
Exploring the Antibacterial Potential of Water Hyacinth
(Eichhornia crassipe (Mart.) Solm) Against
Staphylococcus epidermidis and Propionibacterium acnes
Amelia Febriani
a
, Rosario Trijuliamos Manalu and Novriana Devisari Damanik
Faculty of Pharmacy, Institut Sains dan Teknologi Nasional, Jakarta, Indonesia
Keywords: Antibacteria, Staphylococcus epidermidis, Propionibacterium acnes, Water Hyacinth (Eichhornia Crassipes),
Lake Toba.
Abstract: Water hyacinth (Eichhornia crassipes (Mart.) Solm) is recognized for its antibacterial, antipyretic, anti-
inflammatory, and diuretic properties, containing various active compounds such as saponins, flavonoids,
polyphenols, and alkaloids. The objective of this study was to evaluate the antibacterial activity against
Staphylococcus epidermidis and Propionibacterium acnes a 70% ethanolic extract of water hyacinth from
Lake Toba. The solid diffusion method was employed to determine the minimum inhibition zone, using
tetracycline as a positive control and 10% DMSO as a negative control. The results indicated significant
antibacterial activity of the extract against both bacteria. For Staphylococcus epidermidis, inhibition zones
measured 10.59 ± 0.07 mm (25%), 11.41 ± 0.04 mm (50%), 12.43 ± 0.10 mm (75%), and 14.44 ± 0.01 mm
(100%), with a minimum inhibitory concentration (MIC) of 25%. In comparison, the inhibition zones for
Propionibacterium acnes were 11.20 ± 0.08 mm (25%), 11.44 ± 0.01 mm (50%), 14.51 ± 0.04 mm (75%),
and 19.37 ± 0.12 mm (100%), with an MIC of 20%. These findings highlight the remarkable antibacterial
efficacy of water hyacinth extract at these concentrations and support its potential pharmacological and
therapeutic applications.
1 INTRODUCTION
Water hyacinth is well-known for causing major
environmental harm and imposing a major
management-related financial burden. Nevertheless,
it presents significant opportunities if effectively
utilized, particularly by rural areas. Variables include
high temperatures, eutrophic conditions, and other
environmental factors drive the plant to flourish at
places it has been introduced to. Considered to be one
of the most troublesome invading weeds worldwide,
white horehound is white Its control and eradication
are quite difficult and call for an all-encompassing
plan and community active participation (Harun et al.,
2021). By reducing oxygen levels and preventing
sunlight required for photosynthesis in submerged
aquatic plants, the dense mats created by water
hyacinth disturb water flow and lower fish
populations. Furthermore, these mats provide ideal
circumstances for the spread of disease-carrying
a
https://orcid.org/0009-0006-8015-5169
organisms such as mosquitoes, therefore aggravating
public health issues (Murugesh et al., 2023). Water
hyacinth's expansion in Lake Toba has blocked
sunlight, lowered fish counts, and hampered local
livelihoods depending on the lake's resources. Dense
mats of the plant develop on the surface of the lake,
upsetting the aquatic habitat and thereby reducing the
lake's ecological and aesthetic worth (Tobing &
Harahap, 2024)
Water hyacinth is clearly valuable as a source of
bioactive compounds and in the field of
phytoremediation, despite various challenges. The
plant is quite fit for bioremediation because of its fast
development rate and great biomass. From water
bodies, it can efficiently absorb heavy metals and
other pollutants (Peng et al., 2020). Water hyacinth's
phytochemicals contains several metabolites, such as
vitamins, tannins, saponins, terpenoids, phenolic
compounds, lignins, flavonoids, alkaloids, and
sterols. With the occurrence of all these secondary
metabolites, a wide range of therapeutic values has
Febriani, A., Manalu, R. T. and Damanik, N. D.
Exploring the Antibacterial Potential of Water Hyacinth (Eichhornia crassipe (Mart.) Solm) Against Staphylococcus epidermidis and Propionibacterium acnes.
DOI: 10.5220/0013489400004612
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of BRIN’s 2nd International Conference for Health Research (ICHR 2024), pages 19-27
ISBN: 978-989-758-755-9
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
19

been attributed to the plant, of which the alkaloids,
phenolic compounds, triterpenoids, flavonoids,
tannins, and saponins of the plant exhibited promising
pharmacological effects. (Gebrehiwot et al., 2022)
The search for antimicrobial drugs has been
heightened by the developing issue of antibiotic
resistance. Two known to be causes of skin infections
and inflammatory illnesses including acne vulgaris
are Staphylococcus epidermidis and
Propionibacterium acnes. Gram-positive bacterium
S. epidermidis uses chances to infect patients with
compromised immune systems and creates biofilms
on medical equipment, therefore complicating
therapy (Nguyen, 2017). Gram-positive bacterium P.
acnes causes inflammation and helps comedones to
grow, therefore aggravating acne (Dreno et al., 2015).
Previous investigations have indicated that water
hyacinth extracts have antibacterial qualities and can
fight several kinds of microbes rather successfully.
Indicating its natural antimicrobial action, Asmare
and Gure (2019) showed that extracts obtained from
water hyacinth showed considerable antibacterial
activities against several infections. Recent research
revealed how well methanolic and ethanolic extracts
made from water hyacinth leaves inhibited the growth
of bacteria—more especially, S. aureus and E. coli.
These results show the possible antibacterial
properties of water hyacinth extracts (Jouda et al.,
2016).
