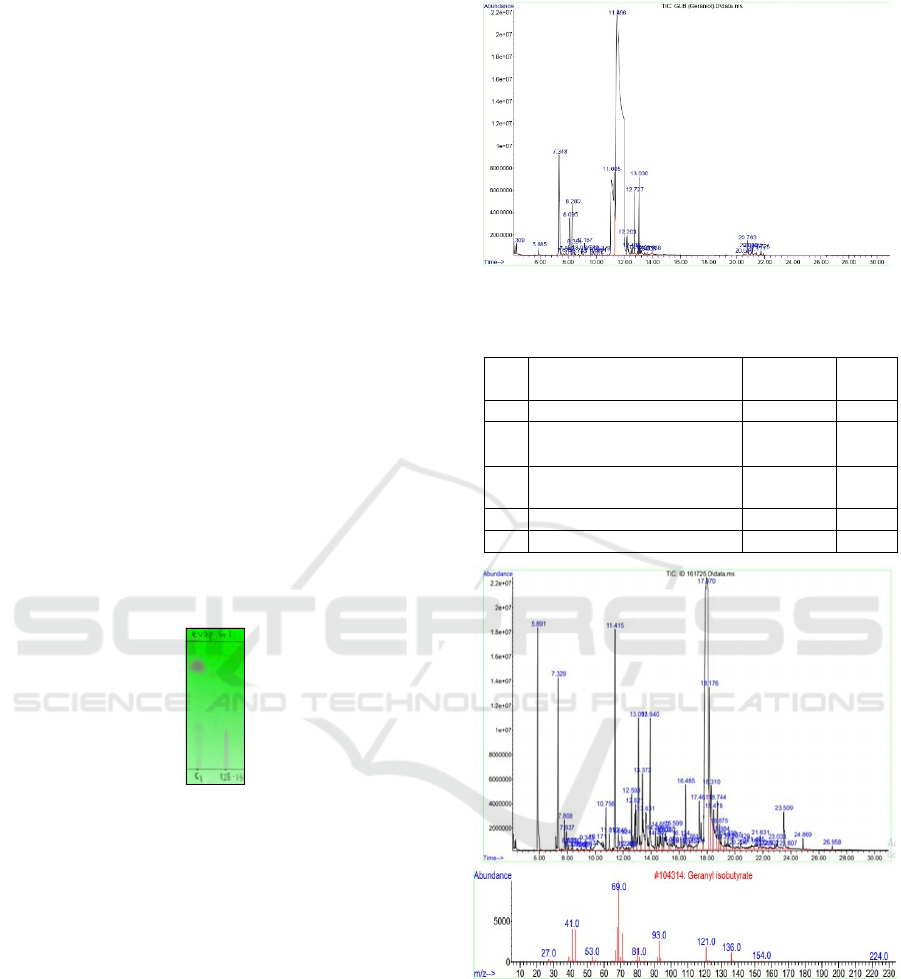
GCMS analysis was performed to identify the
components of the synthesis product based on mass
spectra, area and molecular weight. GC
chromatogram of pure GI showed 62 compound
peaks. It is due to geraniol as starting material itself
had 30 contaminant compounds with an area of
71.56% (Figure 6).
Figure 6: The chromatogram of geraniol
Table 3: Dominant peak in GCMS of GI ester.
No Compound
R
t
(minut1es)
Area
(%)
1 3-Buten-2-ol 17.976 33.89
2
Cyclohexanecarboxylic
Aci
d
18.177 5.75
3
3,7-dimethyl-2,6-
octadien
y
l
13.376 2.77
4 Geraniol 11.409 4.50
5 Oxalic Aci
d
7.326 3.28
Figure 7: GC chromatogram of synthesis product.
The five dominant peaks of GI ester chromatogram
presented in Table 3 and Figure 7. The retention time
(R
T
) of 13.376 minutes corresponds to 3,7-dimethyl-
2,6-octadienyl or geranyl isobutyrate (GI) compound
with the area percentage was 2.77%, as shown in
Table 3 and Figure 7.
