
Deep Learning Approaches for Early Detection and Classification of
Alzheimer's Disease
Diqing Xu
a
School of Computer Science and Engineering & College of Software Engineering & School of Artificial Intelligence,
Southeast University, Nanjing, Jiangsu, China
Keywords: Deep Learning, Alzheimer, VGG16, Inception_v4, ResNet50.
Abstract: Alzheimer's Disease (AD) is a neurodegenerative condition that presents major obstacles to early diagnosis
and classification. This study proposes a new deep learning-based method to classify preprocessed brain MRI
scans, incorporating techniques for transfer learning and data augmentation. Three Convolutional Neural
Network (CNN) models were utilized: the 16-layer Visual Geometry Group network (VGG16), Inception
version 4 (Inception_v4), and the 50-layer Residual Network (ResNet50). The dataset used in this research,
sourced from Kaggle, contains around 6,400 MRI scans, categorized into four classes: mild dementia,
moderate dementia, non-demented, and very mild dementia. A tailored data augmentation pipeline was
developed, utilizing techniques such as rotation, flipping, and intensity modifications. This was combined
with transfer learning by employing pre-trained models from large-scale image datasets, which were then
fine-tuned for AD classification. The performance of the VGG16, Inception_v4, and ResNet50 models was
tested under four experimental scenarios: baseline (without augmentation or transfer learning), data
augmentation alone, transfer learning alone, and a combination of data augmentation and transfer learning.
The findings demonstrated that the integration of transfer learning and data augmentation substantially
enhanced classification accuracy, with the top-performing model achieving an accuracy of 98.49%. This
method can enhance the accuracy and reliability of AD diagnosis, contributing to more timely intervention
and treatment.
1 INTRODUCTION
Alzheimer's disease is a neurological condition that
gradually deteriorates cognitive and memory
functions., ultimately affecting the completion of
simple daily tasks. Currently, it is the seventh leading
cause of death in the United States, making early
diagnosis and intervention crucial (Smithsonian
Magazine, 2023).
Imaging techniques like Computed Tomography
(CT), Magnetic Resonance Imaging (MRI), and
Positron Emission Tomography (PET) are employed
in traditional diagnostic methods to diagnose
Alzheimer's disease (AD). While these methods assist
in diagnosis, they have limitations in accuracy and
cost. CT can detect obvious changes like brain
atrophy but has limited utility in early AD detection
(Adduru et al., 2020). MRI reveals detailed brain
structures, such as atrophy and hippocampal volume
a
https://orcid.org/0009-0007-1886-2003
reduction, but cannot directly identify amyloid
plaques and neurofibrillary tangles (Clark, 2003).
PET can image β-amyloid plaques and tau tangles but
is expensive, poses radiation risks, is primarily used
for research, and has limited long-term sensitivity
(Trudeau, 2018).
Deep learning and artificial intelligence have
made great progress in Alzheimer's disease diagnosis.
Convolutional Neural Networks (CNNs) can identify
brain atrophy patterns, particularly changes in the
hippocampus and entorhinal cortex (Subramoniam,
2022), which are important early features of AD. By
training on large datasets of labeled MRI images,
simplified CNN models can effectively differentiate
between healthy and diseased brains. El-Assy et al.
suggest that CNN models with simple structures can
achieve 95% accuracy in a five-classification task
(El-Assy, 2024).
340
Xu and D.
Deep Learning Approaches for Early Detection and Classification of Alzheimer’s Disease.
DOI: 10.5220/0013517400004619
In Proceedings of the 2nd International Conference on Data Analysis and Machine Learning (DAML 2024), pages 340-348
ISBN: 978-989-758-754-2
Copyright © 2025 by Paper published under CC license (CC BY-NC-ND 4.0)
By assessing model performance across four
cognitive states, this study seeks to increase the
accuracy of early Alzheimer's disease detection by
offering a comprehensive overview of the model's
diagnostic skills at various phases. This research will
provide important evidence for developing early
intervention and treatment strategies for Alzheimer's
disease, thereby improving patients' quality of life.
2 METHODOLOGY
2.1 Dataset
The Alzheimer's Disease MRI dataset that is freely
accessible on the Kaggle platform was used in this
investigation. Images from four categories—Non-
Demented, Very Mild Demented, Mild Demented,
and Moderate Demented—are included in this
dataset. The dataset is compiled from multiple
sources, including various websites, hospitals, and
public databases.
The collection provides approximately 6,400 2D
image slices with an original resolution of 208 x 176
pixels. These images represent patients across various
age groups and genders, as illustrated in Figure 1. For
uniformity and processing efficiency, all images have
been resized to 128 x 128 pixels.