Flavonoids and alkaloids are among the
phytochemical components whose ability to generate
antimicrobial actions via several channels is well
known. Flavonoids can mess with bacterial cell
membranes and stop nucleic acid synthesis.
Conversely, alkaloids can stop bacterial protein
production (Cushnie & Lamb, 2011). This
investigation is to investigate the particular effects of
these compounds on S. epidermidis and P. acnes in
order to acquire a deeper knowledge of their possible
as alternative antibacterial agents.
The dual issue of controlling the invading spread
of water hyacinth and the pressing need for new
antibacterial drugs makes this work current and
important. Using water hyacinth as a source of
antibacterial compounds not only offers a possible fix
for the environmental problems brought about by this
invading species, but also supports the more general
effort against antibiotic-resistant bacteria. Still, there
are few thorough investigations comparing its
efficacy against S. epidermidis and P. acnes. This
work aims to close this gap by evaluating the
antibacterial activity of ethanol extracts obtained
from water hyacinth from lake Toba, North Sumatera,
Indonesia against these two medically important
infections.
2 MATERIAL AND METHOD
2.1 Material
The test microbes used were Staphylococcus
epidermidis and Propionibacterium acnes, which
were obtained from the Microbiology Laboratory,
Faculty of Pharmacy, USU. Mueller Hilton Agar
(Oxoid) was used as the growth medium. The test
material, water hyacinth (Eichhornia crassipes), was
sourced from Lake Toba Haranggaol. The solvent
used in the maceration process was 70% ethanol. For
phytochemical screening, various reagents and
substances were used, including aquadest (Brataco),
2N HCl, Dragendorff reagent, Mayer reagent, 70%
ethanol, Mg, concentrated HCl, anhydrous acetate,
chloroform, and concentrated H2SO4 (Lieberman-
Burchard). Antibiotic discs, tetracycline, and 0.9%
NaCl were used for the microbial test suspension
media. Tetracycline was used as the positive control,
while DMSO served as the negative control
2.2 Method
The leaves of the freshwater hyacinth, collected from
Lake Toba Haranggaol, North Sumatera, Indonesia
were identified as Eichhornia crassipes (Mart.)
Solms by the Herbarium Medanense Laboratory
(MEDA), North Sumatera University, Indonesia with
determination number 6342/MEDA/2021. After
collection, the leaves were cleaned and dried away
from direct sunlight to preserve their secondary
metabolites, followed by slicing. After that prepared
the powder from the dried leaves using the
maceration technique. The solution was filtered via
filter paper, then evaporated on a rotary evaporator to
concentrate the extract.
Water hyacinth extract were tested for
antimicrobial activity using the disc diffusion method
to find MIC at different concentrations like 25%,
50%, 75%, and 100%. The MIC against
Staphylococcus epidermidis and Propionibacterium
acnes was also determined by the solid dilution
method at a concentration of 25%, 20%, 15%, 10%,
and 5%.
ICHR 2024 - BRIN’s International Conference for Health Research (ICHR)
20

2.3 Preparation of Water Hyacinth
Extract
Water hyacinth was obtained from Lake Toba
Haranggaol and dried out of direct sunlight to avoid
damaging the secondary metabolites. To acquire 5 kg
of dried powder, the dried sample (simplicia) was
ground with a blender and sieved through a 60-mesh
sieve. This material was macerated with 25 liters of
70% ethanol, as ethanol concentrations exceeding
70% are less effective in dissolving low molecular
weight flavonoid compounds. The maceration
process entailed the powder being soaked in ethanol
for three days, with intermittent stirring. The resulting
solution was filtered through flannel cloth and re-
filtered using filter paper, followed by re-maceration
until it was clear. The filtrate was evaporated in a
vacuum rotary evaporator and subsequently in a water
bath to produce a thick extract. This extract was
subsequently stored in a dark glass container. (Jimmy
et al., 2019).
2.4 The Ethanol Examination of the
Extract
Testing of the extract for ethanol was performed to
ascertain that the extract to be tested on bacteria was
ethanol-free. Therefore, the inhibitory activity could
not be attributed to residual ethanol in the extract but
to the secondary metabolites of the simplicia. The test
was conducted by gradually adding 1N NaOH to 0.5
g of 70% ethanol extract of water hyacinth leaves,
then allowing it to settle for 3 minutes, then adding 2
mL of 0.1N iodine will result in a yellow precipitate
within 30 minutes, with an iodoform odor if the
extract still contains ethanol. (Saturño,et al, 2019).
2.5 Phytochemical Screening of Water
Hyacinth Extract
The chemical compounds in water hyacinth were
identified through qualitative phytochemical
screening. The subsequent assessments were
implemented during the examination (Baehaki et al.,
2023):
2.5.1 Alkaloid Identification
The mass was immersed in 20 mL of chloroform and
5 mL of 25% ammonia after 1 g of extract and powder
were added. Stir and heat the mixture over a water
boiler, and subsequently filter it. Vaporize the filtrate
until it is half filled. Pour the remaining evaporation
into a test tube and add 1 mL of 2N hydrochloric acid.
Shake the tube and allow it to form two layers. The
clear layer was divided into three test tubes (Tubes I,
II, and III) with equal quantities of Mayer's reagent in
Tube I, Dragendorf's reagent in Tube II, and
Bouchardat's reagent in Tube III. The formation of a
white precipitate with Mayer's reagent, a brown-black
precipitate with Bouchardat's reagent, and a scarlet
precipitate with Dragendorf's reagent were indicators
of the presence of alkaloids.