Based on the mass spectra in Figure 7, the geranyl
isobutyrate compound has a molecular mass (M) of
224 with a base peak at m/z = 69. Other characteristic
ICHR 2024 - BRIN’s International Conference for Health Research (ICHR)
42
peaks appear at m/z = 136 (M+-88), indicating the
loss of isobutyric acid. Another important peak is at
m/z = 93, formed from the loss of ethylene from m/z
= 121, and the peak at m/z = 154 is suspected to be a
fragment of the ester moiety.
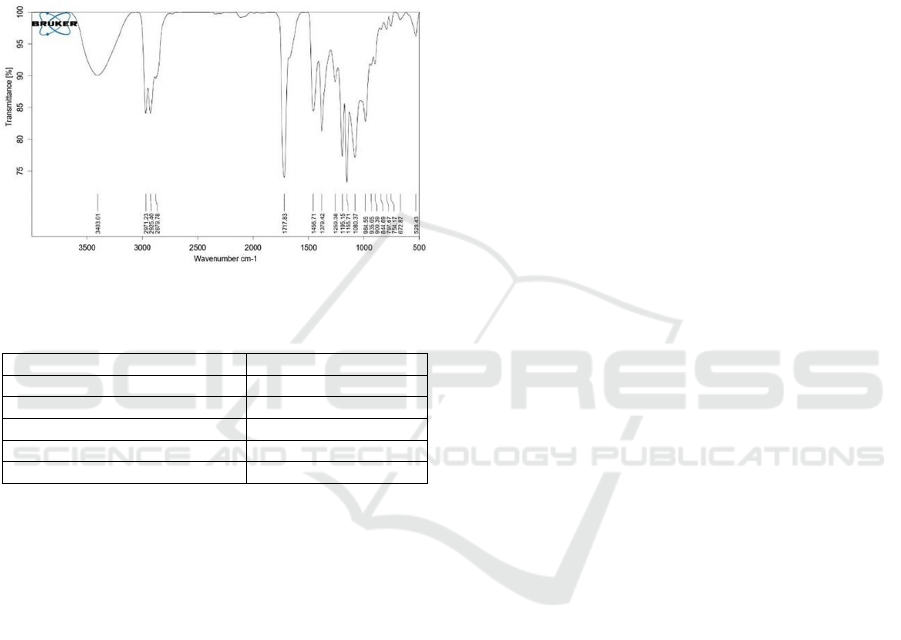
FTIR analysis was conducted to determine the
functional groups present in the ester product. The
FTIR spectrum can be seen in Figure 8. The
interpretation of the wavenumbers for GI is provided
in Figure 8 and Table 4.
Figure 8: FTIR Spectra of GI.
Table 4: The IR Interpretation of GI.
Wavenumber (cm
-1
) Functional Group
3403,01 O-H
s
tretch
2925,40;2971,23;2879,78 C-H s
p
3
1717,83 C=O
1456,707 -C=C- bend
1080,37 C-O
The FTIR results show several characteristic
absorption bands. At a wavenumber of 1717.83 cm
-1
,
there is a significant absorption band corresponding
to the carbonyl (C=O) stretching vibration of the
ester. The presence of this group is further confirmed
by the absorption at 1080.37 cm
-1
, indicating the
presence of the C-O group of the ester. Additionally,
the absorption at 3403.01 cm
-1
shows the presence of
residu of unreacted geraniol. Wang et al. reported that
geranyl esters have an IR peak around 1735.87 cm
-1
,
indicating the presence of a carbonyl (C=O) group,
and a C- O absorption at 1175.32 cm
-1
(Wang et al.,
2019).
3.3 Antibacterial Test Using Diffusion
Method
The diffusion method is carried out using the paper
disk, with the bacterial count for sensitivity testing
ranges from 10
5
to 10
8
CFU/mL. Paper disks
containing antibiotics or samples are placed on a
medium containing the microbes, then incubated and
the results read based on the microbial inhibition
around the disk (Yusmaniar et al., 2017). The basic
principle of the method is the measurement of the
diameter of the clear zone, which indicates the
antibacterial compound's inhibition of bacterial
growth in the test sample (Bhargav et al., 2016.).
GI is a monoterpenoid with good antimicrobial
properties, and its results are optimized with proper
incubation time. The incubation process is conducted
for 18-24 hours as the bacteria are still in the
exponential phase. Interaction with the hydrophobic
structure of bacteria plays a key role in the
antimicrobial effects of hydrocarbons. Bacteria are
more sensitive during the exponential phase
compared to the stationary phase. Several
antimicrobial agents cause significant changes to the
plasma membrane, resulting in total cell lysis.