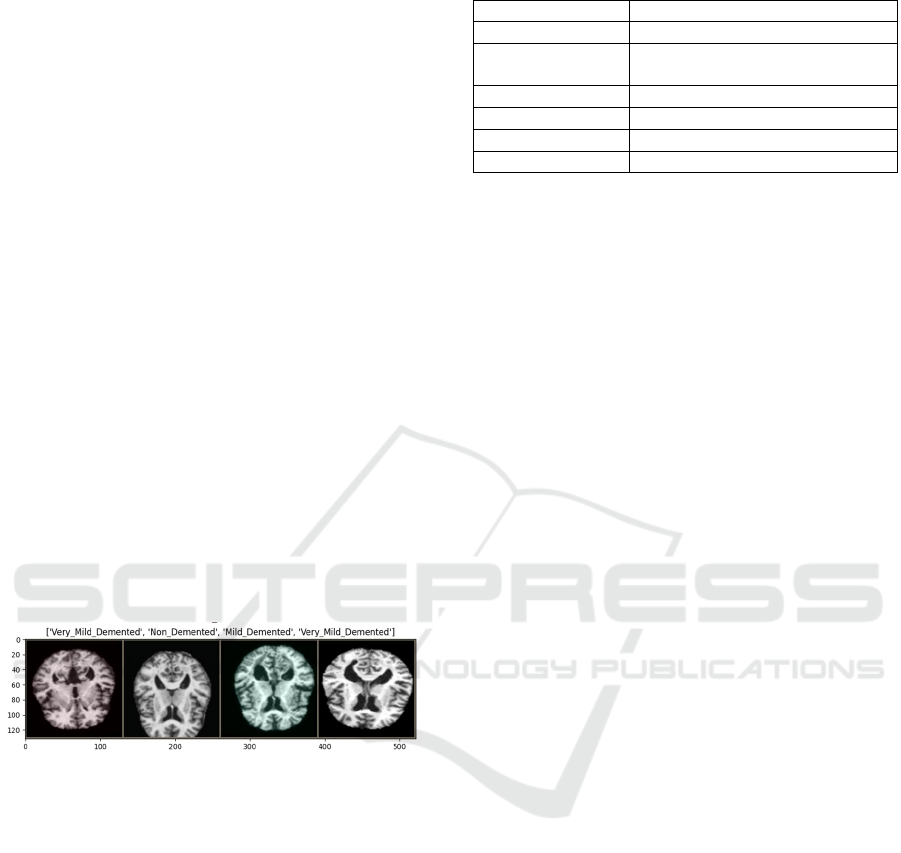
Figure 1: Alzheimer's disease MRI dataset (Photo/Picture
credit: Original).
Figure 1 displays carefully selected slice planes
from comprehensive 3D scan datasets, stored in PNG
format to ensure superior image quality and retain
fine details. All images have undergone extensive
preprocessing to remove any identifiable information,
ensuring strict adherence to ethical guidelines and
protecting patient confidentiality.
2.2 Experimental Setup
The high-performance computer environment used
for all of the study's experiments allowed for the most
effective training and assessment of the deep learning
models. Table 1 provides a summary of all the
experimental setup's detailed specifications.
Table 1: Experimental environment configuration
Name Confi
g
uration Information
O
p
eratin
g
S
y
stem Ubuntu 22.04
Programming
Language
Python 3.10
Framewor
k
P
y
Torch 2.1.0
CUDA 12.1
CPU Intel
(
R
)
Xeon
(
R
)
Platinum 8362
GPU RTX 3090(24GB)
The integration of hardware and software
configurations established an optimal computing
environment for the experiments, enabling efficient
model training, optimization, and evaluation.
Moreover, it ensured the consistency and
reproducibility of the experimental results.
2.3 Data Preprocessing
2.3.1 Dataset Partitioning
The original dataset was split into training and
validation sets using a random sample strategy to
ensure the efficacy and generalizability of the models.
The split ratio of 70:30, which is frequently utilized
in deep learning and machine learning studies, was
utilized. This ratio ensures an ample amount of
training data while preserving a sufficient number of
validation samples for model assessment. The
detailed dataset distribution is as follows:
Training set: 4,479 images (approximately 70%)
Validation set: 1,919 images (approximately
30%)
The dataset comprises a total of 6,398 images.
This splitting strategy ensures that the training set
includes a sufficient number of samples to capture
complex feature patterns, while the validation set
remains large enough to accurately assess model
performance and detect potential overfitting.A
stratified random sampling technique was employed
to preserve the original class distribution (Non-
Demented, Very Mild Demented, Mild Demented,
and Moderate Demented) across both the training and
validation sets. This method reduces sampling bias
and enhances the model's stability across all
categories.
To evaluate the model's capacity for
generalization further, an independent test set was
reserved. This test set remained completely unused
during the training and tuning processes and was
exclusively applied for the final model evaluation.
Detailed information about this test set will be
provided in subsequent sections.
Deep Learning Approaches for Early Detection and Classification of Alzheimer’s Disease
341
2.3.2 Data Augmentation
Extensive data augmentation techniques were used on
the training set to improve the model's generalization
and lower the possibility of overfitting. These
strategies were implemented using the argumentation
library, as detailed in Table 2. For the training set,
multiple augmentation methods were applied to
diversify the data.