2.5.2 Identification of Flavonoids
Three mL of 70% ethanol was mixed with about 1 mL
of powder and it was stirred, warmed and then shaken
once more. The solution was then filtered. The filtrate
was added with 2 drops of concentrated HCl and 0.1
g Mg. The colours red, orange and green showed up
in the ethanol layer, which indicated the existence of
flavonoids.
2.5.3 Identification of Steroids and
Terpenoid
For a period of two hours, 20 mL of ether was
combined with 2 g of powder and extract. Next, it was
filtered and evaporated in an evaporation dish until
residue was obtained. The residual was subsequently
combined with 2 drops of anhydrous acetate and 2 mL
of chloroform. Subsequently, it was transferred to a
test tube and gradually introduced with 1 mL of
concentrated H2SO4 (Lieberman-Burchard) through
the tube wall. The presence of terpenoids was
indicated by the formation of a purple ring, while the
presence of steroids was indicated by a green color.
2.5.4 Tannin Identification
Ethanol was added to 2 g of powder until it was
completely submerged. Next, 1 mL of the solution
was transferred to a test tube and mixed with 2-3
drops of a 1% FeCl3 solution. The formation of a
blue-black or green color was indicative of a positive
outcome.
2.5.5 Identification of Saponin
A test vial was filled with 1 g of powder and extract,
which was subsequently added to 10 mL of hot water,
cooled, and vigorously shaken for 10 seconds. The
presence of saponins was indicated by the formation
of stable froth of 1–10 cm within less than 10 minutes,
which did not dissipate upon the addition of 1 drop of
2N hydrochloric acid.
Exploring the Antibacterial Potential of Water Hyacinth (Eichhornia crassipe (Mart.) Solm) Against Staphylococcus epidermidis and
Propionibacterium acnes
21

2.6 Antibacterial Activity
The antibacterial activity was evaluated using the disc
diffusion technique. 0.1 mL of bacterial suspension
was applied to petri dishes containing sterile Mueller
Hinton Agar (MHA) media. Test solutions at
concentrations of 100%, 75%, 50%, and 25% were
applied to paper discs and then placed on the agar
surface that had been previously inoculated with
bacteria. The plates were placed in an incubator set at
a temperature of 37°C for a duration of 24 hours. The
antibacterial activity was assessed by quantifying the
diameter of the transparent areas surrounding the
discs using calipers (EUCAST, 2019).
The solid dilution method was utilized to
determine the minimum inhibitory concentration
(MIC). Bacterial proliferation was detected starting
from the minimum concentration of the extract that
resulted in the formation of zones of inhibition.
Following the acquisition of the inhibition zone
results, the minimum inhibitory concentration (MIC)
was established using the indicated concentrations.
Solutions (1 mL), bacterial suspension (1 mL), and
MHA medium were combined and placed in petri
dishes. The solution was placed in a controlled
environment at a temperature of 37°C for a duration
of 24 hours. The minimum inhibitory concentration
(MIC) refers to the lowest concentration of the
antibacterial solution that effectively prevented the
development of microorganisms. (Thakur et al.,
2018)
3 RESULT AND DISCUSSION
3.1 Extraction and Pytochemical
Screening
The water hyacinth was extracted using the
maceration process, which was selected for its
simplicity, ease of use, and non-destructive effects on
the sample's constituents. Maceration is the process
of immersing powdered simplicia in a solvent that is
appropriate, and then extracting the active ingredients
at room temperature. The solvent utilized for this
technique was 70% ethanol. Ethanol concentrations
higher than 70% have been found to reduce the
extraction efficiency of total flavonoids. This is
because higher ethanol concentrations are less
effective at dissolving low molecular weight
flavonoid compounds. This observation aligns with
the findings of Sudirman et al. (2024).
Recent research has investigated several methods
and compounds for extracting phytochemicals from
water hyacinth, focusing on the efficiency and
productivity of various techniques. Nguyen et al.
(2019) examined the effectiveness of using
ultrasound-assisted extraction for the extraction of
flavonoids from water hyacinth. As observed in their
study, UAE showed a better extraction yield and was
efficient compared to the conventional method of
maceration. The UAE technique utilizes ultrasonic
waves to enhance the penetration of the solvent into
the plant material, offering a more efficient extraction
process.
Kumar and Singh, in their work 2020, have
studied different solvent systems for flavonoid
extraction from water hyacinth. It has been observed
that the solution containing ethanol and water in a
ratio of 50:50 showed maximum content of
flavonoids. This suggests that a balanced-polarity
solvent system can increase the efficacy of extraction.
This finding supports the principle that extraction of
flavonoids requires ethanol of a middle level, about
50-70%, since this concentration exhibits moderate
polarity.
The maceration technique in this study has
resulted in a yield of 160 grams of the concentrated
extract from 5 kg water hyacinth powder, which
works out to approximately 32% of the extract. The
yield obtained in this study is comparable to the
results published by Nguyen et al. (2019), who used
ultrasound-assisted extraction to obtain a yield of
35%, and Kumar and Singh, who in 2020 obtained
30% yield utilizing a mixture of ethanol and water in
a ratio of 50:50. These comparisons indicate that,
although maceration is an effective and easy
technique, other processes like UAE could give
slightly higher yields and efficiency.
The 70% ethanol extract of water hyacinth did not
exhibit any detectable ethanol in the ethanol-free test,
as evidenced by the absence of an iodoform odor and
the absence of yellow precipitate formation. This
confirmation indicates that the extract is able to
advance to the subsequent stage. The test was crucial
due to ethanol's antiseptic characteristics, which may
inhibit the growth of microorganisms. Thus, the lack
of ethanol guaranteed that any inhibitory effects were
solely caused by the plant's chemical components
rather than any remaining ethanol.