Although the activation of autolytic enzymes may be
responsible for this effect, lysis can also be caused by
the weakening of the cell wall and disruption of the
cell membrane due to osmotic pressure, rather than
specific action on the membrane (El Kolli et al.,
2016).
The antibacterial test conducted on crude ester
and pure GI produced at optimum esterification
process at a 1:1.1 molarity ratio and 80°C temperature
compared to reactant of geraniol (G). Tetracycline
(TS), streptomycin (MS), and dimethyl sulfoxide
(DMSO) were used as positive and negative controls,
respectively, at a concentration of 16,000 ppm. This
activity test was performed against gram-positive
bacteria, namely B. subtilis and S. aureus, as well as
gram-negative bacteria, P. aeruginosa and E. coli.
The results showed at Table 4. It can be seen that
DMSO, as a negative control, exhibited no
antimicrobial activity, with a consistent inhibition
zone of 6 mm. This confirms that the observed
antimicrobial activity is due to GI ester compound.
Tetracycline and streptomycin were used as positive
controls due to their broad-spectrum activity against
various gram-positive and gram-negative bacteria
and their use in both veterinary and human medical
treatments (Araby et al., 2020). Comparatively, GI
showed fairly good antibacterial activity, indicating
potential as a therapeutic agent. Visualization of the
inhibition zones in the antibacterial disk diffusion test
is shown in Figure 9.
Study of the Temperature and Molarity Ratio Effects in Geraniol Esterification and Testing Its Antibacterial Activity
43
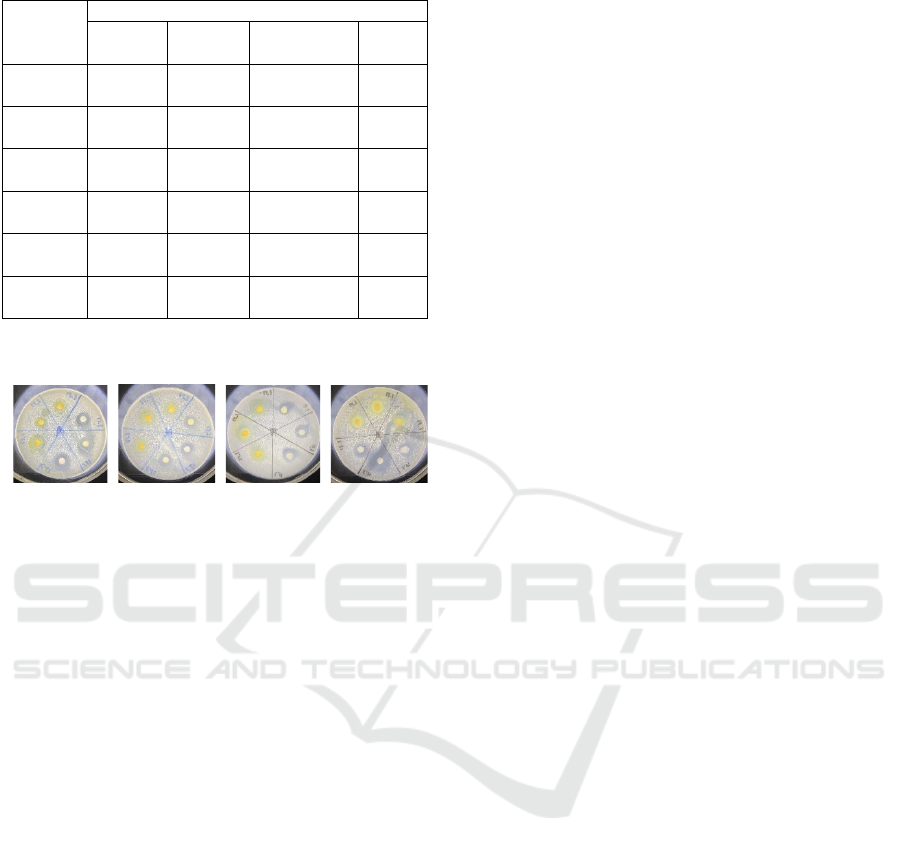
Table 5: The antibacterial activity of samples.