In contrast, for the validation set, only center
cropping and standardization to maintain the original
characteristics of the data. This approach ensures that
the validation process accurately reflects the model's
output with unknown data, without the influence of
additional data augmentation. This carefully designed
data preparation and augmentation strategy, it aims to
maximize the value of available data while ensuring
the fairness and reliability of model evaluation.
Table 2: Data augmentation techniques
Feature Training set Validation set
Number of
Images
4479 1919
Proportion 70% 30%
Center Cro
pp
in
g
Yes
(
128x128
)
Yes
(
128x128
)
Random
Horizontal Flip
Yes (50%
p
robability)
No
Color Jitte
r
Yes (±10%) No
Random Rotation Yes (-15° to
15°)
No
Random
Translation and
Scaling
Yes (max 5%) No
Normalization Yes Yes
2.3.3 Data Normalization
Using the mean values (0.485, 0.456, 0.406) and
standard deviations (0.229, 0.224, 0.225) calculated
from the ImageNet dataset, all images in this study
were normalized. These values are widely used in the
field of computer vision for transfer learning tasks.
The normalization process helps the models in this
experiment converge faster and improves their
generalization ability, while also ensuring
compatibility with pre-trained models.
2.3.4 Class Balancing
To ensure that every class is fairly represented in the
training set, oversampling was applied to the minority
classes, especially for the "Moderate Dementia"
class, which was significantly oversampled. This
ensured that the model could sufficiently learn the
characteristics of each class during training, thus
improving overall model performance and
generalization ability. Specifically, the "Moderate
Dementia" class was augmented 100 times, while the
"Non-Demented" class was only augmented twice.
Following the process of class balancing, the dataset
comprised 8,960 photos categorized as Non-
Demented, 6,663 images classified as Very Mildly
Demented, 8,778 images classified as Mildly
Demented, and 8,800 images classified as Moderately
Demented.
2.4 Transfer Learning Strategy and
Early Stopping Mechanism
This study conducted comparative experiments on
transfer learning and non-transfer learning using three
deep learning models: ResNet50, VGG16, and
Inception_v4. The training process was further
optimized by incorporating an early stopping
mechanism. The following is a comprehensive
analysis of the experimental results.
2.4.1 Advantages of Transfer Learning
Transfer learning greatly sped up the training process
and enhanced the model's performance on the
validation set by using pre-trained model weights
from massive datasets like ImageNet. Transfer
learning showed definite benefits for all three models:
ResNet50, VGG16, and Inception_v4.
Specifically, the training loss and validation loss of
the transfer learning models decreased rapidly, and
the validation accuracy reached a high level within
the early epochs (see Figures 2-3). This rapid
convergence indicates that the pre-trained features
provided by transfer learning effectively guided the
model’s optimization direction early on, allowing the
models to adapt to the target task more quickly and
accurately.
DAML 2024 - International Conference on Data Analysis and Machine Learning
342
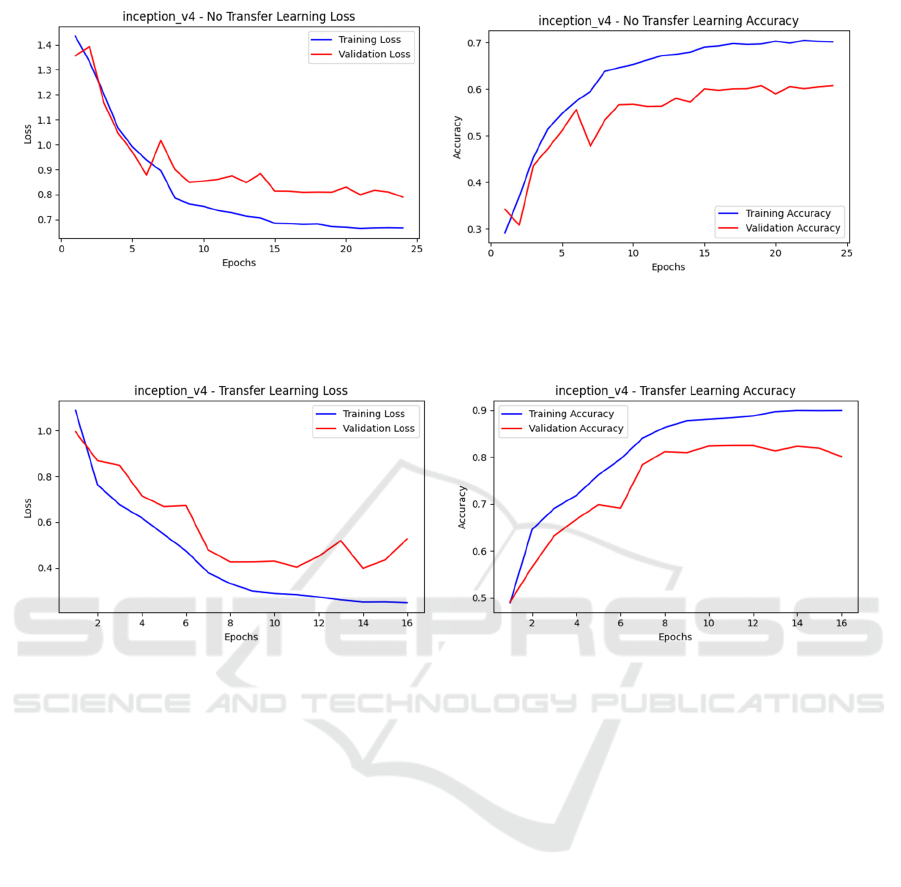
(a) (b)
Figure 2: Inception_v4 - non-transfer learning loss and accuracy curves, (a) non-transfer learning loss results; (b) non-transfer
learning accuracy curve results (Photo/Picture credit: Original).
