
Advances and Challenges of Machine Learning in Brain Medical
Imaging Data Analysis
Yinuo Zhang
Beijing Normal University-Hong Kong Baptist University United International College,
No. 2000 Jintong Road, Zhuhai, China
Keywords: Machine Learning, Deep Learning, MRI, Brain Tumor Detection, Brain Tumor Segmentation.
Abstract: In recent years, machine learning has become a critical method for medical imaging data analysis and has
solved many problems in medical imaging. This study focuses on brain medical imaging data analysis, and
summarizes the advances and challenges of machine learning in this field. The methods range from traditional
machine learning models to emerging machine learning models. These approaches have been improved and
solved many problems, such as image quality problem, generalization problem and so on. However, there are
still many challenges for machine learning in the field of brain medical imaging, including data annotation
and model training problem, non-interpretability of the model and Cross-domain and cross-site data
integration problem. These challenges not only affect the depth of research, but also hinder the clinical
translation of new technologies. Therefore, how to overcome these obstacles has become the key to promote
the further development of this field. This study also proposes some possible solutions to these challenges
that could be further explored in the future.
1 INTRODUCTION
Nowadays, brain medical imaging is becoming a
significant field because of the increasing number and
complexity of brain disorders. And the appearance of
machine learning has greatly contributed to the
development of this field. As noted by Shrivastava et
al. in recent studies, including preprocessing,
segmentation, feature extraction, and classification,
are helpful to the development of brain medical
imaging analysis. However, new brain diseases keep
coming up over time, and the existing machine
learning methods also have some challenges to
analysis the brain medical imaging specifically and
accurately.
This review aims to summarize the recent
advances of machine learning in brain medical
imaging and also the challenges faced by this field.
The advances represent the progress of machine
learning in brain medical imaging over the years,
including the improvement of old models and the
proposal of new models. The challenges represent
that there is still room for progress in machine
learning in this field, and there are still areas that need
to be improved and strengthened. This study has
sorted out these advances and challenges, which also
has great implications for future research and point
the way.
The rest of this paper is organised as follows:
Section 2,3,4 reviews the advancements and
discusses the challenges of machine learning in brain
medical imaging analysis, Section 5 summarizes the
advances and challenges and also has a discussion of
future research trends.
2 EFFECTS OF MACHINE
LEARNING IN BRAIN
MEDICAL IMAGING
This section will introduce the effects of machine
learning in the processing tasks of medical images,
which mainly focused on technical aspects.
2.1 Basic Overview
Machine learning and even the deep learning have a
mass of effects in the medical image of the brain, such
as image segmentation, classification, and
reconstruction. Fully convolutional networks (FCNs)
are being found useful. Compared with convolutional
Zhang and Y.
Advances and Challenges of Machine Learning in Brain Medical Imaging Data Analysis.
DOI: 10.5220/0013526400004619
In Proceedings of the 2nd International Conference on Data Analysis and Machine Learning (DAML 2024), pages 483-487
ISBN: 978-989-758-754-2
Copyright © 2025 by Paper published under CC license (CC BY-NC-ND 4.0)
483

neural networks (CNNs), FCNs return to full-
resolution images, which is better at biomedical
image segmentation.
Previous research by Barragán-Montero and
colleagues highlighted a new concept, adversarial
learning, means that the models are trained in the
presence of adverse samples. Generative adversarial
networks (GANs) have been improved step by step
and is widely used in the realm of medical imaging.
As shown by Barragán-Montero et al., GANs are
mostly used in multi-modality image translation and
data augmentation in synthetic picture synthesis.
There are also many other architectures such as
Support Vector Machine (SVM), Long Short-Term
Memory (LSTM), and U-NET (U-Net: Convolutional
Networks for Biomedical Image Segmentation) etc.,
have also been applied in medical imaging field.
2.2 Deep Learning for MRI
Reconstruction
As noted by Yadav et al. in recent studies, the use of
medical imaging is helpful for the recognition of
brain tumors, which could strengthen patient care and
lessen suffering. This is because magnetic resonance
imaging (MRI) uses no ionizing radiation. As shown
by Yadav et al., MRI could display many tissues in
high resolution and great contrast.
A study has shown that Hue, Saturation, Value
(HSV) Histogram and Gabor Wavelet could improve
the accuracy of the MRI imaging analysis. As shown
in Figure 1, the procedure includes preprocessing,
segmentation and clustering, and others.