This discovery is consistent with recent
investigations that have shown that 70% ethanol is
efficient in extracting active chemicals while limiting
the amount of leftover ethanol. An investigation
conducted by Lim et al. (2022) demonstrated that
70% ethanol exhibited the most effective antibacterial
ICHR 2024 - BRIN’s International Conference for Health Research (ICHR)
22
activities while minimizing the presence of residues
that could potentially interfere with following testing
stages. A separate investigation has verified that
extracts made using a 70% ethanol solution exhibited
notable effectiveness in maintaining the bioactive
components, which are accountable for the
antibacterial characteristics, without any interference
from residual ethanol (Nguyen et al., 2019).
Phytochemical screening was done on both water
hyacinth powder and its 70% ethanol extract. Tests
were positive for the presence of various bioactive
compounds, including alkaloids, tannins, saponins,
and steroids in both powder form and ethanol extract.
The results of the phytochemical screening of the
powdered form and the 70% ethanol extract of water
hyacinth are summarized in Table 1.
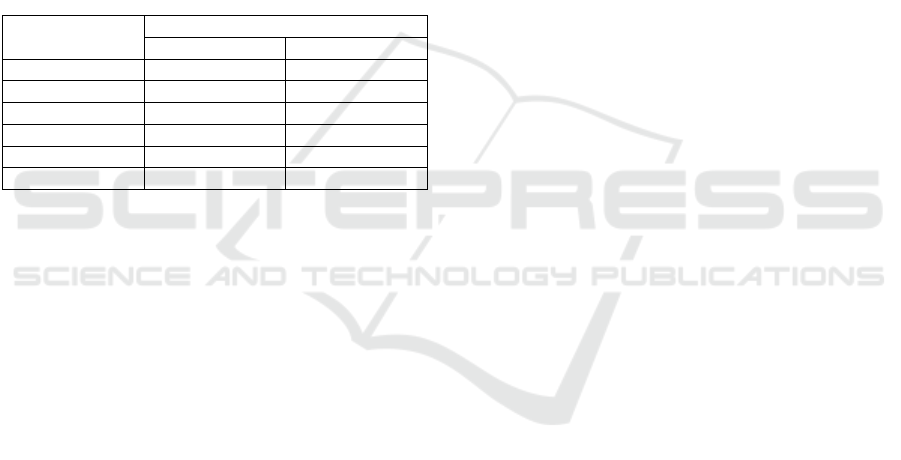
Table 1: Phytochemical screening of powder and 70%
ethanol of water hyacinth extract.
Chemical
compounds
Result
Extract Powde
r
Flavonoids - -
Alkaloids + +
Tanins + ++
Sa
p
onin ++ +
Steroi
d
++ +
Triterpenoids - -
(-): indicates the absence of compounds
(+): indicates the presence of compounds
Results for the saponin test were positive in
forming a stable foam of about 1 cm in height. This
foam persisted even after the injection of 2N HCl.
The test for triterpenoids was positive; it was
indicated by the formation of a purple ring.
The tannin test was positive as a black-green color
developed following the addition of 1% FeCl3. The
flavonoid test was also positive as an orange-yellow
color developed after the addition of 1N NaOH in the
testing of flavonoids. However, the results turned out
to be negative for alkaloids on both powder form and
extract. On the contrary, Mayer's reagent did not form
a white precipitate. Dragendorf's and Bouchardat's
reagents did not form red and brown precipitates,
respectively. Absence of the appearance of a green
tint indicates negativity in steroid testing.
Ben Bakrim et al., (2021) conducted a recent
investigation where they found that water hyacinth
extracts had comparable phytochemical profiles.
Their investigation substantiated the existence of
saponins, tannins, and flavonoids, while also
documenting discrepancies in alkaloid levels based
on the geographic source of the specimens. This
diversity agrees with the findings reported by Wang
et al. 2020, which also reported adverse outcomes for
both alkaloids and steroids. They proposed that
environmental parameters related to soil composition,
water quality, and climate conditions generally have
a strong bearing on phytochemical composition in the
water hyacinth.
Moreover, a study conducted by Yan et al. (2022)
emphasized the significance of various extraction
techniques in identifying the amount and composition
of phytochemicals. The researchers discovered that
ethanol extracts contained a wide range of bioactive
chemicals, but specific alkaloids were more
effectively extracted using other solvents like
methanol or chloroform. These results confirm that
using ethanol extraction with a concentration of 70%
effectively separated tannins, saponins, and
flavonoids, but did not isolate alkaloids or steroids.
3.2 Antibacterial Activity
3.2.1 Inhibition Zone Diameter
In the antimicrobial activity test of the 70% ethanol
extract of water hyacinth using the disc diffusion
method, each disc was impregnated with 20 µL of
extract at concentrations of 25%, 50%, 75%, and
100%. The 10% DMSO was chosen as the solvent
because it can dissolve both polar and nonpolar
substances and does not possess antibacterial
properties, ensuring that any observed antimicrobial
effects are due to the extract itself
The results showed a clear concentration-
dependent increase in the inhibition zones, with
higher concentrations producing larger zones of
inhibition against Staphylococcus epidermidis and
Propionibacterium acnes. Detailed data on the
inhibition zones can be seen in Table 2, while
antimicrobial activity of ethanol extract of water
hyacinth can bee seen in Figure 1, highlighting the
inhibition zones against Staphylococcus epidermidis
and Propionibacterium acnes.