(a) (b)
Figure 3: Inception_v4 - transfer learning loss and accuracy curves, (a) Transfer learning loss results; (b) Transfer learning
accuracy curve results. (Photo/Picture credit: Original).
Figure 2 shows the trends in loss and accuracy for
the model without transfer learning (No Transfer
Learning). In (a), the training loss gradually decreases
with the increasing number of epochs and eventually
stabilizes, indicating that the model's performance on
the training set is progressively improving. However,
the validation loss, after an initial decline, stabilizes
and exhibits some fluctuations. This implies that the
model can be overfitting if it exhibits strong
performance on the training set but poor
generalization on the validation set.
The accuracy curves in (b) further confirm this.
The training accuracy increases significantly with
more epochs, eventually approaching 0.7, while the
validation accuracy, after a rapid initial rise,
fluctuates and ultimately settles around 0.6. This
suggests that perhaps as a result of the model's poor
generalization capacity, the model's performance on
the validation set is not as strong as it was on the
training set.
In contrast, Figure 3 shows the training results of
the model under transfer learning. In (a), the training
loss rapidly decreases and eventually approaches zero,
while the validation loss also exhibits a significant
downward trend. Although there is slight fluctuation
in the later stages, the overall validation loss remains
at a relatively low level. This suggests that transfer
learning improves the model's performance on the
validation set and successfully addresses the
overfitting problem.
The accuracy curves in (b) further support this
conclusion. In comparison to the non-transfer
learning model, the training accuracy increases
significantly under transfer learning and finally
approaches 0.9, while the validation accuracy
stabilizes around 0.8. This demonstrates that transfer
learning greatly improves the model's generalization
ability, enabling it to achieve higher accuracy on the
validation set.
Similarly, in the ResNet50 model, the validation
accuracy of the model using transfer learning
ultimately reached approximately 0.95, whereas the
model without transfer learning exhibited greater
fluctuations in validation accuracy and was unable to
Deep Learning Approaches for Early Detection and Classification of Alzheimer’s Disease
343
achieve the same level. Both the VGG16 and
Inception_v4 models showed similar trends, where
the models with transfer learning demonstrated faster
decreases in training and validation losses, along with
significantly higher accuracy on the validation set.
2.4.2 Early Stopping Mechanism
The early stopping mechanism played a critical role
in the training process of all models, especially when
the performance on the validation set plateaued or
began to deteriorate. By setting a patience parameter,
the early stopping mechanism was able to terminate
training when no further improvements were
observed on the validation set, thus preserving the
model with the highest performance on the validation
set and preventing overfitting.
During the training of all transfer learning models,
the early stopping mechanism effectively reduced
unnecessary training time, ensuring that training was
halted once the model reached its optimal
performance. This approach not only improved
training efficiency but also sustained optimal model
performance on the validation set. For example, the
ResNet50 and Inception_v4 transfer learning models
ceased training when validation accuracy reached
approximately 0.95 and 0.85, respectively, while
validation loss remained consistently low.
2.4.3 Performance Comparison of Different
Models
While ResNet50, VGG16, and Inception_v4 have
distinct architectures, all three models showed
marked performance improvements when transfer
learning was paired with the early stopping
mechanism. However, differences in their
performance on specific tasks became evident. For
example, ResNet50 outperformed both VGG16 and
Inception_v4 on the validation set, likely due to the
strengths of its residual network in deep feature
extraction.
VGG16 displayed greater instability in non-
transfer learning conditions, resulting in lower
validation accuracy. This indicates that deeper
models may face challenges in learning effective
features from small datasets without pre-trained
weights. In contrast, Inception_v4 demonstrated
greater adaptability, achieving high accuracy
relatively quickly with transfer learning.
The performance and training efficiency of deep
learning models were significantly increased by the
combination of early stopping and transfer learning.
Models can quickly adjust to new tasks using transfer
learning with little data, and early halting helps avoid
overfitting. All models benefited from this approach,
with ResNet50 achieving the highest validation
accuracy. Future research could explore the
performance of various architectures in transfer
learning or further optimize the early stopping
mechanism to better meet diverse task requirements.