Sample
Inhibition Zone
(
mm
)
B.
s
ubtilus
S.
aureus
P.
aeruginosa
E. coli
TS
23.67
±0.47
30.00
±0.00
18.00
±0.00
20.00
±0.00
SM
21.33
±1.89
30.00
±0.00
23.33
±2.36
26.33
±0.94
DMSO
6.00
±0.00
6.00
±0.00
6.00
±0.00
6.00
±0.00
Pure GI
18.33
±2.62
15.67
±0.47
10.67
±0.47
16.67
±2.36
Crude
GI
16.67
±0.94
17.00±
1.63
16.33
±0.47
16.67
±1.25
G
12.33
±1.25
11.67±
1.25
11.33
±0.47
10.00
±0.00
B. subtilus S. aureus
P.
aeruginosa
E. coli
Figure 9: Inhibition zone of pure GI.
Additionally, Table 5 shows stronger antibacterial
activity against gram-positive bacteria, with
inhibition zones of 18.33 mm for B. subtilis and 15.67
mm for S. aureus. Based on these inhibition zones GI,
is categorized as a strong antibacterial (Ullah & Ali,
2017). Zabin declared that essential oils with geranyl
isobutyrate as the main product also exhibit good
activity against gram-positive bacteria (Zabin, 2018).
Previous studies have shown that the antibacterial
effect of monoterpenoids is weaker against gram-
negative bacteria due to their hydrophilic nature,
which prevents the contact of hydrophobic
monoterpenoid components with bacterial cells. In
contrast, gram-positive bacteria can directly damage
their cell membranes, leading to cell membrane
rupture, inhibition of enzyme systems, and increased
ion permeability (Lang, 2010). Pure GI showed a
significant increase in inhibition zones compared to
geraniol as starting material. However, there was a
decrease in against P. aeruginosa and S. aureus when
compared to crude GI (1:1.1). This may be due to the
some compounds present in GI were separated during
purification, which may contribute to antibacterial
activity against P. aeruginosa and S. aureus.
Additionally, P. aeruginosa is challenging to
eradicate due to its biofilm-forming ability
(Srivastava et al., 2021). Meanwhile, S. aureus is a
major concern due to its high resistance levels (Zabin,
2018). Consequently, pure GI showed decreased
antibacterial activity against these bacteria compared
to crude GI, which contains more compounds with
potential antibacterial activity against these bacteria.
5 CONCLUSIONS
The study indicate that both temperature and reactant
molarity ratio significantly influence the efficiency of
GI ester formation. The optimum reaction of
esterification was at a 1:1.1 of molarity ratio and a
temperature of 80°C which produces the largest crude
GI of 4.248 g (94.78%), nevertheless the pure GI
produced was only 52 mg (1,48%). One of the
dominant peaks on the GCMS chromatogram
revealed the presence of GI compound at R
T
of
13.376 minutes with an area of 2.77% as well as the
FTIR spectra displayed functional groups of carbonyl
(C=O) at 1717.82 cm
-1
from ester compound.
Antibacterial activity test demonstrated that GI
exhibited significant antibacterial activity against
gram-positive bacteria, with inhibition zones of
18.33±2.62 mm for B. subtilis and 15.67±0.47 mm for
S. aureus, and against gram-negative bacteria, with
inhibition zones for P. aeruginosa 10.67±0.47 mm
and for E. coli 16.67±2.36 mm. Therefore GI is
classified as an strong antibacterial agent against
gram-positive bacteria.
Further purification is needed to increase the pure
ester yield, as well as minimum inhibitory
concentration (MIC) and minimum bactericidal
concentration (MBC) analysis.
ACKNOWLEDGEMENTS
The authors would like to express their deepest
gratitude to Advanced Characterization Laboratories
Serpong, BRIN for their facilities support, and the
Research Organization of Health BRIN for
supporting this research with the funding number
5/III.9/HK/2024.
Galuh Widiyarti who had the research idea and
Stefanie Sugiarto who helped conduct the research by
doing synthesis and antibacterial analysis of GI are
the main contributors. Whilst Novita Ariani who
helped antibacterial test and Elvina Dhiaful Iftitah
who gave comments on the draft manuscript are co
contributors.