Table 2 displays the measuring results of the
inhibition zone diameter of the 70% ethanol extract
of water hyacinth against Staphylococcus epidermidis
and Propionibacterium acnes. The obtained
inhibition zone diameters of Staphylococcus
epidermidis were 10.59± 0.07 mm, 11.41±0.04 mm,
12,43±0.10mm, and 14,44±0.01 mm for
concentrations of 25%, 50%, 75%, and 100%
respectively. The positive control, tetracycline,
successfully suppressed and eradicated Gram-
positive bacteria, whereas the negative control, 10%
Exploring the Antibacterial Potential of Water Hyacinth (Eichhornia crassipe (Mart.) Solm) Against Staphylococcus epidermidis and
Propionibacterium acnes
23
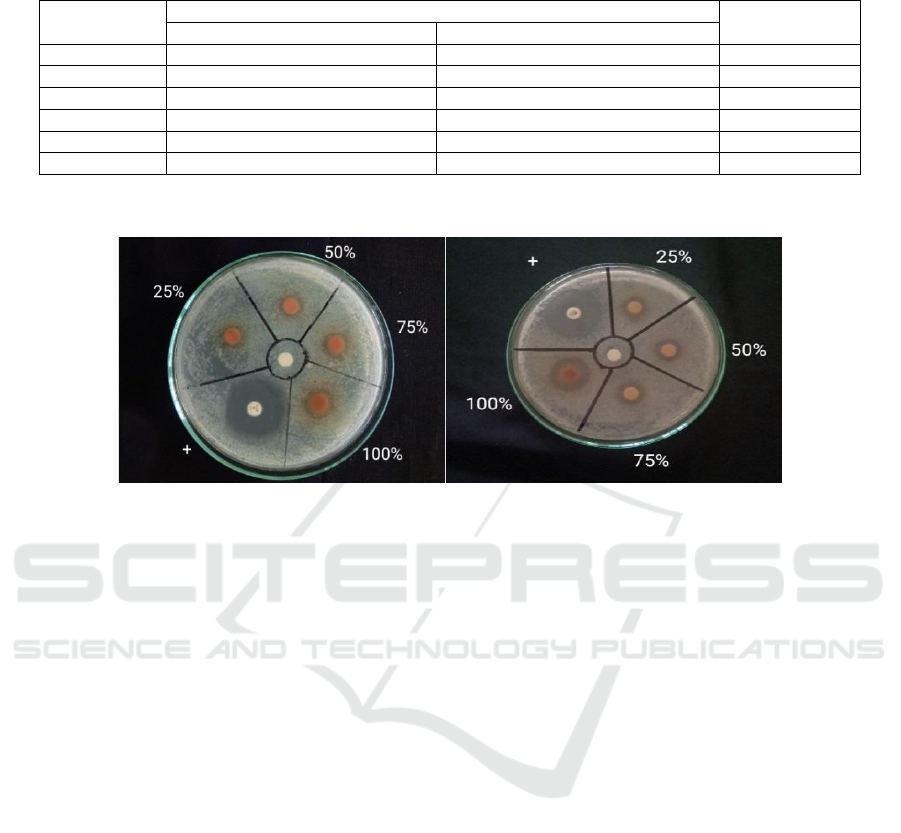
Table 2: Antimicrobial Activity Test Results of 70% Ethanol Extract of Water Hyacinth Against Staphylococcus epidermidis
and Propionibacterium acnes.
Concentration Inhibition Zone Diameter (mm) Inhibitory
response
Staphylococcus epidermidi
s
Propionibacterium acnes
WHE 25% 10.59± 0.07 11.20± 0.08 Moderate
WHE 50% 11.41±0.04 11.44±0.01 Moderate
WHE 75%
12,43±0.10 14,51±0.04 Moderate
WHE 100% 14,44±0.01 19,37±0.12 Moderate
Tetrac
y
cline 28,53±0.14 26,31±0.05 Stron
g
DMSO 10% - - None
(-): No inhibition zone formed; WHE: Water Hyacinth Extract
Data are means of three replicates (n = 3) ± standard error.
Figure 1: Inhibition Zone of Ethanol Extract of Water Hyacinth Against Staphylococcus epidermidis (right) and
Propionibacterium acnes (left).
DMSO, had no inhibitory effects due to the absence
of antibacterial characteristics in DMSO. Niu et al.
(2017) classified variation in inhibition zone diameter
into three categories: mild activity (0-9 mm),
moderate activity (10-14 mm), and strong activity
(>15 mm). The results indicate that the 70% ethanol
extract of water hyacinth, at concentrations of 25%,
50%, 75%, and 100%, has moderate activity. In
contrast, the positive control (tetracycline)
demonstrates significant activity
.
The measurement results of the inhibition
diameter of 70% ethanol extract of water hyacinth
against Propionibacterium acnes as shown in Table
1 were 11.20± 0.08 mm at 25% concentration,
11.44±0.01 mm at 50% concentration, 14,51±0.04
mm at 75% concentration, and 19,37±0.12 mm at
100% concentration. The negative control yielded
negative results compared to the positive control. The
positive control (tetracycline) was effective in
inhibiting and killing Gram-positive bacteria, while
the negative control (10% DMSO) did not show any
inhibition as DMSO does not have antibacterial
properties. These results categorize the 70% ethanol
extract of water hyacinth as having moderate activity
at concentrations of 25%, 50%, and 75%, and as
having strong activity at 100% concentration. The
positive control (tetracycline) is categorized as
having strong activity. Gram-positive bacteria tend to
be more sensitive to antibacterials due to their simpler
cell wall structure compared to Gram-negative
bacteria, allowing antibacterial compounds to enter
Gram-positive bacterial cells more easily. Gram-
positive bacteria have cell walls with more
peptidoglycan, fewer lipids, and contain
polysaccharides (teichoic acids) (Alhumaid et al.,
2021).