2.5 Comparison During the Training
Process
The experiment employed 4,479 training images and
1,919 validation images. The models' performance
was assessed by contrasting VGG16, Inception_v4,
and ResNet50 under four distinct experimental
conditions: baseline (no data augmentation or transfer
learning), data augmentation only, transfer learning
only, and a combination of both data augmentation
and transfer learning.
2.5.1 Validation Accuracy Under Different
Experimental Conditions
Table 3: Accuracy comparison of different models under
various experimental conditions
Experimental
Condition
VGG16 Inception_v4 ResNet50
Baseline (No
Data
Augmentation
or Transfer
Learning)
0.5020 0.5800 0.5690
Data
Augmentation
Only
0.5477 0.6071 0.6154
Transfer
Learnin
g
Onl
y
0.8800 0.8300 0.9050
Data
Augmentation
+ Transfer
Learnin
g
0.9823 0.8551 0.9922
Based on four experimental settings (Basis, Data
Augmentation Only, Transfer Learning Only, and
Data Augmentation + Transfer Learning), the
validation accuracy of the VGG16, Inception_v4, and
ResNet50 models was compared (Table 3). The
results show that the integration of transfer learning
and data augmentation yielded the maximum
precision for all models, with ResNet50 performing
best, achieving a validation accuracy of 0.9922.
VGG16 also demonstrated significant improvement,
reaching an accuracy of 0.9823 under this condition,
largely attributed to the augmentation techniques.
Inception_v4, though showing improvement,
experienced a relatively smaller gain, reaching an
accuracy of 0.8551 with the combined approach.
DAML 2024 - International Conference on Data Analysis and Machine Learning
344
2.5.2 Comparison of Loss Values Under
Different Experimental Conditions
Table 4: Loss values under four experimental conditions
Experimental
Condition
VGG16 Inception_v4 ResNet50
Baseline (No
Data
Augmentation
or Transfer
Learning)
1.0000 0.9500 0.9000
Data
Augmentation
Only
0.8804 0.8092 0.7841
Transfer
Learning Onl
y
0.5000 0.4500 0.3500
Data
Augmentation
+ Transfer
Learnin
g
0.1322 0.4073 0.0447
The trend in the loss values is consistent with the
validation accuracy results. When combining data
augmentation and transfer learning, the loss values
for all models decreased significantly, as shown in
Table 4. ResNet50 achieved the lowest loss value
under this condition, at 0.0447. VGG16's loss value
also dropped considerably to 0.1322. In comparison,
Inception_v4's loss value decreased to 0.4073, but the
improvement was not as pronounced as in the other
models.
2.5.3 Comparison of Training Time Under
Different Experimental Conditions
Table 5: Training time under four experimental conditions
Experimental
Condition
VGG16 Inception_v4 ResNet50
Baseline (No
Data
Augmentation
or Transfer
Learnin
g)
10m 0s 12m 30s 6m 45s
Data
Augmentation
Only
12m
23s
15m 24s 8m 10s
Transfer
Learnin
g
Onl
y
20m
15s
22m 40s 5m 30s
Data
Augmentation
+ Transfer
Learning
25m 1s 29m 17s 7m 2s
In terms of training time, Table 5 illustrates how
all models' training times rose with the introduction
of data augmentation and transfer learning. ResNet50
had the shortest training time under all conditions,
particularly in the combined condition, where it took
only 7 minutes and 2 seconds. VGG16 and
Inception_v4 had relatively longer training times,
with 25 minutes and 1 second and 29 minutes and 17
seconds, respectively, under the combined condition.