ICHR 2024 - BRIN’s International Conference for Health Research (ICHR)
44

REFERENCES
Araby, E., Nada, H. G., Abou El-Nour, S. A., & Hammad,
A. (2020). Detection of tetracycline and streptomycin
in beef tissues using Charm II, isolation of relevant
resistant bacteria and control their resistance by gamma
radiation. BMC Microbiology, 20(1), 186.
https://doi.org/10.1186/s12866-020-01868-7.
Badan Pusat Statsitik. (2021). Statistik Perdagangan Luar
Negeri Indonesia Ekspor Menurut Kode SITC, 2020-
2021. BPS RI.
Bhargav, H.S., Sachin, D.S., Poornav, S.P., Darshan K. M.,
Mahendra, M.N. (2016). Measurement of the Zone of
Inhibition of an Antibiotic. 6th International Advanced
Computing Conf. 978-1-4673-8286 https://doi.org/10.
1109/IACC.2016.82
Capelezzo, A. P., Celuppi, L. C. M., Kuhn, K. Z., Sanaiotto,
O., Scapinello, J., Zanetti, M., Zeferino, R. C. F.,
Müller, L. G., Fiori, M. A., & Riella, H. G. (2023).
Acute toxicity study of antibacterial organophilic
bentonite incorporated with geranyl acetate in mice and
geranyl acetate liberation in simulated gastric fluid.
Toxicon, 224, 107027. https://doi.org/10.1016
/j.toxicon.2023.107027
Direktorat Jendral Perkebunan. (2020). Serai Wangi: Kaya
Akan Manfaat Dan Peluang Yang Menjanjikan. https://
ditjenbun.pertanian.go.id/serai-wangi-kaya-akan-man
faat-dan-peluang-yang-menjanjikan/, accessed on 2
July 2024
El Kolli, M., Laouer, H., El Kolli, H., Akkal, S., & Sahli, F.
(2016). Chemical analysis, antimicrobial and anti-
oxidative properties of Daucus gracilis essential oil and
its mechanism of action. Asian Pacific Journal of
Tropical Biomedicine, 6(1), 8–15. https://doi.org/1
0.1016/j.apjtb.2015.08.004
Hossain, T.J. (2024). Methods for screening and evaluation
of antimicrobial activity: A review of protocols,
advantages, and limitations. European Journal of
Microbiology and Immunology. 14 (2), 97–115
https://doi.org/10.1556/1886.2024.00035
Jin, Z., Ntwali, J., Han, S.-Y., Zheng, S.-P., & Lin, Y.
(2012). Production of flavor esters catalyzed by CALB-
displaying Pichia pastoris whole-cells in a batch
reactor. Journal of Biotechnology, 159(1–2), 108–114.
https://doi.org/10.1016/j.jbiotec.2012.02.013
Khan, Z., Javed, F., Shamair, Z., Hafeez, A., Fazal, T.,
Aslam, A., Zimmerman, W.B., & Rehman, F. (2021).
Current developments in esterification reaction: A
review on process and parameters. Journal of Industrial
and Engineering Chemistry, 103, 80-103. https://doi
.org/10.1016/j.jiec.2021.07.018
Kohsaka, Y., Homma, K., Sugiyama, S., & Kimura, Y.
(2018). Esterification with Aromatic Acyl-1,2,4-
triazole Catalyzed by Weak Base at the Rate
Comparable to Acyl Chloride. Chemistry Letters, 47(1),
100–102. https://doi.org/10.1246/cl.170975
Lade, B.D., Patil, A.S., Paikrao, H.M., Kale, A.S., Hire,
K.K. (2014). A Comprehensive Working, Principles
and Applications of Thin Layer Chromatography.
Research Journal of Pharmaceutical, Biological and
Chemical Sciences, 486-503. https://www.researchgate
.net/publication/264497360
Lira, M. H. P. D., Andrade Júnior, F. P. D., Moraes, G. F.
Q., Macena, G. D. S., Pereira, F. D. O., & Lima, I. O.
(2020). Antimicrobial activity of geraniol: An
integrative review. Journal of Essential Oil Research,
32(3), 187–197. https://doi.org/10.1080/10412905.
2020.1745697
Mazubert, A., Taylor, C., Aubin, J., & Poux, M. (2014).