Triterpenoid chemicals suppress bacterial growth
by interacting with the porins, which are
transmembrane proteins located in bacterial cell walls
and which have been shown to ultimately result in the
formation of robust polymer bonds leading to
structural damage of the porins. This phenomenon
results in cellular damage that decreases the
permeability of the cell wall, which eventually leads
to a lack of nutrients and hence inhibiting or causing
the death of bacterial growth. (Wrońska et al., 2022).
Similarly, steroids demonstrate antibacterial activity
through their interaction with membrane lipids,
resulting in the release of bacterial liposomes. The
interaction between cell membrane phospholipids
and lipophilic substances renders the membranes
permeable, leading to a decrease in membrane
integrity and resulting in cell lysis (Yan et al., 2022)
Susceptibility to antibacterials depends on the
type of cell wall construction; high susceptibility is
seen in Gram-positive bacteria because of the simpler
ICHR 2024 - BRIN’s International Conference for Health Research (ICHR)
24
makeup of the cell wall when compared with the
complex makeup in Gram-negative bacteria. Other
factors that may influence antibacterial assays include
the quantity of the extract, the presence of other
secondary metabolites, incubation time, room
conditions, sterility of equipment, the number of
persons in the room, and how well asepsis is
maintained by the experimenter (Breijyeh et al., 2020.
These variables are essential for guaranteeing precise
and replicable outcomes in antibacterial research.
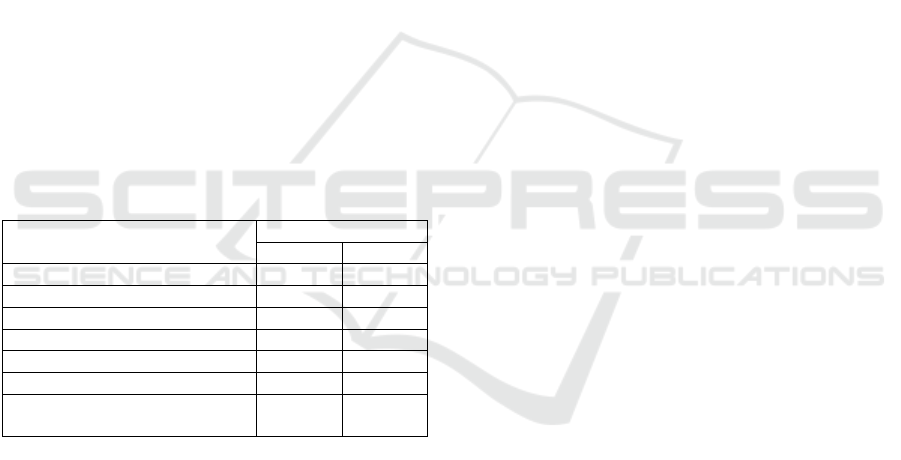
3.2.2 Minimum Inhibitory Concentration
(MIC) Test
The minimum inhibitory concentration, MIC, was
determined using the solid dilution test. This test was
carried out to establish the lowest concentration of the
sample that exhibited antibacterial activity against the
test microorganisms. The minimum inhibitory
concentration, MIC of the water hyacinth extract
concentration, was determined, whereby the zone of
inhibition considered to be the smallest was 25% and
then followed by further reduction to 20%, 15%,
10%, and lastly 5%.
Table 3: Minimum Inhibitory Concentration (MIC) Test
Results of 70% Ethanol Extract of Water Hyacinth Against
Staphylococcus epidermidis and Propionibacterium acnes.
Concentration Result
S.aureus P.acnes
WHE 25% - -
WHE 20% + -
WHE 15% + +
WHE 10% + +
WHE 5% + +
Negative Control (MHA Media) - -
Positive Control (Media +
Bacteria)
+
WHE: Water Hyacinth extract
(+) Indicates bacterial growth
(-) Indicates no bacterial growth
The study demonstrated that the 70% ethanol
extract of water hyacinth (Eichhornia crassipes)
exhibited significant antibacterial activity against
Staphylococcus aureus, with a Minimum Inhibitory
Concentration (MIC) determined at 25%. Bacterial
growth was observed at lower concentrations (5%,
10%, 15%, and 20%), indicating that higher
concentrations are necessary for effective inhibition.
This finding aligns with previous research
highlighting the potent antibacterial properties of
water hyacinth extracts against pathogens
(Kavinkumar et.al., 2023)).
Similarly, the extract showed effectiveness
against Propionibacterium acnes, with an MIC of
20%. Growth was detected at lower concentrations
(5%, 10%, and 15%) but not at 20% and 25%. This
suggests that the extract can effectively inhibit the
proliferation of P. acnes, a key contributor to acne
development. The results indicate that the bioactive
compounds in water hyacinth may disrupt bacterial
cell membranes or metabolic processes, preventing
further growth (Padmarini et.al, 2022). In conclusion,
the findings suggest that the 70% ethanol extract of
water hyacinth has potential as a natural antibacterial
agent against both Staphylococcus aureus and
Propionibacterium acnes. Further research is needed
to identify specific bioactive compounds responsible
for this activity and to explore their potential
applications in treating bacterial infectionstions in
treating bacterial infections.