3 RESULT
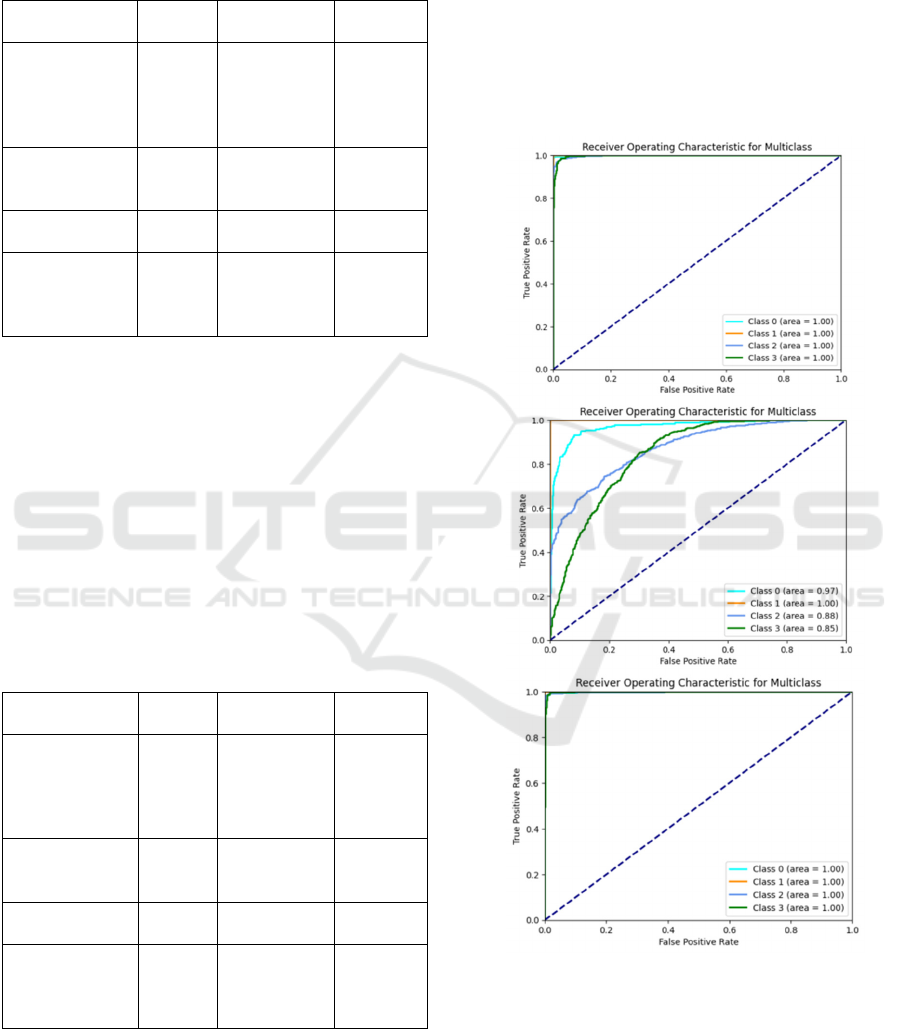
3.1 ROC Curve
Figure 4: ROC curves for the three models (Photo/Picture
credit: Original).
Through the analysis of the multi-class ROC
curves for the VGG16, Inception_v4, and ResNet50
models, this study found that the integration of
transfer learning with data augmentation significantly
improved the classification performance of the
Deep Learning Approaches for Early Detection and Classification of Alzheimer’s Disease
345
models, as shown in Figure 4. Both VGG16 and
ResNet50 achieved an AUC of 1.00 across all
categories, demonstrating extremely high
classification accuracy and generalization ability,
making them suitable for complex task scenarios. In
contrast, Inception_v4 showed slight shortcomings in
some categories. Although its performance was
significantly enhanced by combining transfer
learning with data augmentation, the AUC values for
certain categories still did not reach the optimal level.
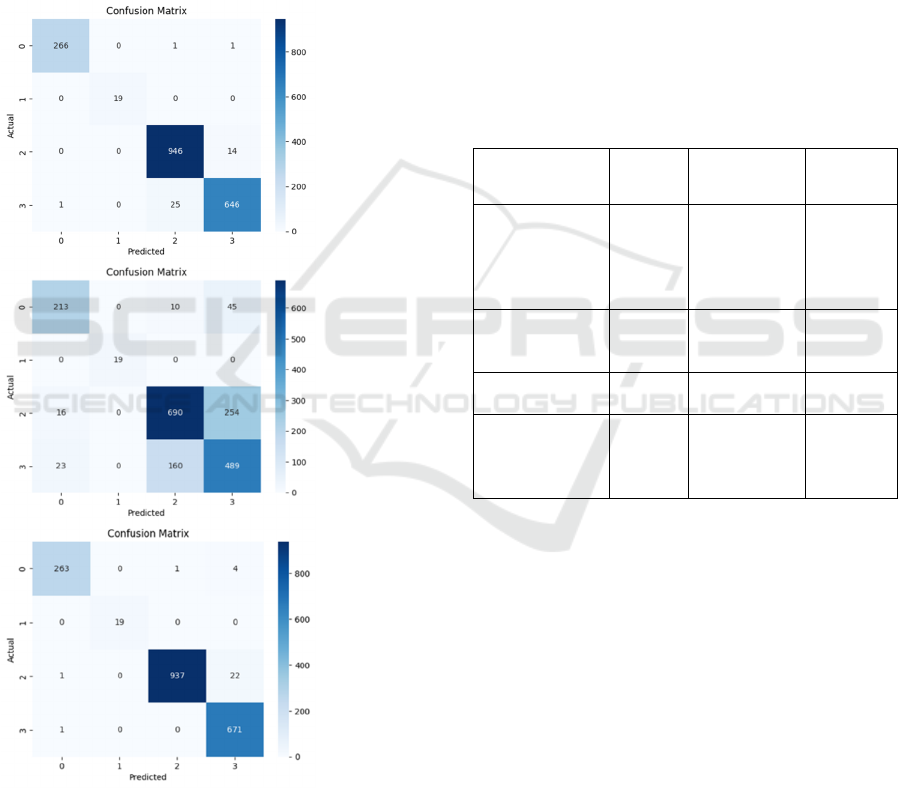
3.2 Confusion Matrix
Figure 5: Confusion matrix analysis of the three models
(Photo/Picture credit: Original).