Key role of temperature monitoring in interpretation of
microwave effect on transesterification and
esterification reactions for biodiesel production.
Bioresource Technology, 161, 270–279. https://doi.
org/10.1016/j.biortech.2014.03.011
Melvine, D., Marissa, D., Juniarti, L., Kartika, N., & Vicry,
V. (2021). Senyawa Asam Karboksilat Dan Ester.
Pavan, B., Dalpiaz, A., Marani, L., Beggiato, S., Ferraro,
L., Canistro, D., Paolini, M., Vivarelli, F., Valerii, M.
C., Comparone, A., De Fazio, L., & Spisni, E. (2018).
Geraniol Pharmacokinetics, Bioavailability and Its
Multiple Effects on the Liver Antioxidant and
Xenobiotic-Metabolizing Enzymes. Frontiers in
Pharmacology, 9, 18. https://doi.org/10.3389/fpha
r.2018.00018
Peris, M. (2021). Understanding Le Châtelier’s principle
fundamentals: five key questions. Chemistry Teacher
International, 1-3.https://doi.org/10.1515/cti-2020-0030.
Schneider, Y. K., Jørgensen, S. M., Andersen, J. H., &
Hansen, E. H. (2021). Qualitative and Quantitative
Comparison of Liquid–Liquid Phase Extraction Using
Ethyl Acetate and Liquid–Solid Phase Extraction Using
Poly-Benzyl-Resin for Natural Products. Applied
Sciences, 11(21), 10241. https://doi.org/10.3390/app
112110241
Setyaningsih, L. W. N., Rizkiyaningrum, U. M., & Andi, R.
(2017). Pengaruh Konsentrasi Katalis Dan Reusability
Katalis Pada Sintesis Triasetin Dengan Katalisator
Lewatit. Teknoin, 23(1). https://doi.org/10.20885/te
knoin.vol23.iss1.art7
Srivastava, N., Singh, A., Kumari, P., Nishad, J. H., Gautam,
V. S., Yadav, M., Bharti, R., Kumar, D., & Kharwar, R.
N. (2021). Advances in extraction technologies: Isolation
and purification of bioactive compounds from
biological materials. In Natural Bioactive Compounds
(pp. 409–433). Elsevier. https://doi.org/10.1016/B978-
0-12-820655-3.00021-5
Tolvanen, P., Kilpiö, T., Mäki-Arvela, P., Murzin, D. Yu.,
& Salmi, T. (2014). Esterification of Fatty Acids and
Short-Chain Carboxylic Acids with Stearyl Alcohol
and Sterols. ACS Sustainable Chemistry &
Engineering, 2(3), 537–545. https://doi.org/10.1021
/sc400467z
Wang, L., Chen, G., Tang, J., Ming, M., Jia, C., & Feng, B.
(2019). Continuous biosynthesis of geranyl butyrate in
a circulating fluidized bed reactor. Food Bioscience, 27,
60–65. https://doi.org/10.1016/j.fbio.2018.05.007
Widiyarti, G., Megawati, M., & Hanafi, M. (2019). The
Potential use of Geraniol Esters from Citronella Oil as
Anticancer Agents. Oriental Journal of Chemistry,
35(3), 987–996. https://doi.org/10.13005/ojc/350310
Study of the Temperature and Molarity Ratio Effects in Geraniol Esterification and Testing Its Antibacterial Activity
45

Yusmaniar, Wardiyah, & Nida, K. (2017). Mikrobiologi
dan Parasitologi. Kementrian Kesehatan Republik
Indonesia, 1-78.
Zabin, S. A. (2018). Antimicrobial, Antiradical Capacity
and Chemical Analysis of Conyza incana Essential Oil
Extracted from Aerial Parts. Journal of Essential Oil
Bearing Plants, 21(2), 502–510. https://doi.org/10.10
80/0972060X.2018.1465362
Zhaoshuang, L., Xinan, W., Wang Peng, W., Shanglin, C.,
Fan Guorong, F., & Zongde, W. (2016). Antimicrobial
activity of natural citral derivatives on food
deterioration bacteria. Journal of Zhejiang Agricultural
Sciences, 28(11), 1928–1933. https://doi.org/10.3969/
j.issn.1004-1524.2016.11.19
ICHR 2024 - BRIN’s International Conference for Health Research (ICHR)
46