4 CONCLUSION
The 70% ethanol extract of water hyacinth
(Eichhornia crassipes) demonstrated significant
antibacterial activity against Staphylococcus
epidermidis and Propionibacterium acnes. For S.
epidermidis, the inhibition zones were 10.59 ± 0.07
mm at a concentration of 25%, 11.41 ± 0.04 mm at
50%, 12.43 ± 0.10 mm at 75%, and 14.44 ± 0.01 mm
at 100%. The minimum inhibitory concentration
(MIC) was determined to be 25%, as bacterial growth
was effectively inhibited at this concentration.
Similarly, for P. acnes, the inhibition zones measured
11.20 ± 0.08 mm at 25%, 11.44 ± 0.01 mm at 50%,
14.51 ± 0.04 mm at 75%, and 19.37 ± 0.12 mm at
100%. The MIC values were 20%, indicating that the
extract exhibited strong antibacterial efficacy at these
concentrations.
ACKNOWLEDGEMENTS
The authors are grateful to the Department of
Pharmacy, National Institute of Science and
Technology, Jakarta, Indonesia and Department of
Pharmacy, North Sumatra University, Indonesia for
the encouragement and continuous support that
ultimately resulted in the fulfillment of this study.
Exploring the Antibacterial Potential of Water Hyacinth (Eichhornia crassipe (Mart.) Solm) Against Staphylococcus epidermidis and
Propionibacterium acnes
25

REFERENCES
Alhumaid, S., Al Mutair, A., Al Alawi, Z., Alzahrani, A. J.,
Tobaiqy, M., Alresasi, A. M., Bu-Shehab, I., Al-
Hadary, I., Alhmeed, N., Alismail, M., Aldera, A. H.,
AlHbabi, F., Al-Shammari, H., Rabaan, A. A., & Al-
Omari, A. 2021. Antimicrobial susceptibility of Gram-
positive and Gram-negative bacteria: A 5-year
retrospective analysis at a multi-hospital healthcare
system in Saudi Arabia. Annals of Clinical
Microbiology and Antimicrobials, 20(1), 43.
https://doi.org/10.1186/s12941-021-00450-x
Asmare, M., & Gure, A. 2019. Antibacterial and Antifungal
Properties of Water Hyacinth (Eichhornia crassipes): A
Review. Journal of Pharmacy and Pharmacology, 7(4),
212-220.
Baehaki, A., Lestari, S., Agustina, W., & Putri, S. D. 2023.
Phytochemical analysis and antioxidant activity of
water hyacinth flowers (Eichhornia crassipes) extract.
Pharmacognosy Journal, 14(1), 196-202.
https://doi.org/10.5530/pj.2022.14.196
Ben Bakrim, W., Ezzariai, A., Karouach, F., Sobeh, M.,
Kibret, M., Hafidi, M., Kouisni, L., & Yasri, A. 2022.
Eichhornia crassipes (Mart.) Solms: A comprehensive
review of its chemical composition, traditional use, and
value-added products. Frontiers in Pharmacology, 13,
842511. https://doi.org/10.3389/fphar.2022.842511
Breijyeh, Z., Jubeh, B., & Karaman, R. 2020. Resistance of
Gram-negative bacteria to current antibacterial agents
and approaches to resolve it. Molecules, 25(6), 1340.
https://doi.org/10.3390/molecules25061340
Cushnie, T. P. T., & Lamb, A. J. 2011. Recent advances in
understanding the antibacterial properties of
flavonoids. International Journal of Antimicrobial
Agents, 38(2), 99-107.
Dreno, B., Gollnick, H., Kang, S., Thiboutot, D., Bettoli,
V., Torres, V. A., & Leyden, J. J. 2015. Understanding
innate immunity and inflammation in acne:
Implications for management. Journal of the European
Academy of Dermatology and Venereology, 29(6),
1128-1134. https://doi.org/10.1111/jdv.13190
EUCAST/ The European Committee on Antimicrobial
Susceptibility Testing (2019). Routine and extended
internal quality control for MIC determination and disk
diffusion as recommended [Internet]. In: Ded by
EUCAST v 9.0. The European Committee on
Antimicrobial Susceptibility Testing. Available from:
http://www.eucast.org/ast_of_bacteria/qc_tables/
Gebrehiwot, H., Dekebo, A., & Endale, M. 2022. Chemical
composition, pharmacological activities, and biofuel
production of Eichhornia crassipes (water hyacinth): A
review. Journal of Environmental Science and
Technology. https://doi.org/10.1080/251758394
Harborne, A. J. 1998. Phytochemical methods a guide to
modern techniques of plant analysis. springer science &
business media
Harun, I., Pushiri, H., & Zulkeflee, Z. 2021. Invasive water
hyacinth: Ecology, impacts, and prospects for the rural
economy. Plants, 10(8), 1613. https://doi.org/
10.3390/plants10081613
Jimmy, J., Widiputri, D., & Gunawan, P. 2019. Study of the
pharmacological activity and heavy metal content of
Eichhornia crassipes extract. ICONIET Proceedings.
https://doi.org/10.33555/ICONIET.V2I2.17
Jouda, M.M., Elbashiti, T., & Masad, A. 2016. The
Antibacterial Effect of Some Medicinal Plant Extracts
and their Synergistic Effect with Antibiotics. Advances
in Life Science and Technology, 46, 59-69.
Kavinkumar, M. C., Praveena, S., Sivaranjani, S.,
Periyanayaki, B., Harsath, J. M., & Ayyappan, K. 2023.