Through the analysis of the confusion matrices, as
shown in Figure 5, it is evident that both VGG16 and
ResNet50 performed more consistently in the
classification task, particularly in distinguishing
between Class 2 and Class 3, where the
misclassification rate was significantly lower than
that of Inception_v4. Inception_v4 tended to struggle
when dealing with small differences between classes,
with a noticeable increase in misclassifications
between Class 2 and Class 3.
Overall, ResNet50 and VGG16 exhibited
excellent generalization and accuracy by utilizing a
blend of data enrichment and transfer instruction,
making them very successful for complex multi-class
classification tasks. By contrast, Inception_v4
exhibited a higher misclassification rate in certain
categories, revealing potential limitations in handling
more challenging or nuanced tasks.
3.3 Accuracy
Table 6: Comparison of accuracy under four experimental
conditions
Experimental
Condition
VGG16 Inception_v4 ResNet50
Baseline (No
Data
Augmentation
or Transfer
Learning)
0.5010 0.5504 0.6001
Data
Augmentation
Onl
y
0.5753 0.6326 0.6274
Transfer
Learning Onl
y
0.8354 0.7185 0.9592
Data
Augmentation
+ Transfer
Learning
0.9781 0.7353 0.9849
Table 6 compares the accuracy results of VGG16,
Inception_v4, and ResNet50 across four experimental
conditions. ResNet50 consistently outperformed the
other models in all scenarios, especially when both
transfer learning and data augmentation were applied,
achieving an accuracy of 0.9849. VGG16 also
showed notable improvement, reaching 0.9781 under
the same conditions. In contrast, despite the
enhancements from data augmentation,
Inception_v4’s highest accuracy reached only
0.7353, even when combined with transfer learning.
DAML 2024 - International Conference on Data Analysis and Machine Learning
346

3.4 Cohen's Kappa Coefficient
Table 7: Comparison of Cohen's kappa coefficients under
four experimental conditions
Experimental
Condition
VGG16 Inception_v4 ResNet50
Baseline (No
Data
Augmentation
or Transfer
Learning)
0.3507 0.3834 0.438
Data
Augmentation
Only
0.3034 0.4203 0.4169
Transfer
Learning Onl
y
0.5512 0.5816 0.696
Data
Augmentation
+ Transfer
Learnin
g
0.9639 0.5686 0.9752
When combining data augmentation with transfer
learning, ResNet50 achieved a precision of 0.9849
and a coefficient of Cohen's Kappa of 0.9752,
outperforming the other models (Table 7). This aligns
with Hasanah et al.'s findings, which also highlighted
the strong performance of ResNet in medical image
classification (Hasanah, 2023). However, ResNet50’s
longer training time, likely due to its deeper
architecture, was noted compared to simpler models.
VGG16 also showed notable improvement under
similar conditions, reaching a precision of 0.9781 and
a Cohen's Kappa coefficient of 0.9639, reflecting its
robust feature extraction capabilities, particularly
with transfer learning. However, the model's
extended training time could limit its applicability in
scenarios requiring rapid processing or real-time
updates.
Inception_v4, on the other hand, underperformed
compared to ResNet50 and VGG16, both in accuracy
and Cohen’s Kappa. Its highest accuracy was 0.7353,
with a Cohen's Kappa of 0.5686, likely due to the
complexity of its architecture, which made it less
efficient for MRI image analysis. Neshat et al.
similarly suggested that the Inception architecture
may require additional fine-tuning for certain medical
imaging tasks (Neshat, 2023). ResNet50 could
benefit from the incorporation of attention
mechanisms, which have been shown to enhance
model focus in medical image classification (Zhou et
al., 2022). For VGG16, knowledge distillation could
be a potential solution to its longer training time.
Hinton et al. demonstrated that this technique
effectively transfers knowledge from larger models to
smaller, faster ones, reducing training time while
maintaining accuracy (Hinton, 2015).
One limitation of this study is the diversity of the
dataset. Although approximately 6,400 MRI images
were included, this may not be enough to represent all
possible cases and variations. Future studies should
aim to expand both the diversity and size of the
dataset to better assess model performance across
various populations and Alzheimer's disease stages.
Additionally, this study primarily focused on 2D MRI
images, which may have overlooked crucial spatial
information available in 3D MRI data, potentially
impacting diagnostic accuracy.
Based on these findings and limitations, several
future research directions are recommended. First,
expanding the dataset to include more diverse MRI
data from patients of varying ethnicities, age groups,
and disease stages would enhance the model’s
generalizability. Second, the potential of 3D CNN
models should be explored, as Folego’s research
showed that 3D CNNs offer significant advantages in
diagnosing brain diseases (Folego, 2020).