Figure 1: The Proposed Work Flow (Yadav, D. C., Sharma,
N., & Kudari, J. M., 2023)
A technology proposed by another research has
also contributed to the improvement of MRI image
technology. Previous research by Zhao and
colleagues highlighted that three schemes make up
the technology: U-Net, modified Akima segmented
cubic Hermite interpolation (MASCHI) scheme, and
parallel semi-connected back-propagation neural
network (SJ-BPNN) scheme, which significantly
increased the image resolution.
2.3 Innovations in Deep Learning
Architecture
Based on the attention mechanism, a study by Yang
and colleagues has presented a multi-offset
reconstruction method (AMO-CEST), which can not
only accelerate Chemical Exchange Saturation
Transfer Magnetic Resonance Imaging (CEST-MRI)
acquisition, but also maintain suitable image quality.
This technology improves the quality of MRI image
technology to some extent.
3 MACHINE LEARNING IN
DISEASES DIAGNOSIS
This section will introduce the practical clinical
application of machine learning, especially the
specific tasks in the diagnosis of brain diseases. This
part mainly takes brain tumor disease as an example.
3.1 Deep Learning in Brain Tumor
Detection
A study by Tang and colleagues has proposed a
methodology based on Residual Network with 18
layers (ResNet18) deep learning architecture. In the
study, the authors evaluate neural network by testing
dataset and found that the accuracy, specificity, and
precision are better than previous models in deep
learning. This result indicates that the ResNet18
model can correctly classify the most of the images,
identify healthy brain images and avoid false
positives. One of the reasons is that the ResNet18
model is trained on a large dataset and then use
transfer learning to fine-tuned on a smaller dataset
(Tang et al., 2023). Additionally, ResNet18 model
have residual blocks, which are helpful to solve
gradient degradation problem. As a result, the
ResNet18 is crucial for the classification of brain
diseases, and then the analysis of medical imaging.
Another research by Zubair Rahman and
colleagues has presented a novel Artificial
Intelligence-driven (AI-driven) methodology based
on Efficient Neural Network B2 (EfficientNetB2)
DAML 2024 - International Conference on Data Analysis and Machine Learning
484
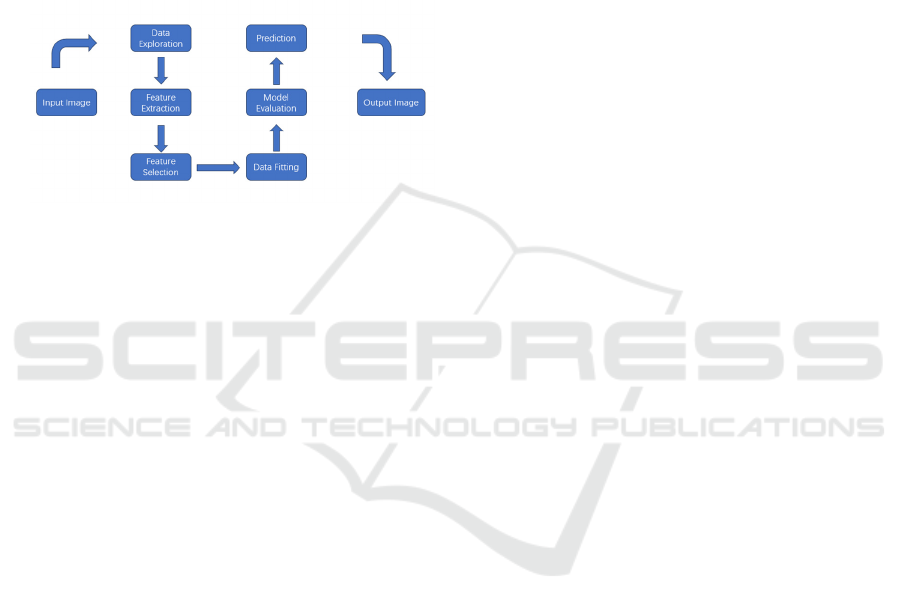
deep learning architecture. And the architecture
diagram of the model is shown in Figure 2. The
method could deal with a mass of problems that brain
tumor detection faced, such as noise and changes in
image quality, which is helpful for the detection of
brain tumors from MRI images (Zubair Rahman etal.,
2024). The study was tested on a mass of publicly
available data sets and show high accuracy. This
indicates that AI-driven tumor detection models are
not only innovative in theory, but also have wide
application potential in practice.
Figure 2: The Architectural Diagram of Model (Zubair
Rahman etal., 2024)
3.2 Deep Learning in Brain Tumor
Segmentation
As noted by Dong et al. in recent studies, good image
segmentation methods are crucial for the 3D
geometric modelling while diagnosing and operating.