Invitro anti-bacterial activity of leaf extract of
Eichhornia crassipes (Mart.) against Streptococcus
pneumoniae.
International Journal of Innovative
Research in Technology, 10(2), 173.
Kumar, R., & Singh, A. K. (2020). Comparative study of
flavonoid extraction from water hyacinth using different
solvents and techniques. Journal of Phytochemistry,
12(1), 45-52.
Lim K, Li WY, Dinata A, Ho ET. 2023. Comparing the
antibacterial efficacy and functionality of different
commercial alcohol-based sanitizers. PLoS ONE 18(3):
e0282005. https://doi.org/10.1371/journal.pone.0282005
Murugesh, V., Devi, S. K., Unni, P. M., Hemalatha, S., &
Venugopalan, V. 2023. Chemical properties of water
hyacinth plant ash. Journal of Advanced Zoology,
44(3), 922. https://doi.org/10.17762/jaz.v44i3.922
Nguyen, T. H., Park, M. D., & Otto, M. 2017. Host response
to Staphylococcus epidermidis colonization and
infections. Frontiers in Cellular and Infection
Microbiology, 7, 90. https://doi.org/10.3389/fcimb.
2017.00090
Nguyen, T. T. H., Lee, M. H., Lee, S. Y., & Kim, J. 2019.
Optimization of ultrasound-assisted extraction
conditions for water hyacinth (Eichhornia crassipes)
leaves. Journal of Food Science and Technology, 56(2),
768-777.
Niu, Y., Yang, C., Zhou, J., Huang, S., & Liu, J. 2017. Two
new compounds with antimicrobial activities from the
seeds of Voacanga africana. Phytochemistry Letters,
22, 80-84. https://doi.org/10.1016/j.phytol.2016.10.019
Padmarini, H. N., Voletta, R. S., Fauzia, S., Ulfah, N.,
Wijaksana, I. K. E., & Krismariono, A. 2022. Inhibition
activity of water hyacinth (Eichhornia crassipes) leaf
extract against Prevotella intermedia. World Journal of
Advanced Research and Reviews, 16(1), 735–741.
https://doi.org/10.30574/wjarr.2022.16.1.1099
Peng, H., Wang, Y., Tan, T. L., & Chen, Z. 2020. Exploring
the phytoremediation potential of water hyacinth by
FTIR spectroscopy and ICP-OES for treatment of
heavy metal contaminated water. International Journal
of Phytoremediation, 22(9), 935-945. https://doi.
org/10.1080/15226514.2020.1774499
Saturño, J. O., Maxion, M. T., & Bartolome, R. M. 2019.
Antibacterial activity of water hyacinth (Eichhornia
crassipes) extracts against Aeromonas hydrophila in-
vitro and in-vivo. International Journal of Biology
Pharmacy and Allied Sciences, 8(1), 1-10.
https://doi.org/10.31032/ijbpas/2019/8.1.4609
Sudirman, S., Herpandi, H., Rinto, R., Lestari, S., Harma,
M., & Aprilia, C. 2024. Effects of extraction
ICHR 2024 - BRIN’s International Conference for Health Research (ICHR)
26

temperature on bioactive compounds and antioxidant
activity of yellow velvetleaf (Limnocharis flava) and
water lettuce (Pistia stratiotes) leaf extract. Food
Research, 8(1), 113. https://doi.org/10.26656/fr
.2017.8(1).113
Thakur, S. G. A., Agrawal, P. K., & Shah, R. M. 2018.
Antimicrobial susceptibility testing of bacteria using
the solid dilution method. Journal of Clinical
Microbiology. https://doi.org/10.1128/JCM.01795-17
Tobing, N. S., & Harahap, R. H. 2024. Dampak adanya
pertumbuhan eceng gondok dalam skala besar terhadap
ekosistem di kawasan Danau Toba. Jurnal Ilmu Sosial
dan Ilmu Politik, 5(2). https://doi.org/
10.56552/jisipol.v5i2.133
Wang, H., Zhang, X., & Liu, Y. (2020). Influence of water
quality and environmental factors on the phytochemical
properties of water hyacinth (Eichhornia crassipes).
Environmental Science and Pollution Research, 27(6),
7154-7165. https://doi.org/10.1007/s11356-020-
08750-2
Wrońska, N., Szlaur, M., Zawadzka, K., & Lisowska, K.
2022). The synergistic effect of triterpenoids and
flavonoids—New approaches for treating bacterial
infections? Molecules, 27(3), 847. https://doi.org/
10.3390/molecules27030847
Yan, K., Cheng, X. J., Bian, G. L., Gao, Y. X., & Li, D. Q.
2022. The influence of different extraction techniques
on the chemical profile and biological properties of
Oroxylum indicum: Multifunctional aspects for
potential pharmaceutical applications. Evidence-Based
Complementary and Alternative Medicine, 2022,
8975320. https://doi.org/10.1155/2022/8975320
Yang, Y., Chen, K., Wang, G., Liu, H., Shao, L., Zhou, X.,
Liu, L., & Yang, S. 2023. Discovery of novel
pentacyclic triterpene acid amide derivatives as
excellent antimicrobial agents dependent on generation
of reactive oxygen species. International Journal of
Molecular Sciences, 24(13), 10566. https://doi.org/
10.3390/ijms241310566
Exploring the Antibacterial Potential of Water Hyacinth (Eichhornia crassipe (Mart.) Solm) Against Staphylococcus epidermidis and
Propionibacterium acnes
27