Incorporating 3D MRI data could improve the
precision of Alzheimer’s diagnosis. Lastly, exploring
multimodal data fusion is essential. Song et al. found
that combining data from PET scans, genetics, and
other sources significantly improves diagnostic
accuracy in neurodegenerative diseases (Song et al.,
2021). These advancements could enhance the
reliability of models for deep learning in early
Alzheimer’s detection and offer more robust support
for clinical practice.
4 CONCLUSIONS
To identify and classify AD early on, this paper
proposes a deep learning-based method based on
brain MRI data. Through the combination of data
augmentation and transfer learning, the study
demonstrated considerable gains in classification
accuracy by comparing three CNN architectures:
ResNet50, Inception_v4, and VGG16. ResNet50, the
best-performing model, achieved 98.85% accuracy,
surpassing the baseline. These findings highlight the
potential of this approach to improve AD
classification accuracy with broad application
potential.
The innovation of this study lies in integrating
customized data augmentation techniques with
transfer learning to efficiently utilize small MRI
datasets, excelling in neurodegenerative disease
classification. This approach not only improves AD
classification but also serves as a reference for other
medical imaging tasks.
Deep Learning Approaches for Early Detection and Classification of Alzheimer’s Disease
347

Future research will focus on enhancing model
generalization and incorporating multimodal data
fusion. To improve generalization, the study aims to
collect multicenter data, standardize preprocessing
workflows, and apply domain adaptation techniques
to ensure robustness across clinical environments. For
multimodal data fusion, future efforts will integrate
biomarkers like MRI, PET scans, and cerebrospinal
fluid analysis, while developing architectures to
process heterogeneous data and explore optimal
fusion strategies. These efforts are expected to further
improve AD diagnosis and provide new insights into
diagnosing other neurodegenerative diseases, leading
to more accurate and comprehensive diagnostic
systems.
REFERENCES
Adduru, V., Baum, S. A., Zhang, C., Helguera, M., Zand,
R., Lichtenstein, M., Griessenauer, C. J. and Michael,
A. M., 2020. A method to estimate brain volume from
head CT images and application to detect brain atrophy
in Alzheimer disease. American Journal of
Neuroradiology, 41(2), pp.224-230.
Clark, C. M., Xie, S., Chittams, J., Ewbank, D., Peskind, E.,
Galasko, D., Morris, J. C., McKeel, D. W., Farlow, M.,
Weitlauf, S. L., Quinn, J., Kaye, J., Knopman, D., Arai,
H., Doody, R. S., DeCarli, C., Leight, S., Lee, V. M.-Y.
and Trojanowski, J. Q., 2003. Cerebrospinal fluid tau
and β-amyloid: How well do these biomarkers reflect
autopsy-confirmed dementia diagnoses? Archives of
Neurology, 60(12), pp.1696–1702.
El-Assy, A. M., Amer, H. M., Ibrahim, H. M. and
Mohamed, M. A., 2024. A novel CNN architecture for
accurate early detection and classification of
Alzheimer’s disease using MRI data. Scientific
Reports, 14(1), 3463.
Folego, G., Weiler, M., Casseb, R. F., Pires, R. and Rocha,
A., 2020. Alzheimer's disease detection through whole-
brain 3D-CNN MRI. Frontiers in Bioengineering and
Biotechnology, 8, 534592.
Hasanah, S. A., Pravitasari, A. A., Abdullah, A. S., Yulita,
I. N. and Asnawi, M. H., 2023. A deep learning review
of ResNet architecture for lung disease identification in
CXR images. Applied Sciences, 13(24), 13111.
Hinton, G., Vinyals, O. and Dean, J., 2015. Distilling the
knowledge in a neural network. arXiv.
Neshat, M., Ahmed, M., Askari, H., Thilakaratne, M. and
Mirjalili, S., 2023. Hybrid Inception Architecture with
residual connection: Fine-tuned Inception-ResNet deep
learning model for lung inflammation diagnosis from
chest radiographs. arXiv.
Smithsonian Magazine, 2023. Here’s where the highest
rates of Alzheimers are in the United States.
Song, J., Zheng, J., Li, P., Lu, X., Zhu, G. and Shen, P.,
2021. An effective multimodal image fusion method
using MRI and PET for Alzheimer's disease diagnosis.
Frontiers in Digital Health, 3, 637386.
Subramoniam, M., Aparna, T. R., Anurenjan, P. R. and
Sreeni, K. G., 2022. Deep learning-based prediction of
Alzheimer’s disease from magnetic resonance images.
In M. Saraswat, H. Sharma and K. V. Arya (Eds.),
Intelligent vision in healthcare, pp.183-198. Springer.
Trudeau, V. L., 2018. Facing the challenges of
neuropeptide gene knockouts: Why do they not inhibit
reproduction in adult teleost fish? Frontiers in
Neuroscience, 12, Article 302.
Zhou, Z., Lu, C., Wang, W., Dang, W. and Gong, K., 2022.
Semi-supervised medical image classification based on
attention and intrinsic features of samples. Applied
Sciences, 12(13), 6726.
DAML 2024 - International Conference on Data Analysis and Machine Learning
348