To divide primary brain tumors from normal brain
tissues, a study proposed a deep learning method
based on a 3D U-net with deep supervision and multi-
scale in continuous experiments and innovations. 3D
U-net is used to process 3D medical image data,
making full use of spatial information in volume data
and preserving detailed features through jump
connections. The integration of deep supervision, that
is, supervised learning at multiple layers of the
network, not just at the final output layer, ensures that
multiple layers can be effectively trained.
Additionally, the multi-scale inputs enable the model
to handle tumor regions of different sizes, thus
improving the model's ability to adjust to intricate
tumor form.The algorithm performs exceptionally
well in terms of segmentation accuracy and
processing speed, according to experimental results.
The model is helpful for the clinical diagnosis.
Research by Taleb and colleagues has developed
five 3D self-supervised methods: 3D Contrastive
Predictive Coding, 3D Rotation prediction, 3D
Jigsaw puzzles, Relative 3D patch location, and 3D
Exemplar networks. Based on experimental results,
3D Self-supervised model performs better than 2D
models, in particular, significantly outperforms
models trained from scratch when using fewer
training samples. With 3D self-supervised pre-
training, the model is able to learn richer contextual
information, which is crucial for medical image
segmentation. And 3D Self-supervised model has the
ability to isolate tumors from MRIs accurately.
Additionally, 3D task for pre-training demonstrates
good cross-domain generalization, especially with
less labelled data. Future expansion could extend this
3D pre-training method to other 3D medical imaging
areas such as Computed Tomography (CT) scans,
Positron Emission Tomography (PET), etc. As a
result, self-supervised pretraining is particularly
suitable for scenarios where medical image data is
abundant but annotation is scarce.
4 MACHINE LEARNING
CHALLENGES IN BRAIN
IMAGING
This section will list some of the challenges of
machine learning in brain image data.
4.1 Data Annotation & Training Issues
As shown by Taleb et al., the scarcity of data and
annotations is a major challenge in model
development and application in the medical imaging
field. Acquiring medical image data is complex,
which require high-cost medical equipment and the
operation of professional personnel. Labelling of
these data consumes a mass of time and requires the
domain experts to participate. The accuracy of
labelling directly affects the performance of the
model. It is possible that transfer learning can be used
to reduce the reliance on large-scale labelled medical
data.
As noted by Liu et al. in recent studies, class
imbalances are also challenges of medical image
analysis. Proposing new loss functions has the
potential to solve this problem.
Additionally, as shown by Li et al., although the
machine learning-based brain image analysis
methods proposed by this study have good
performance, it is still difficult to find a balance
between efficiency and accuracy. The computational
complexity could be reduced and the inference speed
improved while maintaining model performance
through model compression technology.
Advances and Challenges of Machine Learning in Brain Medical Imaging Data Analysis
485

4.2 Model Non-Interpretability
As shown by Eder et al., the internal decision-making
process of machine learning cannot be explained at all
when working with complex data, which makes it
difficult for healthcare professionals to trust and
verify Artificial Intelligence (AI) results.
Black box algorithms, for example, whose opacity
leads to a host of problems, including potential bias,
attribution of responsibility, patient autonomy, and
erosion of trust (Durán, J. M., & Jongsma, K. R.,
2021). Computer reliability theory supports the
reliability of algorithms without necessarily requiring
their transparency. However, it is crucial to note that
ethical concerns remain important. The doctors must
take the best care based on trust.
4.3 Cross-Domain & Cross-Site
Integration
When data is collected at multiple sites, differences
between the data can interfere with model training
due to different equipment, experimental conditions,
and participant characteristics (Bostami, B.,
Espinoza, F. A., van der Horn, H. J., Van Der Naalt,
J., Calhoun, V. D., & Vergara, V. M.,2022). The site
effect can reduce the generalization ability of the
model. Harmonization may solve this problem to
some extent, which can standardize the data and
improve the reliability of the model.
Datasets from different domains are difficult to
integrate because they differ in collection methods,
labelling standards, and formats (Said, A., Bayrak, R.,
Derr, T., Shabbir, M., Moyer, D., Chang, C., &
Koutsoukos, X., 2023). And data preprocessing
requirements may be different from domain to
domain. It is possible to solve this problem by
unifying data formats or creating flexible
preprocessing frameworks.
5 CONCLUSIONS
This study has discussed the advances in brain
medical imaging and a mass of innovative methods
and models. Although significant advances have
made in the field of brain medical imaging based on
machine learning, the scarcity of data and annotations,
non-interpretability of the model and the problem of
cross-domain and cross-site data integration limit the
broader application of medical learning in this area.
Future research should develop more generalizable
models and combine with interpretable technology.
By solving these problems, brain medical imaging
analysis will make more contributions to personalized
medical and precision medical.
REFERENCES
Barragán-Montero, A., Javaid, U., Valdés, G., Nguyen, D.,
Desbordes, P., Macq, B., ... & Lee, J. A. (2021).
Artificial intelligence and machine learning for medical
imaging: A technology review. Physica Medica, 83,
242-256.
Bostami, B., Espinoza, F. A., van der Horn, H. J., Van Der
Naalt, J., Calhoun, V. D., & Vergara, V. M. (2022, July).
Multi-site mild traumatic brain injury classification
with machine learning and harmonization. In 2022 44th
Annual International Conference of the IEEE
Engineering in Medicine & Biology Society
(EMBC) (pp. 537-540). IEEE.
Dong, Y., Wang, T., Ji, X., Li, Z., & Ma, C. (2023,
September). Primary brain tumors Image segmentation
based on 3D-UNET with deep supervision and 3D brain
modeling. In 2023 5th International Conference on
Robotics and Computer Vision (ICRCV) (pp. 53-57).
IEEE.
Durán, J. M., & Jongsma, K. R. (2021). Who is afraid of
black box algorithms? On the epistemological and
ethical basis of trust in medical AI. Journal of Medical
Ethics, 47(5), 329-335.
Eder, M., Moser, E., Holzinger, A., Jean-Quartier, C., &
Jeanquartier, F. (2022). Interpretable machine learning
with brain image and survival
data. BioMedInformatics, 2(3), 492-510.
Li, Z., Zhang, X., Müller, H., & Zhang, S. (2018). Large-
scale retrieval for medical image analytics: A
comprehensive review. Medical image analysis, 43,
66-84.
Liu, X., Gao, K., Liu, B., Pan, C., Liang, K., Yan, L., ... &
Yu, Y. (2021). Advances in deep learning-based
medical image analysis. Health Data Science, 2021.
Said, A., Bayrak, R., Derr, T., Shabbir, M., Moyer, D.,
Chang, C., & Koutsoukos, X. (2023). Neurograph:
Benchmarks for graph machine learning in brain
connectomics. Advances in Neural Information
Processing Systems, 36, 6509-6531.
Shrivastava, P., & Sharma, D. K. (2023, December). A
Review: Medical Image Analysis Using Deep Learning
Models. In 2023 12th International Conference on
System Modeling & Advancement in Research Trends
(SMART) (pp. 659-662). IEEE.
Taleb, A., Loetzsch, W., Danz, N., Severin, J., Gaertner, T.,
Bergner, B., & Lippert, C. (2020). 3d self-supervised
methods for medical imaging. Advances in neural
information processing systems, 33, 18158-18172.
Tang, M. C. S., & Teoh, S. S. (2023, March). Brain tumor
detection from mri images based on resnet18. In 2023
6th International conference on information systems
and computer networks (ISCON) (pp. 1-5). IEEE.
Yadav, D. C., Sharma, N., & Kudari, J. M. (2023,
December). Maximizing Insights from MRI Brain
DAML 2024 - International Conference on Data Analysis and Machine Learning
486

Images Segmentation through HSV Histogram and
Gabor Wavelet Transform, and Machine Learning-
Assisted Image Retrieval. In 2023 IEEE International
Conference on ICT in Business Industry & Government
(ICTBIG) (pp. 1-5). IEEE.
Yang, Z., Shen, D., Chan, K. W., & Huang, J. (2024).
Attention-Based MultiOffset Deep Learning
Reconstruction of Chemical Exchange Saturation
Transfer (AMO-CEST) MRI. IEEE Journal of
Biomedical and Health Informatics.
Zhao, L. Y., Xiao, L. Y., Cheng, Y., & Liu, Q. H. (2022,
July). Combined Machine Learning-Inversion Scheme
for Super-Resolution 3-Dimensional Microwave
Human Brain Imaging. In 2022 IEEE International
Symposium on Antennas and Propagation and USNC-
URSI Radio Science Meeting (AP-S/URSI) (pp. 894-
895). IEEE.
Zubair Rahman, A. M. J., Gupta, M., Aarathi, S., Mahesh,
T. R., Vinoth Kumar, V., Yogesh Kumaran, S., &
Guluwadi, S. (2024). Advanced AI-driven approach for
enhanced brain tumor detection from MRI images
utilizing EfficientNetB2 with equalization and
homomorphic filtering. BMC Medical Informatics and
Decision Making, 24(1), 113.
Advances and Challenges of Machine Learning in Brain Medical Imaging Data Analysis
487
